Back to Journals » OncoTargets and Therapy » Volume 13
LukS-PV Inhibits Hepatocellular Carcinoma Cells Migration via the TNNC1/PI3K/AKT Axis
Authors Ma F, Wang Z, Qiang Y, Xu L, Ding P, Wang Y, Ma X
Received 24 August 2020
Accepted for publication 24 September 2020
Published 12 October 2020 Volume 2020:13 Pages 10221—10230
DOI https://doi.org/10.2147/OTT.S278540
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Fan Ma,1,* Ziran Wang,2,* Yawen Qiang,3 Liangfei Xu,1 Pengsheng Ding,4 Yangyan Wang,1 Xiaoling Ma1,4
1Department of Clinical Laboratory, Affiliated Provincial Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China; 2Department of Clinical Laboratory, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 3Department of Obstetrics and Gynecology Laboratory, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China; 4Department of Clinical Laboratory, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoling Ma
Department of Clinical Laboratory, Affiliated Provincial Hospital of Anhui Medical University, No. 17 Lujiang Road, Hefei, Anhui 230001, People’s Republic of China
Email [email protected]
Purpose: Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide. LukS-PV is the S component of Panton-Valentine leucocidin (PVL), a toxin secreted by Staphylococcus aureus. We aimed to investigate the role of LukS-PV in HCC cell migration and the specific molecular mechanism involved.
Methods: We used scratch assays to detect the mobility of liver cancer cells treated with LukS-PV. Quantitative real-time PCR and Western blot analysis were performed to detect the expression levels of related genes. RNA sequencing and quantitative proteomics sequencing were used to assess the transcriptional and proteomic alterations of target genes. RNA sequencing and Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Set Enrichment Analysis (GSEA) pathway analyses revealed the downstream signaling pathway targets of LukS-PV.
Results: Our results demonstrated that LukS-PV could inhibit HCC cell migration in a concentration-dependent manner. LukS-PV could also downregulate the expression of TNNC1, which was highly expressed in HCC cells. Additionally, the study showed that LukS-PV inhibited HCC cell migration by downregulating TNNC1. Further studies showed that LukS-PV inhibited the phosphorylation of PI3K/AKT pathway by targeting TNNC1, thereby inhibiting HCC cell migration.
Conclusion: Our study demonstrated that LukS-PV has an inhibitory role in the migration of liver cancer cells through the TNNC1/PI3K/AKT axis.
Keywords: hepatocellular carcinoma, LukS-PV, migration, TNNC1/PI3K/AKT axis
Introduction
Hepatocellular carcinoma (HCC) is a clinically common malignant tumor with an incidence rate that ranks it as the sixth highest solid tumor worldwide.1 HCC is prone to metastasis2 and the 5-year survival rate of patients is low.3 Both surgery and chemotherapy have certain limitations, with the latter exerting notable toxicity and side effects.4 Moreover, relapse frequently occurs after surgical treatment, and thus it is urgent to identify new targeted drugs.
In recent years, because of their specificity and cytotoxicity in binding to target cells, bacterial toxins have become a hot spot in the research and development of antitumor drugs, such as dCD133KDEL, a recombinant Pseudomonas exotoxin that can specifically kill CD133+ tumor-initiating cells and inhibit the proliferation of head and neck carcinoma cells.5 A bispecific ligand-directed toxin (BLT), another type of recombinant deimmunized Pseudomonas exotoxin (dEGF4KDEL), can have striking effects against systemic human pancreatic cancer.6 Listeriolysin O toxin produced by Listeria monocytogenes can exhibit anticancer activities in leukemia7 and melanoma,8 and diphtheria toxin produced by the bacterium Corynebacterium diphtheriae can treat cutaneous T-cell lymphoma.9 LukS-PV is the S component of Panton-Valentine leucocidin (PVL) secreted by Staphylococcus aureus. We previously found that LukS-PV can induce the apoptosis and differentiation of human acute myeloid leukemia cells.10 LukS-PV can also inhibit HCC progression.11
TNNC1, which is known as slow troponin C, contains a calcium-binding subunit and facilitates the interaction between actin and myosin in muscle cells.12 However, in non-muscle cells, TNNC1 may act as a regulatory protein for cellular locomotion. For example, in ovarian cancer, TNNC1 overexpression can promote tumor cell invasion and metastasis.13 In early-stage tongue cancer, TNNC1 can predict the prognosis of TSCC and its occult cervical lymphatic metastasis.14 In liver cancer, we identified several tumor-related proteins and related metabolic pathways in HepG2 cells treated with LukS-PV altered using quantitative proteomic sequencing, some forms of which demonstrated antitumor activity.15 High-throughput transcriptomic and proteomic analyses of HepG2 cells treated with LukS-PV indicated that LukS-PV could downregulate the expression of TNNC1 at both the mRNA and protein level. Thus, TNNC1 may also be a target for regulating HCC cell movement.
Our previous studies have shown that LukS-PV could inhibit the proliferation and promote the apoptosis of liver cancer cells,11 but its effect on the migration of HCC cells is still unclear. In this study, the role of LukS-PV in the migration of HCC cells and its molecular basis were further explored. The study results revealed that LukS-PV inhibited HCC cell migration by targeting TNNC1 and activating the PI3K/AKT signaling pathway. These results indicated that LukS-PV harbors great potential significance as a therapeutic drug for HCC.
Materials and Methods
Cell Culture
The human normal liver cell line L02 and liver cancer cell lines HepG2, Hep3B and Huh-7 were purchased from the Shanghai Cell Bank (Chinese Academy of Sciences, Shanghai, China). L02 cell were cultured in RPMI-1640 (Gibco, USA) containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C in 5% CO2. HepG2, Hep3B and Huh-7 cells were cultured in DMEM (Gibco, USA) containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C in 5% CO2. Our study was approved by the Ethics Committee and Institutional Review Board of University of Science and Technology of China, Anhui, China.
Production, Purification, and Quantification of LukS-PV
The sequence of LukS-PV was amplified from PVL-positive Staphylococcus aureus isolates by PCR, and recombinant LukS-PV was generated as described previously.16 Recombinant LukS-PV was purified using a His-Bind Purification Kit (Millipore, USA) and quantified using a BCA Kit (Beyotime, China).
Quantitative Real-Time PCR Analysis
Total RNA from HCC cells was extracted using Trizol reagent (Invitrogen, USA) and cDNA from total RNA was prepared by reverse transcription. PCR was performed using SYBR Green Master Mix (Takara, China) according to the manufacturer’s instructions. The primers were as follows: TNNC1 (5ʹ-ATG ATG GTT CGG TGC ATG AAG GAC-3ʹ and 5ʹ-TCC GTG ATG GTC TCG CCT GTA G-3ʹ); and GAPDH (5ʹ-GGA GCG AGA TCC CTC CAA AAT-3ʹ and 5ʹ-GGC TGT TGT CAT ACT TCT CAT GG-3ʹ). The relative mRNA expression levels of target genes were quantified using the 2−ΔΔCt method, and GAPDH was used as an internal reference. All experiments were performed in triplicate.
Western Blot Analysis
Proteins were lysed with RIPA lysis buffer and quantified with a BCA kit. The soluble protein was separated on 10% and 12.5% SDS-PAGE gels and transferred to nitrocellulose membranes. Membranes were blocked with 5% skim milk for 1.5 h and then incubated with anti-GAPDH (Abclonal, China), anti-TNNC1 (Proteintech, China), anti-MMP2 (Abclonal), anti-MMP9 (Abclonal), anti-PI3K (Proteintech), anti-p-PI3K (Proteintech), anti-AKT (Cell Signaling Technology, USA), and anti-p-AKT (Cell Signaling Technology) antibodies at 4°C overnight. Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1.5 h at room temperature.
Transfection
TNNC1 plasmid and corresponding empty vector were purchased from GenePharma Biotechnology (Shanghai, China). SiRNA-TNNC1 and the corresponding negative control were purchased from General Biotechnology (Anhui, China). HCC cells were transfected with the expression plasmid at a final concentration of 20 nmol/L using Lipofectamine 2000 (Invitrogen, USA) and siRNA at a final concentration of 70 nmol/L using Lipofectamine 2000 (Invitrogen, USA). Transfection efficiency was detected by qRT-PCR and Western blotting. The target sequence for siRNA-TNNC1 was as follows: sense, 5ʹ-GCA CCA AGG AGC UGG GCA ATT-3ʹ; antisense, 5ʹ-UUG CCC AGC UCC UUG GUG CTT-3ʹ.
Cell Scratch Assay
An appropriate number of HCC cells was cultured to full confluence in 12-well plates. After culturing for 12 h in complete medium, wounds were made using 10-μL pipette tips and the cells were cultured in fresh serum-free medium. After 0 h, 48 h from scratching, the wounds were photographed with an inverted microscope and the width changes were recorded to enable the scratch heal rate of HCC cells in the control and experimental groups to be calculated. Three wells were used in each group and three independent experiments were performed.
RNA Sequencing
Total RNA of the samples was isolated using an RNeasy Mini kit (Qiagen, Germany). Paired-end libraries were synthesized using a TruSeq™ RNA sample preparation kit (Illumina, USA) according to the manufacturer’s instructions. Purified libraries were quantified by a Qubit®2.0 Fluorometer (Life Technologies, USA) and validated by an Agilent 2100 bioanalyzer (Agilent Technologies, USA) to confirm the insert size and calculate the molar concentration. The library construction and sequencing were performed at the Shanghai Sinomics Corporation.
Quantitative Proteomic Sequencing
HepG2 cells treated with various concentrations of LukS-PV and PBS were sonicated three times on ice using a high intensity ultrasonic processor (Scientz, China) in lysis buffer, then the supernatant was collected and the protein concentration was quantified with a BCA kit. For digestion, the protein solution was reduced with 5 mM dithiothreitol and alkylated with 11 mM iodoacetamide, and the sample was diluted by adding 100 mM TEAB to a urea concentration of less than 2M. Finally, trypsin was added for overnight digestion.
After digestion, the peptide was reconstituted in 0.5 M TEAB and treated with a TMT kit/iTRAQ kit according to the manufacturer’s protocol. The peptide was fractionated using an Agilent 300Extend C18 column by high pH reverse-phase HPLC. Tryptic peptides were dissolved in 0.1% formic acid, directly loaded onto a home-made reversed-phase analytical column, and subjected to a NSI source followed by tandem mass spectrometry (MS/MS) in Q Exactive™ Plus (Thermo) coupled online to the UPLC. Finally, the resulting MS/MS data were analyzed using the Maxquant search engine (v.1.5.2.8).
Statistical Analysis
Differences between the two groups were analyzed by the unpaired Student’s t-test, and differences between multiple groups were assessed by ANOVA. A P-value of P<0.05 (*), P<0.01 (**), or P<0.001 (***) was considered statistically significant.
Results
LukS-PV Inhibited the Migration of HCC Cells
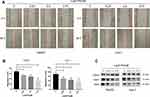
To investigate whether LukS-PV has a role in HCC cell migration, we treated HepG2 and Huh-7 cells with different concentrations of LukS-PV for 48 h. Then, scratch assays were undertaken to measure the migration rate of the HCC cells. Compared with the control group, the migration capacity of HepG2 and Huh-7 cells treated with LukS-PV was reduced after 48 h of wounding. Furthermore, the reduction rate was positively correlated with the concentration of LukS-PV (Figure 1A and B). Additionally, Western blot results also showed that LukS-PV could reduce the expression of the migration-related proteins MMP-2 and MMP-9 in HepG2 and Huh-7 cells (Figure 1C). These results indicated that LukS-PV could inhibit the migration of HCC cells in a concentration-dependent manner.
LukS-PV Downregulated the High Expression of TNNC1 in HCC Cells
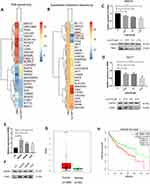
To further investigate the potential mechanism of LukS-PV inhibition in HCC cell migration, we used RNA sequencing and quantitative proteomic sequencing to analyze HepG2 cells treated with LukS-PV and PBS. Among a series of downregulated tumor-related genes, we identified TNNC1 (Figure 2A and B). TNNC1 has also been reported to been associated with the invasion and metastasis of ovarian cancer,13 and therefore we chose to study it further. QRT-PCR and Western blotting were used to further verify that LukS-PV could downregulate TNNC1 expression in HCC cells (Figure 2C and D). To further confirm the role of TNNC1 in HCC, qRT-PCR and Western blotting were performed to detect the mRNA and protein expression levels of TNNC1 in the normal liver cell line L02 and liver cancer cell lines HepG2, Hep3B, and Huh-7, proving that TNNC1 was highly expressed in HCC cells (Figure 2E and F). We also used GEPIA17 to analyze TNNC1 expression in liver cancer in cases from The Cancer Genome Atlas (TCGA) database. These data showed TNNC1 was highly expressed in tumor samples compared with normal tissues (Figure 2G). Further analysis of TCGA database revealed that high TNNC1 expression was associated with poor overall survival (Figure 2H). These results showed that LukS-PV could decrease TNNC1 expression in HCC cells and TNNC1 could be a prognostic marker for liver cancer.
LukS-PV Inhibited HCC Cell Migration by Downregulating TNNC1
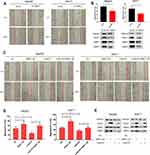
To define the role of TNNC1 in HCC cell migration, we first knocked down TNNC1 in HepG2 and Huh-7 cells. SiRNA-mediated knockdown of TNNC1 resulted in a dramatic decrease in the migration of HepG2 and Huh-7 cells (Figure 3A and B). In contrast, scratch assays under ectopic TNNC1 expression revealed that the TNNC1-overexpressing cells displayed a marked increase in migration (Figure 3C–E). Moreover, ectopic expression of TNNC1 significantly attenuated the inhibitory effects of LukS-PV on the migration of HepG2 and Huh-7 cells compared with that of the LukS-PV treatment group (Figure 3C–E). These results indicated that LukS-PV inhibited HCC cell migration by downregulating TNNC1.
LukS-PV Inhibited HCC Cell Migration via the TNNC1/PI3K/AKT Axis
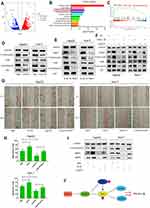
To further explore the molecular mechanism of LukS-PV inhibition of HCC cell migration by downregulating TNNC1, RNA sequencing was performed. LukS-PV treatment promoted the upregulation of 1678 genes and the downregulation of 1008 genes compared with the PBS group; the downregulated genes included TNNC1 (Figure 4A). After running the differentially expressed genes through Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Set Enrichment Analysis (GSEA) pathway analyses, the PI3K/AKT signaling pathway attracted our attention (Figure 4B and C). The PI3K/AKT signaling pathway has been reported to be involved in the migration and invasion of various tumor types.18,19 Therefore, we hypothesized that LukS-PV might inhibit the activation of the PI3K/AKT signaling pathway by downregulating TNNC1. To verify this hypothesis, Western blot analysis was undertaken to detect the expression of these pathway proteins. The results revealed that PI3K and AKT phosphorylation was decreased compared with the control group after LukS-PV treatment for 24 h (Figure 4D). The role of TNNC1 in the PI3K/AKT pathway was further investigated and revealed that the phosphorylation levels of PI3K and AKT were significantly reduced in the TNNC1 knockdown group (Figure 4E). Conversely, when TNNC1 was overexpressed, the phosphorylation levels of PI3K and AKT were increased (Figure 4F). Furthermore, the effect of LukS-PV on p-PI3K and p-AKT could be reversed by TNNC1 overexpression (Figure 4F). These results demonstrated that LukS-PV could inhibit the phosphorylation of PI3K/AKT signaling molecules by downregulating TNNC1. To further prove that LukS-PV inhibited the migration of HCC cells by inhibiting the activation of the PI3K/AKT pathway, we performed scratch assays and Western blot analysis to detect the migration rates and migration-associated proteins of HCC cells treated in different ways. Rescue experiments revealed that the inhibitory effect of LukS-PV on the migration of HCC cells could be reversed by AKT phosphorylation agonist (Figure 4G–I). This study demonstrated that LukS-PV inhibited the phosphorylation of the PI3K/AKT pathway by downregulating TNNC1, thereby inhibiting the migration of liver cancer cells (Figure 4J).
Discussion
Hepatocellular carcinoma is the most common primary liver cancer with high morbidity and mortality.1 The treatment of liver cancer includes surgical resection, liver transplantation, liver-directed therapy, and systemic therapy. However, only surgical resection and liver transplantation are considered as potentially curative approaches.20 The therapeutic effect of hepatocellular carcinoma is affected by multiple factors, including liver function, performance status, and tumor stage. Hence, only 30% of patients can receive curative therapies (surgery or ablation).21 In recent years, bacterial toxins have played an increasing role in tumor therapy.9 LukS-PV is the S component of PVL secreted by Staphylococcus aureus. Our previous study found that LukS-PV could inhibit leukemia cell proliferation and induce cell cycle arrest and apoptosis.22,23 Further studies have shown that LukS-PV could also inhibit HCC cell proliferation and induce cell cycle arrest and apoptosis,11 but the role of LukS-PV in HCC cell migration and its molecular mechanism were unclear. To investigate the role of LukS-PV in HCC cell migration, we performed scratch assays by stimulating HCC HepG2 and Huh-7 cells with different concentrations of LukS-PV. Compared with the control groups, LukS-PV could inhibit HCC cell migration in a concentration-dependent manner. To further explore the molecular mechanism of LukS-PV inhibition of HCC cell migration, we performed transcriptome and proteomic sequencing to explore the downstream target genes of LukS-PV. Sequencing results showed TNNC1 ranked higher in the degree of downregulation in both “omic” studies, and therefore we chose TNNC1 for further study. A literature review revealed that TNNC1 could promote ovarian cancer cell migration and invasion,13 as well as serve as a potential marker for the early occult cervical lymph node metastasis and prognosis of tongue cancer.14 Further analysis of TCGA database showed that TNNC1 was highly expressed in HCC and was associated with poor overall survival. Therefore, we hypothesized that TNNC1 was a downstream target gene of LukS-PV that inhibited HCC cell migration. First, we used qRT-PCR and Western blot analysis to verify that LukS-PV could downregulate TNNC1 in HCC cells. Knockdown of TNNC1 could reduce the migration ability of liver cancer cells, while TNNC1 overexpression could increase the migration ability of liver cancer cells and reverse the inhibitory effect of LukS-PV on liver cancer cell migration. These results demonstrated that LukS-PV inhibited HCC cell migration by downregulating TNNC1. To further explore the molecular mechanism of LukS-PV inhibition of HCC cell migration, RNA sequencing was undertaken. KEGG and GSEA pathway analysis identified the PI3K/AKT signaling pathway as being significant. The PI3K/AKT pathway is an important signaling cascade that is implicated in multiple oncogenic processes, including cell proliferation, apoptosis, differentiation, epithelial–mesenchymal transition, migration, and invasion.24–31 We first used Western blot analysis to detect the phosphorylation of PI3K/AKT proteins in liver cells treated with LukS-PV as well as TNNC1 knockdown and overexpression. LukS-PV could inhibit the phosphorylation of PI3K/AKT pathway proteins by downregulating TNNC1. Finally, scratch assays demonstrated that AKT phosphorylation agonist could reverse the inhibitory effect of LukS-PV on HCC cell migration. These results ultimately proved that LukS-PV inhibited liver cancer cell migration via the TNNC1/PI3K/AKT axis.
There are some limitations in our study. We first proved that LukS-PV inhibited HCC cell migration via the TNNC1/PI3K/AKT axis, but the specific molecular mechanism of LukS-PV regulating TNNC1 has not been fully clarified. We will continue to investigate this in future studies. Second, the expression and prognosis of TNNC1 in liver cancer were analyzed only with TCGA database. In future experiments, clinical samples will be collected and analyzed to verify the expression of TNNC1 in HCC and its correlation with the prognosis and clinicopathological features of liver cancer. Finally, we undertook cell experiments to prove that LukS-PV could inhibit HCC cell migration in vitro, but whether LukS-PV can inhibit HCC cell migration in vivo remains to be elucidated. In the future, we will also construct a patient-derived tumor xenograft nude mouse model for verification.
In summary, we first proved that LukS-PV could inhibit HCC cell migration in vitro. Second, there have been few reports on TNNC1 in liver cancer. Our results proved that TNNC1 could promote the migration of liver cancer cells and LukS-PV inhibited HCC cell migration by downregulating TNNC1. Finally, our study results showed that LukS-PV inhibited HCC cell migration through the TNNC1/PI3K/AKT axis.
Conclusion
We showed that LukS-PV inhibited HCC cell migration, probably by downregulating TNNC1 and inhibiting the PI3K/AKT signaling pathway. Our results confirm a tumor-suppressive role for LukS-PV in HCC cells, suggesting its use as a therapeutic drug.
Author Details
Fan Ma, Email: [email protected];
Ziran Wang, Email: [email protected];
Yawen Qiang, Email: [email protected];
Liangfei Xu, Email: [email protected];
Pengsheng Ding, Email: [email protected];
Yangyan Wang, Email: [email protected];
Xiaoling Ma, Email: [email protected]
Acknowledgments
We thank H. Nikki March, PhD, from Liwen Bianji, Edanz Editing China, for editing the English text of a draft of this manuscript. This work was supported by the National Science Foundation of China (81972001), the Anhui Natural Science Foundation (1808085QH259) and the Fundamental Research Funds for the Central Universities (WK9110000007, WK9110000107).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
2. Huang R, Yan G, Sun H, et al. Identification of prognostic and metastasis-related alternative splicing signatures in hepatocellular carcinoma. Biosci Rep. 2020;40(7). doi:10.1042/BSR20201001
3. Giannini EG, Farinati F, Ciccarese F, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology. 2015;61(1):184–190. doi:10.1002/hep.27443
4. Roxburgh P, Evans TR. Systemic therapy of hepatocellular carcinoma: are we making progress? Adv Ther. 2008;25(11):1089–1104. doi:10.1007/s12325-008-0113-z
5. Waldron NN, Kaufman DS, Oh S, et al. Targeting tumor-initiating cancer cells with dCD133KDEL shows impressive tumor reductions in a xenotransplant model of human head and neck cancer. Mol Cancer Ther. 2011;10(10):1829–1838. doi:10.1158/1535-7163.MCT-11-0206
6. Oh S, Todhunter DA, Panoskaltsis-Mortari A, Buchsbaum DJ, Toma S, Vallera DA. A deimmunized bispecific ligand-directed toxin that shows an impressive anti-pancreatic cancer effect in a systemic nude mouse orthotopic model. Pancreas. 2012;41(5):789–796. doi:10.1097/MPA.0b013e31823b5f2e
7. Stachowiak R, Lyzniak M, Budziszewska BK, et al. Cytotoxicity of bacterial metabolic products, including listeriolysin O, on leukocyte targets. J Biomed Biotechnol. 2012;2012:954375. doi:10.1155/2012/954375
8. Provoda CJ, Stier EM, Lee KD. Tumor cell killing enabled by listeriolysin O-liposome-mediated delivery of the protein toxin gelonin. J Biol Chem. 2003;278(37):35102–35108. doi:10.1074/jbc.M305411200
9. Weldon JE, Pastan I. A guide to taming a toxin–recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J. 2011;278(23):4683–4700. doi:10.1111/j.1742-4658.2011.08182.x
10. Sun XX, Zhang SS, Dai CY, et al. LukS-PV-regulated MicroRNA-125a-3p Promotes THP-1 macrophages differentiation and apoptosis by down-regulating NF1 and Bcl-2. Cell Physiol Biochem. 2017;44(3):1093–1105. doi:10.1159/000485415
11. Wang Z, Yu W, Qiang Y, et al. LukS-PV inhibits hepatocellular carcinoma progression by downregulating HDAC2 expression. Mol Ther Oncolytics. 2020;17:547–561. doi:10.1016/j.omto.2020.05.006
12. Pinto JR, Siegfried JD, Parvatiyar MS, et al. Functional characterization of TNNC1 rare variants identified in dilated cardiomyopathy. J Biol Chem. 2011;286(39):34404–34412. doi:10.1074/jbc.M111.267211
13. Leung CS, Yeung TL, Yip KP, et al. Calcium-dependent FAK/CREB/TNNC1 signalling mediates the effect of stromal MFAP5 on ovarian cancer metastatic potential. Nat Commun. 2014;5:5092. doi:10.1038/ncomms6092
14. Yang X, Wu K, Li S, et al. MFAP5 and TNNC1: potential markers for predicting occult cervical lymphatic metastasis and prognosis in early stage tongue cancer. Oncotarget. 2017;8(2):2525–2535. doi:10.18632/oncotarget.12446
15. Zhao CC, Yu WW, Qi YJ, et al. Quantitative proteomic analysis reveals that Luks-PV exerts antitumor activity by regulating the key proteins and metabolic pathways in HepG2 cells. Anticancer Drugs. 2020;31(3):223–230. doi:10.1097/CAD.0000000000000866
16. Ma X, Chang W, Zhang C, Zhou X, Yu F. Staphylococcal panton-valentine leukocidin induces pro-inflammatory cytokine production and nuclear factor-kappa B activation in neutrophils. PLoS One. 2012;7(4):e34970. doi:10.1371/journal.pone.0034970
17. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi:10.1093/nar/gkx247
18. Zhang Y, Jia J, Jin W, et al. Lidocaine inhibits the proliferation and invasion of hepatocellular carcinoma by downregulating USP14 induced PI3K/Akt pathway. Pathol Res Pract. 2020;216(8):152963. doi:152963.doi:10.1016/j.prp.2020.152963
19. Zhou F, Wang J, Chi X, Zhou X, Wang Z. lncRNA TM4SF1-AS1 activates the PI3K/AKT signaling pathway and promotes the migration and invasion of lung cancer cells. Cancer Manag Res. 2020;12:5527–5536. doi:10.2147/CMAR.S254072
20. Kim DW, Talati C, Kim R. Hepatocellular carcinoma (HCC): beyond sorafenib-chemotherapy. J Gastrointest Oncol. 2017;8(2):256–265. doi:10.21037/jgo.2016.09.07
21. Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010;15(Suppl 4):42–52. doi:10.1634/theoncologist.2010-S4-42
22. Zhang P, Yu WW, Peng J, et al. LukS-PV induces apoptosis in acute myeloid leukemia cells mediated by C5a receptor. Cancer Med. 2019;8(5):2474–2483. doi:10.1002/cam4.2137
23. Shan W, Bu S, Zhang C, et al. LukS-PV, a component of Panton-Valentine leukocidin, exerts potent activity against acute myeloid leukemia in vitro and in vivo. Int J Biochem Cell Biol. 2015;61:20–28. doi:10.1016/j.biocel.2015.01.007
24. Chen CH, Lu PJ, Chen YC, et al. FLJ10540-elicited cell transformation is through the activation of PI3-kinase/AKT pathway. Oncogene. 2007;26(29):4272–4283. doi:10.1038/sj.onc.1210207
25. Chen CH, Lai JM, Chou TY, et al. VEGFA upregulates FLJ10540 and modulates migration and invasion of lung cancer via PI3K/AKT pathway. PLoS One. 2009;4(4):e5052. doi:10.1371/journal.pone.0005052
26. Jia Y, Xiao Z, Gongsun X, et al. CEP55 promotes the proliferation, migration and invasion of esophageal squamous cell carcinoma via the PI3K/Akt pathway. Onco Targets Ther. 2018;11:4221–4232. doi:10.2147/OTT.S168861
27. Jiang C, Cheng Z, Jiang T, Xu Y, Wang B. MicroRNA-34a inhibits cell invasion and epithelial-mesenchymal transition via targeting AXL/PI3K/AKT/Snail signaling in nasopharyngeal carcinoma. Genes Genomics. 2020;42(8):971–978. doi:10.1007/s13258-020-00963-3
28. Zhou YM, Yao YL, Liu W, Shen XM, Shi LJ, Wu L. MicroRNA-134 inhibits tumor stem cell migration and invasion in oral squamous cell carcinomas via downregulation of PI3K-Akt signaling pathway by inhibiting LAMC2 expression. Cancer Biomark. 2020;29(1):51–67. doi:10.3233/CBM-191362
29. Li L, Liu JD, Gao GD, Zhang K, Song YW, Li HB. Puerarin 6″-O-xyloside suppressed HCC via regulating proliferation, stemness, and apoptosis with inhibited PI3K/AKT/mTOR. Cancer Med. 2020. doi:10.1002/cam4.3285
30. Ni J, Chen L, Ling L, Wu M, Ren Q, Zhu W. MicroRNA-196a promotes cell proliferation and inhibits apoptosis in human ovarian cancer by directly targeting DDX3 and regulating the PTEN/PI3K/AKT signaling pathway. Mol Med Rep. 2020;22(2):1277–1284. doi:10.3892/mmr.2020.11236
31. Zheng Y, Guo C, Zhang X, Wang X, Ma A. Garcinol acts as an antineoplastic agent in human gastric cancer by inhibiting the PI3K/AKT signaling pathway. Oncol Lett. 2020;20(1):667–676. doi:10.3892/ol.2020.11585
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.