Back to Journals » International Journal of Nanomedicine » Volume 12
Lung-targeting drug delivery system of baicalin-loaded nanoliposomes: development, biodistribution in rabbits, and pharmacodynamics in nude mice bearing orthotopic human lung cancer
Authors Wei Y, Liang J, Zheng X, Pi C, Liu H, Yang H, Zou Y, Ye Y, Zhao L
Received 16 August 2016
Accepted for publication 31 October 2016
Published 29 December 2016 Volume 2017:12 Pages 251—261
DOI https://doi.org/10.2147/IJN.S119895
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Yumeng Wei,1 Jing Liang,1 Xiaoli Zheng,2 Chao Pi,1 Hao Liu,1 Hongru Yang,3 Yonggen Zou,4 Yun Ye,1,5 Ling Zhao1
1Department of Pharmaceutics, School of Pharmacy, Southwest Medical University, 2Department of Biochemistry, The Institute of Basic Medical Sciences, Southwest Medical University, Jiangyang District, 3Department of Oncology, The Affiliated Hospital of Southwest Medical University, 4Department of Orthopedics, The Affiliated Hospital of Traditional Chinese Medicine of Southwest Medical University, Longma Tan District, 5Department of Pharmacy, The Affiliated Hospital of Southwest Medical University, Luzhou City, Sichuan Province, People’s Republic of China
Abstract: The present study aims to develop a kind of novel nanoliposomes for the lung-targeting delivery system of baicalin as a Chinese medicine monomer. Baicalin-loaded nanoliposomes were prepared by the effervescent dispersion and lyophilized techniques. Baicalin-loaded nanoliposomes had an average particle size of 131.7±11.7 nm with 0.19±0.02 polydispersity index, 82.8%±1.24% entrapment efficiency and 90.47%±0.93% of yield and sustaining drug release effect over 24 h and were stable for 12 months at least. In vitro no hemolytic activity was observed for the experimental drug concentration. After intravenous administration of baicalin-loaded nanoliposomes to rabbits, drug concentration in the lungs was the highest among the tested organs at all time points and was significantly higher than that of its solution. For the targeting parameters, the relative intake rate and the ratio of peak concentration of lung were 4.837 and 2.789, respectively. Compared with plasma, liver, spleen, and kidney, the ratios of targeting efficacy (Te)liposomes to (Te)injection of lung were increased by a factor of 14.131, 1.893, 3.357, and 3.470, respectively. Furthermore, the results showed that the baicalin-loaded nanoliposomes did not induce lung injury. Importantly, baicalin-loaded nanoliposomes showed better antitumor therapeutic efficacy in the nude mice bearing orthotopic human lung cancer with the median survival time of blank liposomes (11.40±0.16 days), baicalin solution (17.30±0.47 days), and baicalin-loaded nanoliposomes (25.90±0.53 days). Therefore, the liposome is a promising drug carrier with an excellent lung-targeting property and therapeutic effect for the treatment of lung disease, such as lung cancer.
Keywords: liposomes, biodistribution, lung-targeting drug delivery, cancer therapy, baicalin
Introduction
According to the World Health Organization, lung cancer is the leading cause of cancer-related death.1,2 It was estimated that 1.37 million deaths were caused by lung cancer worldwide.3 Although there are some improvements in lung cancer therapy, the 5-year survival rate is as low as 15.9% for all stages combined.4 At present, chemotherapy, surgery, and radiotherapy are the main methods for the treatment of lung cancer. Unfortunately, 75% of patients with lung cancer diagnosed at the first time are found to have advanced disease that is incurable and lose the chance of accepting surgical treatment.4 Therefore, it was suggested that chemotherapy should be the most important measure in the treatment for patients with advanced stage and inoperable lung cancer. However, because most of drugs in conventional dosage forms are distributed throughout the body, the treatment of lung cancer is ineffective and results in serious side effects.3 Thus, lung-targeted drug delivery systems (LTDDS) have been recognized as an ideal strategy for the treatment of lung cancer.5
Baicalin is 7-D-glucuronic acid 5,6-dihydroxyflavone derived from the dried root of Scutellaria baicalensis Georgi, known as radix scutellariae, a basic and key medicinal composition unit in the traditional Chinese medicine.6,7 Extensive studies have shown that baicalin has strong anti-tumor effects.8–10 For the treatment of lung cancer, in vitro experiments, baicalin could suppress cell proliferation of human lung cancer A549 and mouse Lewis lung cancer in a dose- and time-dependent behavior. In in vivo study, baicalin could reduce tumor growth and prolong survival period in C57BL/6 mice bearing Lewis lung carcinoma tumor and nude mice bearing A549 carcinoma.11 In order to improve treatment efficacy of baicalin in lung cancer, it is necessary to develop LTDDS for baicalin.
The LTDDS mainly include liposomes, microspheres and nanoparticles via intravenous route.5,12–17 From the biomedical point of view, the liposomes composed of phospholipids and cholesterol are nontoxic, biocompatible, and biodegradable drug carriers. Liposomes as LTDDS have been extensively studied and have attracted increasing attentions in the past few decades.12,13 To date, no studies have been reported with respect to the LTDDS for baicalin. In this study, lung-targeting baicalin-loaded nanoliposomes were developed for the first time. For in vitro evaluation, baicalin-loaded nanoliposomes were evaluated in terms of mean size, polydispersity index (PDI), encapsulation efficiency, yield, in vitro release, stability, and hemolytic study. For in vivo evaluation, tissue distribution and lung-targeting characterization in rabbits were investigated after intravenous administration of baicalin-loaded nanoliposomes and its solutions as the control. Besides, we also evaluated the potential lung injury induced by baicalin-loaded nanoliposomes in rats and investigated pharmacodynamics in nude mice bearing orthotopic human lung cancer for the first time.
Materials and methods
Materials
The reference substance of baicalin used in the analysis was obtained from the National Institutes for Food and Drug Control of China (Beijing, China). The general baicalin was purchased from Xinxiang Bokai Bio-Technology Co., Ltd. (Henan, China). Rutin used as internal standard was obtained from Chengdu Mansite Pharmaceutical Co., Ltd. (Sichuan, China). Phosopholipon 90H (HSPC) was obtained from Shanghai Toshisun Bio-Technology Co., Ltd. (Shanghai, China). Matrigel was purchased from Shanghai Shenxiang Co., Ltd. (Shanghai, China). Citric acid (injection grade), carbonic acid monosodium salt (NaHCO3), Tween-80 (injection grade), dehydrated alcohol, and ammonium acetate were obtained from Luzhou Juhe Chemical Co., Ltd., (Luzhou, China). Acetonitrile and methanol (high-performance liquid chromatography [HPLC] grade) were obtained from Chengdu Jinghong Co., Ltd., (Chengdu, China). In all, 0.9% of sodium chloride injection was supplied from the Affiliated Hospital of Southwest Medical University (Luzhou, China). Ultrapure water used in this study was produced by water purification system in the laboratory.
Cells and animals
Human lung cancer A549 cells were purchased from Cell Bank, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in the Roswell Park Memorial Institute 1640 medium containing 10% fetal bovine serum, streptomycin (100 μg/mL), and penicillin (100 U/mL) in a humidified 5% CO2 atmosphere at 37°C.
New Zealand rabbits weighing 1.5–2.0 kg and Sprague Dawley rats weighing 150–200 g were provided by the Laboratory Animal Center of Southwest Medical University (Luzhou, China). The Balb/c nude mice in the weight range of 17–20 g were obtained from Jianyang Dashuo Biology Technology Co., Ltd. (Sichuan, China). The animals were housed in a temperature- and moisture-controlled (20°C±2°C and 55%±10%, respectively) room with a 12-h light–dark cycle and allowed free access to food and water. They were fasted for 12 h before experiment. All procedures involving animals complied with the requirements of the National Act on the Use of Experimental Animals (China). This study was approved by the Southwest Medical University Animal Ethical Experimentation Committee (No 2013002).
Preparation of baicalin-loaded nanoliposomes
Baicalin-loaded nanoliposomes were prepared by the effervescent dispersion technique. Briefly, baicalin, HSPC, Tween-80, and citric acid (mass ratio =2:2:1:1, respectively) were dissolved in ethanol. The solution was added dropwise to NaHCO3 water solution (0.5%, w/v) containing mannitol (5.0%, w/v) at 15°C–20°C under continuous stirring. The mixture solution was continuously stirred until it became cheese like. Finally, the cheese-like products were lyophilized and stored at 2°C–8°C for further investigation.
Particle size and PDI
The mean diameter and size distribution (PDI) of baicalin-loaded nanoliposomes were measured by NanoBrook 90Plus Zeta (Brookhaven Instruments, Holtsville, NY, USA) at 25°C. Before measurement, weighed freeze-dried powder was briefly dispersed in the ultrapure water and then was diluted with ultrapure water to achieve a suitable scattering intensity. Each sample was measured in triplicate.
Entrapment efficiency
The entrapment efficiency (EE) was determined by the equilibrium dialysis method with dialysis membranes (cutoff 8,000–10,000; Luzhou Kelong Reagent Co. Ltd., China). The samples of baicalin-loaded nanoliposomes were dispersed with ultrapure water and then dialyzed against phosphate-buffered saline (PBS; pH 7.4) for 4 h. After dialysis, free drug was determined by the HPLC. An aliquot of 0.2 mL of baicalin-loaded nanoliposome suspension was vortex mixed with 0.8 mL of methanol for 5 min. Afterward, the supernatant following centrifuging was measured by HPLC to obtain the total drug content in baicalin-loaded nanoliposome sample.
Briefly, HPLC was performed by using chromatographic system (pump: LPG-3400SD, UV–vis detector: VWD-3100, auto injector: WPS-3000 and column oven: TCC-3000, C18 column: Inertsil ODS-SP [4.6×250 mm, 5 μm particle size, made in Japan] and guard column: Phenomenex C18 [4.0×3.0 mm, 5 μm particle size, made in the USA]). The methanol, acetonitrile, and 0.4% (v/v) aqueous phosphoric acid (7.5:7.5:85, v/v, respectively) were chosen as a mobile phase at the detection wavelength of 278 nm. The flow rate was 1.0 mL/min at 35°C, and the injection volume was 20 μL.
The EE% was calculated by the following equation:
|
Wfree was the amount of free baicalin in liposomal samples, and Wtotal was the total drug amount in baicalin nanoliposomal formulation.
In vitro release
Baicalin released from its nanoliposomal formulation was evaluated by dialysis. The freeze-dried powder of baicalin-loaded nanoliposomes was redissolved with ultrapure water to obtain an appropriate drug concentration (baicalin, 7.5 mg/mL) and sealed in dialysis bags (cutoff 8,000–10,000; Luzhou Kelong Reagent Co. Ltd.). Then the samples were dialyzed against 100 mL of PBS (pH 7.4) as a release medium at a stirring rate of 100 rpm at 37°C. At predetermined time interval, 0.2 mL of release medium was collected and complemented with fresh PBS buffer at the same temperature. The supernatant obtained by centrifuging the sample solution was determined by the HPLC system as described earlier.
Stability studies
Dried baicalin-loaded nanoliposome samples were sealed at 4°C for 12 months. The samples were analyzed for the changes in particle size and EE at fixed time intervals, respectively.
Hemolytic study
Whole blood was obtained from the rabbit and stirred to remove fibrinogen by a glass stick. Afterward, red blood cells (RBCs) obtained by centrifuging at 1,000 rpm for 10 min were washed by normal saline. The same course was repeated to obtain clear and colorless supernatant. The RBCs were resuspended in normal saline to reach 5% (w/v) cell suspension and used further for hemolytic evaluation. The different volumes of baicalin nanoliposome suspension were added to 2.5 mL of the RBCs. The final volume was adjusted to 5 mL with normal saline. In addition, 2.5 mL of the RBCs treated with 2.5 mL of normal saline was considered as negative control, while water was considered as positive control. The mixtures were incubated at 37°C for 0.5, 1.5, and 3 h and then centrifuged at 1,000 rpm. The absorbance values of the supernatants were measured at 570 nm by a spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The percentage of hemolysis was calculated by the following equation:
|
Abs and Ab0 are absorbance values of the baicalin-loaded nanoliposome sample and negative control group, and Ab100 is the value of the positive control group.
Tissue distribution and lung-targeting evaluation
The rabbits were randomly divided into ten groups with five each. Baicalin-loaded nanoliposomes or its solutions as the control were administered intravenously from group 1 to group 5 or from group 6 to group 10 through their ear marginal vein with a single dose of 10 mg/kg body weight of baicalin. At 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 12, and 24 h after the intravenous administration, the whole blood samples were collected and immediately centrifuged at 5,000 rpm for 10 min to obtain plasma samples.
Afterward, the rabbits were sacrificed and heart, liver, spleen, lung, kidney, stomach, and brain were immediately removed at each time point and washed with 0.9% saline solution. The tissue samples were treated with filter paper to remove the redundant liquid, weighed, and homogenized. All plasma and tissue samples were frozen at −20°C until analyzed by HPLC.
In all, 50 μL of rutin internal standard solution containing 80.0 μg/mL of methanol and 250 μL of ammonium acetate buffer (pH 3.5; 1 M) were added to 0.5 mL of plasma or tissue homogenate samples in a centrifuge tube with 10 mL capacity and vortexed for 3 min. Then, 3 mL of acetonitrile was added to the solution by vortex mixing for 5 min. The clear supernatant obtained by centrifuging at 10,000 rpm for 10 min was collected and evaporated to dryness under nitrogen gas stream at 40°C. The residue was then resuspended in 200 μL of mobile phase and centrifuged at 10,000 rpm for 10 min. Then, 20 μL of the clear supernatant was injected into the HPLC system.
For the determination of baicalin in plasma and tissues, HPLC was performed using UltiMate 3000 series chromatographic system (Dionex, CA, USA) with separation on a reverse-phase C18 column Inertsil ODS-SP (4.6×250 mm, 5 μm) protected by a Phenomenex C18 guard column (4.0×3.0 mm, 5 μm). The mobile phase consisted of the mixture of methanol and acetonitrile (1:1, v/v) (A) and 0.4% (v/v) aqueous phosphoric acid (B) using a gradient elution of 85% B at 0–1 min, 85%–30% B at 1–14 min and 30%–85% B at 14–15 min. The flow rate was 1.0 mL/min, and the column temperature was maintained at 35°C. In all, 20 μL of sample solution was injected into HPLC and assayed at the wavelength of 278 nm.
On the basis of pharmacokinetic parameters obtained in this study, three targeting parameters, namely, the intake rate (Re), targeting efficacy (Te), and peak concentration ratio (Ce) were collected to evaluate the lung-targeting effect of baicalin-loaded nanoliposomes.
Evaluation of lung injury
Rats were randomly allocated into two groups with equal gender ratio. Both experimental groups received baicalin-loaded nanoliposomes via tail vein injection with a single dose of 100 mg/kg body weight of baicalin, while the control group received the same volume of normal saline. For each group, the rats were sacrificed immediately at the first, fourth, and seventh days after injection. Then lung tissues were removed and washed with cold saline. The lung tissues were fixed in 10% neutral formalin, embedded in paraffin, sliced at a thickness of 5 μm and stained with hematoxylin and eosin (HE). The histopathological studies were carried out to identify lung injury induced by LTDDS for baicalin-loaded nanoliposomes.
Pharmacodynamics
The Balb/c nude mice were anesthetized using intraperitoneal injection of pentobarbital sodium solution at a dose of 50 mg/kg body weight. Afterward, 1.0×107 human lung cancer A549 cells in the logarithmic growth phase suspended in 100 μL of PBS and Matrigel (1:1, v/v) were injected into right lungs of mice guided by multislice computed tomography (CT). At 7 days after tumor transplantation, the mice were scanned by high-resolution CT. Among the mice, three of them were randomly collected and sacrificed. Then lung was immediately removed and fixed in 10% (v/v) neutral-buffered formalin to process for HE staining. The results showed that the xenotransplantation model of human lung cancer A549 cells could be successfully established by this method.
On day 7, the nude mice bearing orthotopic human lung cancer were randomly divided into three groups. Each group (n=10) was injected intravenously with blank nanoliposomes (0 mg baicalin/kg body weight), baicalin solution (100 mg baicalin/kg body weight), and baicalin nanoliposomes (100 mg baicalin/kg body weight) at the same volume. The treatment of each group was started at 7 days after tumor transplantation, and they continued to die naturally on every other day. During the experimental process, when the mice naturally scarified, lung tumor was excised immediately and weighed accurately.
Data analysis
On the basis of the changes in drug concentration of plasma and tissues as a function time, pharmacology software Drug and Statistics Software 2.0 (Mathematical Pharmacology Professional Committee of China, Shanghai, China) was used to calculate pharmacokinetic parameters. Survival comparison between groups was conducted with Kaplan–Meier survival curves. All results were expressed as mean ± standard deviation (SD), and statistically significant difference between baicalin-loaded nanoliposomes and its injectable solution as control was evaluated using two-tailed t-test. The level of significant difference was set at P<0.05.
Results and discussion
Characteristics of baicalin-loaded nanoliposomes
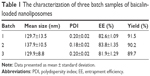
After freeze-drying of baicalin nanoliposomal formulation, the yellow and loose powder or granules were obtained. On the basis of the optimized formulation and technology, three batch samples of baicalin-loaded nanoliposomes were prepared and characterized in terms of particle size, PDI, zeta potential, EE, and yield (Table 1). Particle size and PDI of baicalin-loaded nanoliposomes measured by NanoBrook 90Plus Zeta were 131.7±11.7 nm and 0.19±0.02, respectively. The EE of baicalin-loaded nanoliposomes was 82.8%±1.24%, which was higher than that (34.62%–60.11%) of other results reported in the literature. Hong et al18 and Chen et al19 developed baicalin-loaded nanoliposomes and flexible nanoliposomes with EE of 34.62% and 60.11%, respectively. It was well known that there was a significant difference in EE of liposomes prepared by different formulations and preparation technologies.
In vitro release behavior
In the present study, dialysis bag method was chosen to investigate the in vitro release behavior of baicalin-loaded nanoliposomes. The results of in vitro release of baicalin from liposomal formulation and its solution (baicalin was dissolved in 0.1% NaOH and then HCl was used to adjust the pH value to be 7.3) as control are presented in Figure 1. As shown in Figure 1, baicalin liposomal formulation exhibited a slower release behavior in comparison with its solution. Baicalin released from the liposome carrier was 37.8%±1.9%, 78.5%±2.9%, and 89.6%±2.1% at 2, 12, and 24 h of dialysis, respectively, whereas, baicalin released from its solution was 90.0%±3.6% at 4 h. A similar trend in the vitro release behavior for baicalin-loaded nanoliposomes prepared in this study was observed compared to results reported previously.20,21 No significant difference in the rate of drug release in the case of three batch samples of baicalin-loaded nanoliposomes was observed (P>0.05). It further suggested that the optimal formulation possessed of a good reproduction.
  | Figure 1 Cumulative percent drug release from baicalin-loaded nanoliposomes and its solution (n=3). |
Stability studies
To determine whether baicalin liposomes as freeze-dried powder is more stable, baicalin-loaded nanoliposomes were stored at 4°C over a period of 12 months and sampled periodically to determine the particle size and EE% of the drug. The stability test of baicalin-loaded nanoliposomes is summarized in Table 2. Although a slight change was observed, namely, the mean particle size increased and EE (%) decreased as the storage time prolonged, the results demonstrated that the mean particle size and EE (%) had no significant change during 12 months (P>0.05).
  | Table 2 The stability of baicalin-loaded nanoliposomes (mean ± SD, n=3) |
Hemolytic study
It is well known that hemolytic activity is an important factor to investigate the quality of new drug dosage form for intravenous administration. As shown in Figure 2, baicalin liposomal formulation developed in the present study was found to possess negligible hemolysis of erythrocytes. Therefore, the encapsulation of baicalin in liposome carriers could reduce its hemolytic toxicity to a very low level. For example, maximum drug concentration at a dose of 4.5 mg/mL resulted in <5% hemolysis rate.
  | Figure 2 Hemolysis of erythrocytes by various drug concentrations of baicalin-loaded nanoliposomes (n=3). |
Tissue distribution and lung-targeting evaluation
In order to prove lung-targeting characterization, the in vivo tissue distribution of baicalin was performed after intravenous administration of baicalin-loaded nanoliposomes and its solution as the control with a single dose of 10 mg/kg to rabbits; the results are summarized in Figures 3 and 4. As shown in Figure 3, the biodistribution of baicalin solution was observed in a time-dependent manner. From Figure 4, in the case of baicalin-loaded nanoliposomes, the biodistribution in plasma, heart, and lung was found to be in a time-dependent manner, whereas the biodistribution in liver, spleen, kidney, brain, and stomach was elevated within the first 2 h and afterward decreased at 2–24 h after intravenous administration. After intravenous administration of baicalin-loaded nanoliposomes, drug concentration in the lungs was the highest among the tested organs at all time points; moreover, drug concentration in the lungs was also significantly higher than that of its solution. For example, drug concentration of baicalin-loaded nanoliposomes showed 2.789-fold increase in the lungs at 15 min after intravenous administration in comparison with baicalin solutions. Whereas, drug concentration in the other organs was less than or similar to its solutions, suggesting a rapid and efficient localization of baicalin-loaded nanoliposomes in the lungs. In addition, extremely low plasma drug level and rapid plasma clearance were observed in the case of baicalin-loaded nanoliposomes after injection into rabbits in comparison with its solutions, which further supported the targeting characteristics of baicalin-loaded nanoliposomes to lungs.
  | Figure 3 Distribution in rabbit tissues after intravenous administration of baicalin solution ( |
  | Figure 4 Distribution in rabbit tissues after intravenous administration of baicalin liposomes ( |
To further understand the lung-targeting behavior of the drug when intravenously administered in the liposomal formulation, a comparative lung-targeting study was carried out between baicalin-loaded nanoliposomes and its solutions. On the basis of drug concentration in each tissue or plasma, the area under the curve (AUC) of the drug concentration–time profile of baicalin and targeting parameters (Re, Te, and Ce) was calculated and is shown in Table 3.22 Baicalin liposomal formulation showed the largest value of AUC for lung, which indicated that baicalin-loaded nanoliposomes developed in the present study were preferable for targeting to lungs than other organs.
As shown in Table 3, the value of Re (defined as Re = AUCliposomes/AUCsolution) for lungs was 4.837 in the case of baicalin-loaded nanoliposomes, which indicated that the exposure of the baicalin to lung was significantly increased by encapsulation in liposome carriers. We all know that when the value of Re is >1, the tissue is exposed to the drug to a greater extent when administered in the liposomal form. Thus, it suggested that the liposome carrier was more specific accumulation of the drug in the lungs in comparison to its solutions. The value of Te (defined as Re = AUClung target/AUCother tissue) demonstrated the efficiency of a given delivery system against the nontarget organ through the ratio of target to nontarget organ drug distribution. In this study, the Te values of lung to other organs or plasma in the case of baicalin liposomal formulation all exceeded 1 and the value was 65.045 for plasma. Moreover, the ratios of Te liposomes/Te solution were increased significantly and the value was 14.131 for plasma. Therefore, the liposome carrier significantly increased the lung-targeting effect of baicalin in comparison with its solution. The value of Ce, defined as Ce = (Cmax)liposomes/(Cmax)solution, provided information regarding the efficiency of a delivery system on the change of biodistribution. Compared with baicalin solution, the value of Ce in the lungs was increased by a factor of 2.789 in the case of its liposomal formulation, which indicated that baicalin-loaded nanoliposomes had a preference to concentrate drug in the lungs in comparison with its solutions.
In conclusion, these results of tissue distribution and lung-targeting evaluation demonstrated that baicalin-loaded nanoliposomes developed in this study had obvious lung-targeting characteristics as compared with its solutions. It is well known that the lungs, due to their susceptibility to some diseases such as lung cancer, tuberculosis, and pneumonia, are a suitable target organ to localize drugs.5 Therefore, the liposome carrier offered a possibility for baicalin for the treatment of lung cancer. Many studies showed that the liposomes accumulate mostly in the organs of the reticuloendothelial system such as liver, spleen, and lung within the first 15–30 min after intravenous administration of the liposomal formulation.5,23 In general, the liposomes with a particle size of >5 μm could be trapped passively by the vascular network of the lung to achieve lung-targeting effect.24 Besides the physiochemical characterization of liposomes such as particle size, surface charge, and membrane lipid component,25,26 other factors such as particle–cell contact may also influence the biodistribution behavior of the drug.27 In this study, the highest distribution of baicalin with its nanoliposomes was observed in lungs. Specific lung-targeting mechanism needs further study.
Evaluation of lung injury
In the view of tissue distribution of baicalin, drug concentration and AUC in the lungs were significantly increased after intravenous administration of baicalin liposomal formulation to rabbits in comparison with its solution. In order to confirm the feasibility of baicalin-loaded nanoliposomes for lung-targeting delivery system, the possible lung injury induced by its liposomes was evaluated. The results showed that rats treated with baicalin-loaded nanoliposomes at the first, fourth, and seventh days after injection presented normal lung histology with normal sizes of airspaces and alveolar septa (Figure 5). Compared with the negative control group, no significant difference in lung changes at the first, fourth, and seventh days after injection of baicalin-loaded nanoliposomes to rats was observed. However, a slight broadening of alveolar septa was observed. Hence, the nanoliposomes seemed to induce some minor inflammation that did not increase with the time as observed from Figure 5A–C.
  | Figure 5 The pathological sections of lung at 1 (A), 4 (B), and 7 days (C) after intravenous administration of baicalin-loaded nanoliposomes and saline (D) as the control. Magnification ×200. |
Pharmacodynamics
In order to evaluate the therapeutical efficacy of baicalin-loaded nanoliposomes as a lung-targeting drug delivery system on lung cancer, the nude mice bearing orthotopic human lung cancer were intravenously administered with blank liposomes, baicalin solution, and baicalin-loaded nanoliposomes on every other day after 7 days post-tumor implantation. The results are shown in Figure 6. The total tumor weight of blank liposomes group from 11 to 12 days, baicalin solution group from 15 to 20 days and baicalin-loaded nanoliposomes sample from 23 to 28 days after tumor implantation was 955.00±153.83 mg, 936.00±120.18 mg, and 947.00±105.83 mg, respectively, which was not significantly different (P>0.05; Figure 6A). However, a significant difference in increased tumor weight daily of the saline group, baicalin solution group and baicalin-loaded nanoliposomes sample was observed. A significant decrease in increased tumor weight daily of baicalin-loaded nanoliposomes sample (36.89±6.13 mg tumor weight) was observed compared with blank liposomes (83.49±11.01 mg tumor weight) and baicalin solution groups (54.10±5.64 mg tumor weight; P<0.01; Figure 6B). Importantly, the nude mice bearing orthotopic human lung cancer treated with baicalin-loaded nanoliposomes presented demonstrated significant increased survival rates in comparison with blank liposomes and baicalin solution (P<0.01; Figure 6C). The median survival time of tumor-bearing mice treated with blank liposomes, baicalin solution, and baicalin-loaded nanoliposomes was 11.40±0.16 days, 17.30±0.47 days, and 25.90±0.53 days, respectively. The results were explained that baicalin-loaded nanoliposomes could deliver main drug into lung cancer based on the biodistribution study and greatly increased drug concentration in lung cancer. Therefore, baicalin-loaded nanoliposomes showed better efficacy in lung cancer treatment in comparison with baicalin solution with whole-body biodistribution.
Conclusion
Baicalin-loaded nanoliposomes for lung-targeting delivery system were successfully developed in this study for the first time. For in vitro evaluation, baicalin-loaded nanoliposomes exhibited high encapsulation efficiency, sustained-release behavior, and excellent stability. At the same time, the liposomes did not cause hemolytic activity. For in vivo evaluation, baicalin-loaded nanoliposomes can increase significantly drug concentration in the lungs after single intravenous administration. Moreover, according to the evaluation of lung injury, baicalin-loaded nanoliposomes were safe. Importantly, baicalin-loaded nanoliposomes showed better antitumor therapeutic efficacy in the nude mice bearing orthotopic human lung cancer. Therefore, the liposome is a promising drug carrier with excellent lung-targeting effect for Chinese medicine monomer, like baicalin, for instance.
Acknowledgment
This study was financially supported by the National Natural Science Foundation of China (81101678, 81341124, 31201566), the Science and Technology Support Project of Sichuan Province (2014SZ0071, 2014FZ0105), the Joint Fund of Sichuan Province, Luzhou City and Southwest Medical University (14JC0134, 14ZC0026, 14ZC006-6) and the Joint Fund of Luzhou City and Southwest Medical University (2013LZLY-K80, 2015LZCYD-S09 (4/8)).
Disclosure
The authors report no conflicts of interest in this work.
References
Zhuang B, Du L, Xu H, et al. Self-assembled micelle loading cabazitaxel for therapy of lung cancer. Int J Pharm. 2016;499(1–2):146–155. | ||
Melguizo C, Cabeza L, Prados J, et al. Enhanced antitumoral activity of doxorubicin against lung cancer cells using biodegradable poly(butylcyanoacrylate) nanoparticles. Drug Des Devel Ther. 2015;9:6433–6444. | ||
Li D, Gong L. Preparation of novel pirfenidone microspheres for lung-targeted delivery: in vitro and in vivo study. Drug Des Devel Ther. 2016;10:2815–2821. | ||
Mehta HJ, Patel V, Sadikot RT. Curcumin and lung cancer – a review. Target Oncol. 2014;9(4):295–310. | ||
Wei Y, Zhao L. Passive lung-targeted drug delivery systems via intravenous administration. Pharm Dev Technol. 2014;19(2):129–136. | ||
Zhao L, Wei Y, Huang Y, He B, Zhou Y, Fu J. Nanoemulsion improves the oral bioavailability of baicalin in rats: in vitro and in vivo evaluation. Int J Nanomedicine. 2013;8:3769–3779. | ||
Wei Y, Pi C, Yang G, et al. LC-UV determination of baicalin in rabbit plasma and tissues for application in pharmacokinetics and tissue distribution studies of baicalin after intravenous administration of liposomal and injectable formulations. Molecules. 2016;21(4):E444. | ||
Hu YZ, Wang DH, Luan Y, Gong HD. Antitumor effect of baicalin on rat brain glioma. Zhonghua Zhong Liu Za Zhi. 2013;35(1):11–16. | ||
Wang CZ, Zhang CF, Chen L, Anderson S, Lu F, Yuan CS. Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int J Oncol. 2015;47(5):1749–1758. | ||
Yu Y, Pei M, Li L. Baicalin induces apoptosis in hepatic cancer cells in vitro and suppresses tumor growth in vivo. Int J Clin Exp Med. 2015;8(6):8958–8967. | ||
Du G, Han G, Zhang S, et al. Baicalin suppresses lung carcinoma and lung metastasis by SOD mimic and HIF-1alpha inhibition. Eur J Pharmacol. 2010;630(1–3):121–130. | ||
Meng H, Xu Y. Pirfenidone-loaded liposomes for lung targeting: preparation and in vitro/in vivo evaluation. Drug Des Devel Ther. 2015;9:3369–3376. | ||
Ji C, Na W, Fei X, Sheng-Jun C, Jia-Bi Z. Characterization, lung targeting profile and therapeutic efficiency of dipyridamole liposomes. J Drug Target. 2006;14(10):717–724. | ||
Harsha S, Al-Khars M, Al-Hassan M, et al. Pharmacokinetics and tissue distribution of spray-dried carboplatin microspheres: lung targeting via intravenous route. Arch Pharm Res. 2014;37(3):352–360. | ||
Ramaiah B, Nagaraja SH, Kapanigowda UG, Boggarapu PR, Subramanian R. High azithromycin concentration in lungs by way of bovine serum albumin microspheres as targeted drug delivery: lung targeting efficiency in albino mice. Daru. 2016;24(1):14. | ||
Lee SY, Jung E, Park JH, et al. Transient aggregation of chitosan-modified poly(d,l-lactic-co-glycolic) acid nanoparticles in the blood stream and improved lung targeting efficiency. J Colloid Interface Sci. 2016;480:102–108. | ||
Anselmo AC, Gupta V, Zern BJ, et al. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013;7(12):11129–11137. | ||
Hong Y, He W, Li D, He L, Zhang W. Preparation and in vitro anti-tumor effect of baicalin liposome. Chin J Exp Tradit Med Formulae. 2012;18(3):29–31. | ||
Chen YJ, Jia Y, Jin R, et al. Preparation of baicalin flexible nanoliposomes. Chin J Exp Tradit Med Formulae. 2011;17(5):1–4. | ||
Liu S, Wu WY, Xi ZX, Wang N, Huang J. Determination of in vitro drug release of baicalin liposomes by HPLC. Chin Pharm. 2007;18(33):2594–2596. | ||
Mu YM. Preparation and characterization of liposomes containing baicalin. Chin Tradit Patent Med. 2008;30(7):1077–1079. | ||
Gupta PK, Hung CT. Quantitative evaluation of targeted drug delivery systems. Int J Pharm. 1989;56(3):217–226. | ||
Waser PG, Muller U, Kreuter J, et al. Localization of colloidal particles (liposomes, hexylcyanoacrylate nanoparticles and albumin nanoparticles) by histology and autoradiography in mice. Int J Pharm. 1987;39(3):213–227. | ||
Zhang SX, Gao XJ, Shen KH, Yang PW, Ju XL. Evaluation of poly (D,L-lactideco-glycolide) microspheres for the lung-targeting of yuanhuacine, a novel DNA topoisomerase I inhibitor. J Drug Target. 2009;17(4):286–293. | ||
Hirano K, Hunt CA. Lymphatic transport of liposome-encapsulated agents: effects of liposome size following intraperitoneal administration. J Pharm Sci. 1985;74(9):915–921. | ||
Kim CK, Lee MK, Han JH, Lee BJ. Pharmacokinetics and tissue distribution of methotrexate after intravenous injection of differently charged liposome-entrapped methotrexate to rats. Int J Pharm. 1994;108(1):21–29. | ||
Xiang QY, Wang MT, Chen F, et al. Lung-targeting delivery of dexamethasone acetate loaded solid lipid nanoparticles. Arch Pharm Res. 2007;30(4):519–525. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

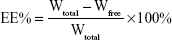
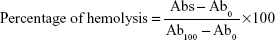
 ± SD, n=5; plasma: μg/mL, tissues: μg/g).
± SD, n=5; plasma: μg/mL, tissues: μg/g).
