Back to Journals » Drug Design, Development and Therapy » Volume 14
Lycopene Inhibits Epithelial–Mesenchymal Transition and Promotes Apoptosis in Oral Cancer via PI3K/AKT/m-TOR Signal Pathway
Received 27 February 2020
Accepted for publication 10 June 2020
Published 24 June 2020 Volume 2020:14 Pages 2461—2471
DOI https://doi.org/10.2147/DDDT.S251614
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Ran Wang,1,* Xinxing Lu,2,* Riyue Yu1
1Department of Stomatology, Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Urology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Riyue Yu
Department of Stomatology, Beijing Shijitan Hospital, Capital Medical University, Beijing 100089, People’s Republic of China
Email [email protected]
Background: Oral cancer (OC) is one of the most common cancers around the world. Despite the progress in treatment, the prognosis of OC remains poor, especially for patients with advanced diseases. It urges the development of novel therapeutic options against OC. Lycopene (LYC) is an antioxidant with chemoprotective properties against cancer. However, little is known about the mechanisms underlying the protective role of LYC in OC tumorigenesis.
Methods: In this study, we investigated the anti-cancer effect of LYC on the progression of OC in vitro and in vivo and explored the underlying mechanisms involved in this process.
Results: LYC inhibited OC cell proliferation, migration, invasion, apoptosis, and xenograft tumor growth in a dose-dependent manner. Furthermore, we found that LYC might inhibit epithelial–mesenchymal transition and induce apoptosis in OC cells by deactivating the PI3K/AKT/m-TOR signaling through increasing the levels of E-cadherin and Bax and downregulating N-cadherin, p-PI3K, p-AKT, p-m-TOR, and bcl-2.
Conclusion: We reported for the first time that LYC exhibited anti-cancer effects on OC development both in vitro and in vivo via regulating EMT process and apoptosis. These findings provide support for the potential clinical use of LYC in OC treatment.
Keywords: oral cancer, lycopene, EMT, apoptosis, AKT
Introduction
Oral cancer (OC) is one of the most common cancers worldwide.1,2 Despite the substantial progress in cancer treatment over the past decades, there has been little improvement in the survival rate of OC.3 Multiple genetic and epigenetic alterations related to cell proliferation, apoptosis, and epithelial–mesenchymal transition (EMT) have been found associated with the tumorigenesis of OC.4–6 Therefore, it is of vital importance to understand the molecular mechanisms underlying oral carcinogenesis and to search for novel effective therapeutic agents for OC patients.
It has been widely reported that cancer cells produce more reactive oxygen species (ROS) compared to normal cells.7 Moreover, increasing evidence has revealed that ROS mediate tumorigenesis through multiple signaling pathways, such as phosphatidylinositol 3-kinases (PI3K)/AKT/mammalian target of rapamycin (m-TOR), Wnt/β-catenin, and NF-kappa B signaling pathways.8–10 The substances that inhibit ROS production may possess therapeutic potential against cancers.
Recently, antioxidants have attracted increasing attention as chemoprotective agents. Lycopene (LYC) is a carotenoid antioxidant widely distributed in tomato, pink grapefruit, pomegranate, and watermelon. The potent antioxidant property of LYC is ascribed to its conjugated double bonds.11 LYC has been shown to exert anti-cancer effects on multiple types of cancers, such as prostate cancer,12 colon cancer,13 lung cancer,14 breast cancer,15 and gastric cancer.16 Previous epidemiologic studies also suggest that higher consumption of tomato products is associated with a reduced risk of oral cancer.17,18 In 2002, Livny et al reported that LYC might be an effective anti-carcinogenic agent in oral carcinogenesis.19 However, little is known about the mechanisms underlying the protective role of LYC in OC tumorigenesis.
This study aimed to explore the anti-cancer effects of LYC on OC progression using in vitro and in vivo models and to explore the mechanisms involved in the regulation of EMT and apoptosis by LYC treatment.
Materials and Methods
Ethics Statement
This study approved by the Committee of Animal Experimentation and the Ethics Committee of Capital Medical University and Beijing Shijitan Hospital. All experiments were performed according to the NIH guidelines for animal care and use.20
Antibodies and Reagents
LYC, complete Freund’s adjuvant (CFA), and corn oil were obtained from Solarbio (Beijing, China). The primary antibodies, including anti-GAPDH, anti-p-PI3K, anti-PI3K, anti-p-Akt, anti-Akt, anti-p-m-TOR, anti-m-TOR, anti-Bax, anti-bcl-2, anti-E-cadherin, anti-N-cadherin antibodies (at 1:1000 dilution respectively), HRP-labelled goat anti-mouse IgGs (at 1:2000 dilution), and antibodies for immunofluorescence staining were purchased from Abcam (Cambridge, MA, USA). Dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO, USA).
Cell Lines and Cell Culture
Human OC cell lines (CAL-27 and SCC-9) were obtained from the Cell Bank of Peking Union Medical College and cultured in RPMI-1640 medium (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) in a humidified atmosphere containing 5% CO2 at 37°C.
Cell Proliferation Assay (CCK-8 Assay)
Cell growth inhibition was evaluated using a CCK-8 kit (KeyGEN BioTECH, China) according to the manufacturer’s instructions. Cells in the logarithmic growth phase were seeded into 96-well plates at a density of 5×103 cells per 200 µL and cultured in a 37°C/5% CO2 atmosphere in triplicate. After a 24 h-incubation to allow cell attachment, the culture medium was replaced with fresh medium containing indicated concentrations of LYC for further 24, 48, or 72 hours. Two hours before each measurement time point, 10 µL CCK-8 was added to each well and co-cultured with cells for another 2 h in a humidified environment containing 5% CO2 at 37°C. The optical density value (absorbance) was recorded at 450 nm using an enzyme-linked immunosorbent assay plate reader (Bio-Rad Laboratories, Inc., Berkeley, CA, USA). The inhibition curves of LYC for each cell line were generated.
Colony Formation Assay
Cells were treated with vehicle control or LYC at indicated concentrations for 48 h. Then cells were trypsinized and dispensed into individual wells of 6-well plates at a density of 200 cells per well and incubated for 14 days without changing the medium. The colonies were fixed with 10% formaldehyde for 10 minutes and stained with Giemsa Stain solution (Solarbio Inc, Beijing, China) for 15 minutes. Then the number of colonies with > 50 cells was counted under a dissecting microscope. The percentage of viable cells was calculated. This experiment was performed in triplicate.
Cell Migration Assay (Wound Healing Assay)
Cell migration capacity was determined by wound healing assay. Cells were plated in a 6-well plate to reach 90% confluency followed by the treatment with vehicle control or LYC at indicated concentrations for 48 h. Subsequently, a sterile 200-μL pipette tip was used to scratch a vertical line in the cell plate. Cell debris was washed off with PBS. Cells were treated with serum-free RPMI-1640 medium for 48 h. Images were captured in triplicate for each condition using an inverted microscope at 0 h (baseline) and 48 h (endpoint). The wound healing area was detected using Image J software (Scion Corp., Frederick, MD).
Cell Invasion Assay (Transwell Assay)
A transwell assay was performed to evaluate cell invasive capability. The 24-well BioCoat cell culture inserts (BD Biosciences, Bedford, MA, USA) with a polyethylene terephthalate membrane (8-μm porosity) coated with Matrigel Basement Membrane Matrix (1 mg/mL; BD Biosciences) were used. The membranes of the upper chambers were coated with Matrigel (BD) and then incubated at 37°C for 6 h. Before each measurement, cells were treated with vehicle control or various concentrations of LYC for 48 h and then seeded in the upper chamber filled with serum-free media, while 0.6 mL culture medium supplemented with 10% FBS was added to the lower chamber. After 24-h incubation, cells were washed twice with PBS, fixed in paraformaldehyde, and then stained with Giemsa Stain solution (Solarbio Inc, Beijing, China). Cells adhered to the upper surface of the chamber were carefully removed using cotton swabs, whereas the ones invaded to the bottom surface of the membrane were photographed. The relative number of invaded cells was calculated as the number of cells counted on the bottom of the chamber in the experimental groups relative to the control group.
TUNEL Assay
The apoptotic cell death was detected by TUNEL assay. Cells were cultured in 12-well plates for 24 h followed by the exposure to various doses of LYC for 48 h. Then cells were harvested for the measurement of apoptosis using a TUNEL cell apoptosis in situ detection kit (KeyGEN, Nanjing, China) according to the manufacturer’s instructions. Positively-stained cells were counted using a microscope.
Western Blot Analysis
Protein lysates were prepared on ice using ice-cold RIPA buffer (Beyotime Institute of Biotechnology, Beijing, China) containing protease inhibitor cocktail (Roche, Switzerland). Protein concentration was quantified by the Bradford Protein Assay (Bio-Rad Laboratories, USA). After denatured at 100°C for 10 min, protein samples were loaded on and separated by SDS-PAGE and transferred onto a PVDF membrane. The nonspecific binding sites were blocked with 5% (w/v) fetal bovine serum at room temperature for 2 h. Then, the membrane was incubated with a mouse monoclonal antibody at 4°C overnight. After three washes with TBST buffer (15 minutes each time), the membrane was further incubated with an HRP-labelled goat anti-mouse IgG (1:2000 dilution; Abcam) at 37°C for 1 h. Finally, the chemo-luminescent signal was detected using the Clarity™ ECL Western Substrate (Bio-Rad Laboratories) and quantified using the Quantity One Software Version 4.1.1 (Bio-Rad Laboratories). GAPDH was used as a loading control for Western blots.
Animal Study
Male Balb/c nude mice were obtained from the Experimental Animal Ministry of Capital Medical University. All animals were allowed to adapt to the new surroundings for one week prior to the experiment. They (n = 20) were subcutaneously inoculated with 1×106 CAL-27 cells suspended in 100 μL mixture of Matrigel and PBS (1:1) through the right axillary fossa. These mice were randomly assigned into four groups (n = 5 per group), namely vehicle control group (Group 1), low-dose LYC group (Group 2), medium-dose LYC group (Group 3), and high-dose group (Group 4), which then received intragastric treatments with corn oil, LYC at 3 mg kg/d, LYC at 6 mg/kg/d, and LYC at 12 mg/kg/d, respectively. LYC was dissolved in corn oil in Groups 1, 2, and 3.21,22 When xenograft tumors reached a volume of approximately 100 mm3, the intervention treatment started. Tumor size was monitored every 3 days using caliper and tumor volume was calculated using the formula: L×S2×0.5, in which L represented the longest diameter of the tumor and S represented the shortest diameter of the tumor. After 28 days, mice were anesthetized with chloral hydrate and sacrificed by cervical dislocation. Tumor tissues were removed, weighted, and stored for further analyses.
Immunofluorescence Staining
At the end of the animal study, xenograft tumors were fixed in 10% phosphate-buffered formalin and embedded in paraffin for immunofluorescence staining. Sections at 5 µm thickness were deparaffinized with xylene, rehydrated in an alcohol gradient, immersed in 3% H2O2. Subsequently, samples were incubated with a primary antibody against PCNA or E-cadherin (1:100 dilution, Abcam) overnight at 4 °C and then with a secondary antibody (Invitrogen). Cells stained with DAPI were imaged and analyzed using a fluorescence microscope.
Statistical Analysis
All statistical analyses were performed using SPSS (22.0) and the data were shown as mean ± standard deviation (SD). The statistical differences among groups were assessed by Student’s t-test. The difference was considered statistically significant when a p-value was less than 0.05.
Results
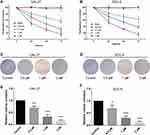
Lycopene Suppressed OC Cell Proliferation in a Dose- and Time-Dependent Manner
To investigate the biological function of LYC in OC cells, CCK-8 proliferation assay and colony formation assay were performed. The CCK-8 assay evaluated the viability of OC cells following LYC treatment. Results showed that LYC inhibited the proliferation of both CAL-27 and SCC-9 cells in a dose- and time-dependent manner (Figure 1A and B) as depicted by gradually decreased cell viability with increasing concentrations of LYC (0 µM, 0.5 µM, 1 µM, 2 µM) and longer treatment time (24h, 48 h, 72). Consistently, the colony formation assay showed that LYC treatment dose-dependently decreased the number of surviving cells in both CAL-27 and SCC-9 cell lines (Figure 1C–F). In other words, the colony formation ability of OC cells improved with the increase in the dosage of LYC.
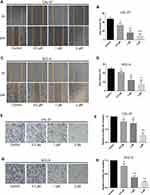
Lycopene Inhibited OC Cell Migration and Invasion in a Dose-Dependent Manner
The migration ability of OC cells exposed to different concentrations of LYC was assessed by wound healing assay. LYC at 0.5, 1, and 2µM inhibited the migration of OC cells in a concentration-dependent manner compared to vehicle-treated controls (Figure 2A–D). The invasive capability of cells in each group was evaluated by transwell assay and the results were consistent with those of the migration assay (Figure 2E–H). Taken together, lycopene inhibited the migration and invasion of human OC cells in a dose-dependent manner.
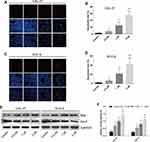
Lycopene Induced Apoptosis of OC Cells in a Dose-Dependent Manner
The apoptosis rates of cells treated with LYC at various concentrations were assessed by TUNEL assay. LYC treatment significantly and dose-dependently increased the apoptosis rate of OC cells (CAL-27 and SCC-9) compared with the vehicle control group (Figure 3A–D). The expressions of apoptotic proteins, Bax and Bcl-2, in all groups of cells were detected by Western blot. We found that LYC significantly increased the ratio of Bax/bcl-2 in comparison to the controls, indicating induced apoptosis in OC cells administered with LYC (Figure 3E and F). Taken together, these results showed that LYC significantly promoted the apoptosis of OC cells in a dose-dependent manner by regulating the expressions of pro-apoptotic protein, Bax, and anti-apoptotic protein, bcl-2, suggesting the pro-apoptotic effect of LYC on OC cells.
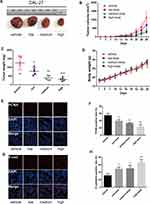
Lycopene Inhibited Tumor Growth and EMT in a Mouse Xenograft Model of OC
To identify the anti-tumor role of LYC on OC progression in vivo, we subcutaneously injected nude mice with CAL-27 cells and then treated them with vehicle or LYC at indicated doses. All mice were treated with vehicle control or LYC every day and the tumor size was recorded every three days. At 28 days after injection, mice were sacrificed and tumors were harvested (Figure 4A). The tumors of LYC-treated mice were smaller compared with the vehicle-treated group. LYC dose-dependently suppressed the growth of xenograft tumors in these animals (Figure 4B and C). Also, LYC was well tolerated by nude mice even at a dosage of 12 mg/kg/day. No side effects such as poor mental state or emaciation were found. The weight loss in LYC-treated mice was not significantly different among groups (Figure 4D), indicating that LYC might inhibit tumor progression in vivo without drug-related toxicity.
In addition, LYC treatment downregulated the expression of PCNA, a marker of proliferation,23 in CAL-27 cell xenograft tumors in a dose-dependent manner (Figure 4E and F), while the level of E-cadherin, a negative marker of EMT,24 was gradually and significantly upregulated in mice treated with LYC (Figure 4G and H). These results implied that LYC inhibited tumor growth and EMT process in mice inoculated with OC cells.
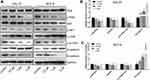
Lycopene Inhibited OC Tumorigenesis via Suppressing EMT and the PI3K/AKT/m-TOR Signaling Pathway
To elucidate the mechanism underlying the regulatory effect of LYC on OC, the EMT markers (E-cadherin and N-cadherin) and the key proteins related to the PI3K/AKT/m-TOR signaling pathway were analyzed by Western blot (Figure 5A).25,26 LYC treatment significantly and dose-dependently decreased the expression ratios of p-PI3K/PI3K, p-AKT/AKT, and p-m-TOR/m-TOR, but increased the ratio of E-cadherin/N-cadherin (Figure 5B and C) in OC cells. The above findings indicated that LYC might suppress EMT process and increase the apoptosis of OC cells via activating the PI3K/AKT/m-TOR signaling pathway.
Discussion
OC is a common fatal cancer that has become a major health burden around the world.27 Although great advances have been made in the management of OC, it still has a poor prognosis, particularly in patients with advanced OC.28 Also, current therapeutic measures cannot give satisfactory results.29,30 Therefore, researchers are investigating naturally-occurring substances that may be used as alternative treatment options for OC patients.
Some epidemiological studies showed that Mediterranean diet consisting of good amounts of vegetables, especially tomato, fruits, olive oil, grains, and fish, might contribute to lower rates of cancer.31–33 LYC is a major component in tomato and is also abundantly found in various fruits and vegetables.34 It acts as a potential chemoprotective agent against several types of cancers with minimum toxicity to normal cells.12 In 2002, Livny et al reported the anticancer impacts of LYC on human OC cell line KB-1.19 They showed that LYC inhibited KB-1 cell proliferation and enhanced gap-junctional communication between cancer cells in vitro. However, whether LYC exerted anti-tumor effects in vivo and the mechanisms involved in the regulation of LYC in OC development remain unclear.
In the present study, we confirmed the inhibitory effects of LYC on the proliferation of two OC cell lines (CAL-27 and SCC-9). Our data also showed that the anti-cancer properties of LYC were associated with a dose-dependent increase in apoptosis, and a reduction in proliferation, colony formation, migration, and invasion of OC cells. Furthermore, we demonstrated the anti-cancer potential of LYC in vivo for the first time. LYC significantly inhibited the growth of xenograft tumors in a nude mice model. Also, LYC treatment was well tolerated by nude mice and showed no toxicity at the doses tested. Then, the mechanisms underlying the regulation of LYC in apoptosis and EMT were further investigated. We found that LYC inhibited OC progression by regulating the expressions of EMT markers, apoptosis markers, and the key proteins related to the PI3K/AKT/m-TOR signaling pathway. These results implied the potential therapeutic value of LYC for OC treatment.
LYC targets multiple signaling pathways during cancer initiation, progression, and metastasis.35–37 The mechanism involved in the anti-cancer property of LYC is complicated. The PI3K/AKT/m-TOR signaling pathway plays an important role in tumorigenesis and cancer progression. Cancer cell survival and the acquisition of EMT are strongly associated with the activation of the PI3K/AKT/mTOR pathway.38–41 AKT is activated by the lipid product of PI3K and then phosphorylates its substrates, such as m-TOR, leading to an increase in cell proliferation and survival.42 The activation of the PI3K/AKT/m-TOR signaling pathway has been shown to upregulate a variety of oncogenes and growth factors, such as VEGF, c-myc, and survivin.43,44 Our results presented that PI3K/AKT/m-TOR signaling pathway was markedly suppressed by LYC in a dose-dependent manner, suggesting that LYC might inhibit OC cell proliferation and promote apoptosis through the PI3K/AKT/m-TOR signaling pathway.
EMT is a process that plays a pivotal role in tumorigenesis and metastasis.45,46 The activation of EMT in cancer cells allows them to dissociate from the primary tumor and invade into blood vessels.47 Cadherin switching (high N-cadherin expression and low E-cadherin expression), which is an indicator of EMT, is significantly associated with multiple endpoints of tumor progression and cancer-specific death in different types of cancer.48–50 In this study, we found that the expression of E-cadherin was upregulated while the level of N-cadherin was decreased by LYC treatment in a dose-dependent manner (Figure 5B and C), indicating the inhibitory effect of LYC on EMT process in OC.
Conclusions
In summary, this study demonstrated the anti-cancer effects of LYC on two OC cell lines and a mouse xenograft model. Our results indicated that LYC might suppress EMT and induce apoptosis in OC cells via activating the PI3K/AKT/m-TOR signaling pathway. These findings might support the development of new therapeutic options for OC patients. The potential clinical use of LYC in OC treatment needs to be further evaluated in clinical trials.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Leemans CR, Tiwari R, Nauta JPJ, Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993;71(2):452–456. doi:10.1002/1097-0142(19930115)71:2<452::AID-CNCR2820710228>3.0.CO;2-B
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
3. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–316. doi:10.1016/j.oraloncology.2008.06.002
4. Liang J, Liang L, Ouyang K, Li Z, Yi X. MALAT 1 induces tongue cancer cells’ EMT and inhibits apoptosis through Wnt/β‐catenin signaling pathway. J Oral Pathol Med. 2017;46(2):98–105. doi:10.1111/jop.12466
5. Attramadal CG, Kumar S, Boysen ME, Dhakal HP, Nesland JM, Bryne M. Tumor budding, EMT and cancer stem cells in T1-2/N0 oral squamous cell carcinomas. Anticancer Res. 2015;35(11):6111–6120.
6. Sasahira T, Kirita T, Kuniyasu H. Update of molecular pathobiology in oral cancer: a review. Int J Clin Oncol. 2014;19(3):431–436. doi:10.1007/s10147-014-0684-4
7. Kumari S, Badana AK, Malla R. Reactive oxygen species: a key constituent in cancer survival. Biomark Insights. 2018;13:1177271918755391. doi:10.1177/1177271918755391
8. Liou G-Y, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–496. doi:10.3109/10715761003667554
9. Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10(3):175–176. doi:10.1016/j.ccr.2006.08.015
10. Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metabol. 2014;2(1):17. doi:10.1186/2049-3002-2-17
11. Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev. 1998;56(2):35–51. doi:10.1111/j.1753-4887.1998.tb01691.x
12. Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94(5):391–398. doi:10.1093/jnci/94.5.391
13. Tang FY, Shih CJ, Cheng LH, Ho HJ, Chen HJ. Lycopene inhibits growth of human colon cancer cells via suppression of the Akt signaling pathway. Mol Nutr Food Res. 2008;52(6):646–654. doi:10.1002/mnfr.200700272
14. Palozza P, Simone RE, Catalano A, Mele MC. Tomato lycopene and lung cancer prevention: from experimental to human studies. Cancers. 2011;3(2):2333–2357. doi:10.3390/cancers3022333
15. Gloria NF, Soares N, Brand C, Oliveira FL, Borojevic R, Teodoro AJ. Lycopene and beta-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res. 2014;34(3):1377–1386.
16. Kim MJ, Kim H. Anticancer effect of lycopene in gastric carcinogenesis. J Cancer Prev. 2015;20(2):92. doi:10.15430/JCP.2015.20.2.92
17. Lu R, Dan H, Wu R, et al. Lycopene: features and potential significance in the oral cancer and precancerous lesions. J Oral Pathol Med. 2011;40(5):361–368. doi:10.1111/j.1600-0714.2010.00991.x
18. De Stefani E, Oreggia F, Boffetta P, Deneo-Pellegrini H, Ronco A, Mendilaharsu M. Tomatoes, tomato-rich foods, lycopene and cancer of the upper aerodigestive tract: a case-control in Uruguay. Oral Oncol. 2000;36(1):47–53. doi:10.1016/S1368-8375(99)00050-0
19. Livny O, Kaplan I, Reifen R, Polak-Charcon S, Madar Z, Schwartz B. Lycopene inhibits proliferation and enhances gap-junction communication of KB-1 human oral tumor cells. J Nutr. 2002;132(12):3754–3759. doi:10.1093/jn/132.12.3754
20. Council NR. Guide for the Care and Use of Laboratory Animals. National Academies Press; 2010.
21. Tang FY, Pai MH, Kuo YH, Wang XD. Concomitant consumption of lycopene and fish oil inhibits tumor growth and progression in a mouse xenograft model of colon cancer. Mol Nutr Food Res. 2012;56(10):1520–1531. doi:10.1002/mnfr.201200098
22. Tang F-Y, Pai M-H, Wang X-D. Consumption of lycopene inhibits the growth and progression of colon cancer in a mouse xenograft model. J Agric Food Chem. 2011;59(16):9011–9021. doi:10.1021/jf2017644
23. Hall P, Levison D, Woods A, et al. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some, neoplasms. J Pathol. 1990;162(4):285–294. doi:10.1002/path.1711620403
24. Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–3654. doi:10.1158/0008-5472.CAN-07-2938
25. Rogers CD, Saxena A, Bronner ME. Sip1 mediates an E-cadherin-to-N-cadherin switch during cranial neural crest EMT. J Cell Biol. 2013;203(5):835–847. doi:10.1083/jcb.201305050
26. Pan S-T, Qin Y, Zhou Z-W, et al. Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK-and PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells. Drug Des Dev Ther. 2015;9:1601.
27. Ramqvist T, Dalianis T. An epidemic of oropharyngeal squamous cell carcinoma (OSCC) due to human papillomavirus (HPV) infection and aspects of treatment and prevention. Anticancer Res. 2011;31(5):1515–1519.
28. Galluzzi L, Senovilla L, Vitale I, Michels J, Kroemer G. Molecular mechanism of cisplatin resistance. Oncogene. 2011;31(15):1869–1883. doi:10.1038/onc.2011.384
29. Tsai JY, Dillon JK. Chemoprevention in oral cancer. In: Improving Outcomes in Oral Cancer. Springer; 2020:13–22.
30. Messadi D, Le AD, Tanaka T, Wilder-Smith P. Oral cancer. In: Oral Diagnosis. Springer; 2020:99–111.
31. Tucker KL, Flanagan K. Differential cancer risk in latinos: the role of diet. In: Advancing the Science of Cancer in Latinos. Springer. 2020:69–77.
32. Obón‐Santacana M, Luján‐Barroso L, Freisling H, et al. Consumption of nuts and seeds and pancreatic ductal adenocarcinoma risk in the European prospective investigation into cancer and nutrition. Int J Cancer. 2020;146(1):76–84. doi:10.1002/ijc.32415
33. Tokudome S, Nagaya T, Okuyama H, et al. Japanese versus Mediterranean diets and cancer. Asian Pac J Cancer Prev. 2000;1(1):61–66.
34. Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274(2):532–538. doi:10.1016/0003-9861(89)90467-0
35. Rao AV, Agarwal S. Role of antioxidant lycopene in cancer and heart disease. J Am Coll Nutr. 2000;19(5):563–569. doi:10.1080/07315724.2000.10718953
36. Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91(4):317–331. doi:10.1093/jnci/91.4.317
37. Preedy VR, Watson RR. Lycopene: Nutritional, Medicinal and Therapeutic Properties. CRC Press; 2019.
38. Chang L, Graham P, Hao J, et al. Acquisition of epithelial–mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4(10):e875–e875. doi:10.1038/cddis.2013.407
39. Guo R, Meng Q, Guo H, et al. TGF-β2 induces epithelial-mesenchymal transition in cultured human lens epithelial cells through activation of the PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 2016;13(2):1105–1110. doi:10.3892/mmr.2015.4645
40. Chang L, Graham P, Hao J, et al. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014;5(10):e1437–e1437. doi:10.1038/cddis.2014.415
41. Zhang D-M, Liu J-S, Deng L-J, et al. Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis. 2013;34(6):1331–1342. doi:10.1093/carcin/bgt060
42. Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 2009;9(2):237–249. doi:10.2174/156800909787580999
43. Zhang Y, Ng PK-S, Kucherlapati M, et al. A pan-cancer proteogenomic atlas of PI3K/AKT/mTOR pathway alterations. Cancer Cell. 2017;31(6):820–832. e823. doi:10.1016/j.ccell.2017.04.013
44. Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy.
45. Nieto MA, Huang RY-J, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166(1):21–45. doi:10.1016/j.cell.2016.06.028
46. Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18(2):128. doi:10.1038/nrc.2017.118
47. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. cell. 2009;139(5):871–890. doi:10.1016/j.cell.2009.11.007
48. Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13(23):7003–7011. doi:10.1158/1078-0432.CCR-07-1263
49. Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147(3):631–644. doi:10.1083/jcb.147.3.631
50. Pontoriero GF, Smith AN, Miller L-AD, Radice GL, West-Mays JA, Lang RA. Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev Biol. 2009;326(2):403–417. doi:10.1016/j.ydbio.2008.10.011
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.