Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Lower neutrophil-to-lymphocyte ratio predicts high risk of multidrug-resistant Pseudomonas aeruginosa infection in patients with hospital-acquired pneumonia
Authors Zhou YQ, Feng DY , Li WJ , Yang HL, Wang ZN, Zhang TT , Chen ZG
Received 5 July 2018
Accepted for publication 24 August 2018
Published 2 October 2018 Volume 2018:14 Pages 1863—1869
DOI https://doi.org/10.2147/TCRM.S179181
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Yu-Qi Zhou,1,2,* Ding-Yun Feng,1,2,* Wen-Juan Li,1,2 Hai-Ling Yang,1,2 Zhao-Ni Wang,3 Tian-Tuo Zhang,1,2 Zhuang-Gui Chen1,3
1Institute of Respiratory Diseases of Sun Yat-Sen University, Guangzhou, Guangdong, China; 2Department of Internal Medicine, Division of Respiratory Diseases, Guangzhou, Guangdong, China; 3Department of Pediatrics, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China
*These authors contributed equally to this work
Background and purpose: Hospital-acquired pneumonia (HAP) remains an important cause of morbidity and mortality despite advances in antimicrobial therapy. The emergence of multidrug resistant (MDR) Pseudomonas aeruginosa (PA) is of major concern. Our aim was to evaluate the risk factors and prognosis of HAP due to MDR-PA infection.
Patients and methods: In a retrospective observational study, we collected data on all episodes of HAP caused by PA (PA-HAP) occurring from January 2013 to December 2016. Characteristics of patients with drug-sensitive PA were compared with those with MDR-PA. Data of demographic, underlying conditions, peripheral neutrophil-to-lymphocyte ratio (NLR), and clinical outcomes were collected and analyzed.
Results: One hundred fifty-seven patients with PA-HAP were included, of which 69 (43.9%) patients were diagnosed with MDR-PA infection. There were significant differences between MDR-PA group and non-MDR-PA group on the following variables: initial inappropriate antibiotic therapy (P<0.001, OR 0.103, 95% CI 0.044–0.244), admission in more than two departments in previous 30 days (P<0.001, OR 0.186, 95% CI 0.072–0.476), and NLR level (P=0.020, OR 0.911, 95% CI 0.843–0.985). The effect of antibiotic treatment was significantly different (P<0.001, OR 4.263, 95% CI 2.142–8.483). The 30-day mortality was higher in MDR-PA group than that in non-MDR-PA group (P<0.001).
Conclusion: We have shown that lower NLR level was identified as a clinical predictor of MDR-PA infection in HAP patients. Even with goal-directed therapy, MDR-PA infection implicates poor outcomes in patients with HPA.
Keywords: risk factors, prognosis, multidrug-resistant Pseudomonas aeruginosa, hospital-acquired pneumonia
Corrigendum for this paper has been published
Introduction
Being highly relevant to longer hospital length of stay, increased costs, and morbidity, pneumonia remains a common but imperative issue of health care. Hospital-acquired pneumonia (HAP) is a pulmonary infectious disease that often develops in patients hospitalized for >48 hours, whether in the intensive care units (ICUs) or in other wards.1 Hospital-acquired pneumonia (HAP) occurs at a rate of 0.5%–1% in hospitalizations, being the second most common nosocomial infection in the USA.2 HAP can dramatically increase the hospital length of stay and economic burden. Besides, mortality caused by pneumonia still remains high.3 It has led to an overall mortality of 27%–51%, which is even poorer in the elderly.1,4,5
Among patients suffering from HAP, Pseudomonas aeruginosa (PA) is one of the most common pathogens found in the respiratory system.6,7 In the USA, PA was rated the second most common pathogen isolated (16.6%) in nosocomial pneumonias, according to the National Healthcare Safety Network 2009–2010.8 In China, PA also ranks among the top pathogens identified from the lower respiratory tract, counting to 12.31% of nosocomial infectious cases from 2002 to 2004 and 13.37% from 2005 to 2007.9
More importantly, resistance to multiple drugs is emerging in PA-infected individuals, while the development of new antibiotics is relatively slow in recent years. Micek et al conducted a worldwide cohort study of HAP caused by PA, demonstrating a high prevalence of multidrug resistance (MDR) of 30.5%.10 Because less antibiotics could be utilized on these patients, infection caused by MDR-PA indicated a high hospital mortality.11 There are several risk factors being reported that could probably increase the infection of MDR organisms, like prior broad-spectrum antimicrobial treatment, immunosuppressive therapy, and long hospital or nursing room stay.12,13 In China, tending to apply a wide-spectrum antibiotic therapy is a nationwide phenomenon because of the delay in identification of microbiological pathogens and drug sensitiveness report. Thus, to well control the prevalence of MDR organism infection, the first step should be to identify the potential risk factors. With indicators predicting whether HAP patients are infected with MDR-PA or not, physicians would not only make a more appropriate clinical decision before the microbial report comes out but also might help reduce the incidence of MDR.
Though there are quite a lot of risk factors that have been demonstrated in other similar studies, most of them were related to the medical history, which sometimes would be forgotten or mistaken by patients, and it is also difficult to make a precise standard. On this ground, we conducted this investigation on the hospitalized patients with PA infectious pneumonia in southern China, and we found that other than some reported risk factors, neutrophil-to-lymphocyte ratio (NLR) also was highly correlated with the MDR-PA infection, which might work as a more intuitive and objective predictor of MDR-PA infection for HAP patients.
Patients and methods
Participants
All patients were admitted in the Third Affiliated Hospital of Sun Yat-sen University between January 2013 and December 2016. Patients who had a microbiological examination of at least one of the following respiratory specimens, including sputum, pleural fluid, flexible bronchoscopy with protected specimen brush, bronchoalveolar lavage, transbronchial biopsy, or tracheobronchial aspirate in intubated patients performed for diagnosed HAP were enrolled in this retrospective study.
Patients with the following conditions were included: 1) age >14 years old; 2) new pulmonary infiltration occurring ≥48 hours after admission, new or progressing cough with/without sputum production, fever (>37.8°C) or hypothermia (<35.6°C), leukocytosis, left shift, or leukopenia based on local normal values.
Patients with the following conditions were excluded: COPD, bronchiectasis, diabetes mellitus, hematopathy, immunosuppressive disease, or receiving immunosuppressive therapy (chemotherapy, chronic usage of steroids, and autoimmune disease treatment). By following this standard, we eliminated the potential confounding factors in NLR analysis.
Bacterial identification was performed using standard methods.14 Susceptibility test was conducted by the microdilution method (MicroScan system; Baxter Healthcare, West Sacramento, CA, USA). Results were interpreted according to the National Committee for Clinical Laboratory Standards guidelines published in 2012 (CLSI, 2012). In this study, MDR-PA was defined as PA resistant to at least one agent in three or more antimicrobial categories in the susceptibility test of isolates from patients with HAP. The antimicrobial categories used in this investigation were aminoglycosides, carbapenems, cephalosporins, gyrase inhibitors, penicillin+β-lactamase inhibitors, epoxide, and polymyxin.15
HAP patients caused by PA were divided into two groups for comparison: (1) MDR-PA HAP, and (2) non-MDR-PA HAP.
Data collection
Information prior to the date of PA infection was collected: prior endotracheal intubation, prior mechanical ventilation, prior ICU stay, antibiotic therapy in previous 90 days, initial inappropriate antibiotic therapy (IAT), and treatment admission in more than two departments in previous 30 days. In addition, age, gender, white blood cells, neutrophils, lymphocytes, monocytes, NLR, and hemoglobin (HGB) in peripheral blood, treatment response, and the 30-day mortality were recorded.
One or more of the following situations can be defined as a change of the managing department: patients moved from outpatient department or emergence room to inpatient department; patients moved from one specialty to the other for getting special treatment such as accepting continuous renal replacement therapy for acute kidney failure; patients moved from normal department to ICU for critical situation. The criteria of changing the treatment department in this study include no improvements of the initial symptoms after 72 hours’ treatments; patients having organ failure need special treatments.
Initial IAT was defined as when the prescribed antibiotics given initially within 48 hours were not sensitive or active according to subsequent culture examination.
The antibiotic was regarded as effective according to the PA susceptibility test and two or more following clinical findings could be observed after 7-day antimicrobial therapy:16 temperature <37.5°C, respiratory rate ≤24 breaths per minute, heart rate <100 beats per minute, systolic blood pressure ≥90 mmHg or more, oxygen saturation >90% without oxygen supplement, normal mental status.
Ethical approval
The study was approved by the Institutional Review Board and the ethics committee of the Third Affiliated hospital of Sun Yat-Sen University. The ethics committee waived the need for informed consent as only deidentified patient data were used, and no human intervention was involved.
Statistical analysis
Statistical analyses were performed with SPSS (version 20.0; SPSS Inc, Chicago, IL, USA). Continuous data such as age were presented as the mean and standard deviation (mean±SD) if normally distributed or the median and interquartile range if not. Categorical data such as gender were presented using frequencies and percentages. Continuous variables were compared by using the Mann–Whitney U-test or Student’s t-test. Categorical variables were compared by using Pearson’s chi-squared test or Fisher’s exact test. Binary logistic regression was used to identify variables significantly associated with HAP caused by MDR-PA. The receiver operating characteristic (ROC) curve was applied to determine whether NLR is a well predictor for MDR-PA infection. Kaplan–Meier survival curves for MDR and non-MDR groups were compared using a log-rank test. All significance testing was two tailed, and P<0.05 was considered statistically significant.
Results
Clinical characteristics and baseline analysis
Two thousand six hundred forty-five patients with positive microbial organisms detected at lower respiratory tract were identified, and 796 (30.1%) were with PA. Previous studies found that PA incidence rates were lower.8 The reason is that PA colonization or causing community-acquired pneumonia (CAP) was collected on the first round. This study excluded patients with COPD, bronchiectasis, diabetes mellitus, and so on. Only 157 cases were included with an average age of 57.80±17.75 years (Figure 1).
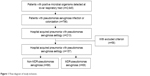  | Figure 1 Flow diagram of study inclusion. |
The median duration of hospitalization before infection was 8 days. Out of the 157 patients, 69 (43.9%) were infected with MDR-PA, 105 (66.9%) with prior endotracheal intubation, 68 (43.3%) with prior mechanical ventilation, 75 (47.8%) with prior ICU stay, 95 (60.5.9%) with treatment department changed in previous 30 days, 71 (45.2%) had received initial IAT, 73 (46.5%) had effective treatment, and 4 (2.5%) died within 30 days since HAP were diagnosed.
Clinical risks and survival analysis
By comparison, there were significant differences on the following variables of the two groups: prior endotracheal intubation, prior mechanical ventilation, prior ICU stay in the preceding 90 days, initial IAT, treatment department changed in the preceding 30 days, low HGB level, and NLR (Table 1). In the multivariable analysis, the occurrence of MDR was found to be closely related to the following factors: IAT (P<0.001, OR 0.103, 95% CI 0.044–0.244), treatment department changed in the preceding 30 days (P<0.001, OR 0.186, 95% CI 0.072–0.476), and NLR (P=0.020, OR 0.911, 95% CI 0.843–0.985) (Table 2). The ROC curve also showed that NLR is a well predictor of MDR-PA infection in HAP patients (area under curve = 0.619, 95% CI 0.531–0.707, P=0.011) (Figure 2). The outcome showed that the effective rates of antibiotic treatment within 7 days between these two groups were significantly different (P<0.001, OR 4.163, 95% CI 2.142–8.483) (Table 3).
  | Figure 2 Receiver operating characteristic curve for neutrophil-to-lymphocyte ratio. |
Except 33 patients with empirical treatment, 124 patients accepted an anti-PA therapy based on susceptibility tests in vitro. Among these 124 patients, 13 of 42 MDR-PA patients and 51 of 82 non-MDR-PA patients had effective treatment. The effective ratio of antibiotic treatment was significantly different (P=0.001, OR 3.670, 95% CI 1.698–8.198) between MDR-PA and non-MDR-PA in this study (Table 4). Survival analysis showed that MDR-PA patients had a lower 30-day survival than non-MDR-PA group (P=0.001) (Figure 3).
  | Figure 3 Kaplan–Meier survival curves according to MDR-PA infection. |
Discussion
PA is highly endemic in ICUs, where it causes urinary tract infections, bacteremia, and pneumonia.17 It is the second most frequent pathogen in ICUs.18 The carriage of PA in respiratory tract can increase under long ICU stays.19
In this study, we investigated several potential risk factors of MDR-PA infection, and we proved that lower NLR, IAT, and treatment department changing was strongly correlated to high risk of MDR-PA infection in HAP patients.
Among these tested risk factors, NLR was the less reported one. Though it has been demonstrated as a prognosis predictor in many infectious diseases, especially in bloodstream infections,20–23 few have reported its role in predicting MDR-PA infection. Interestingly, we found that MDR-PA HAP group had lower NLR than the counterpart group. It is reported that the early hyperdynamic phase of infection is characterized by a proinflammatory state mediated by neutrophils and other inflammatory cells.20 This systemic inflammatory response is associated with the suppression of neutrophil apoptosis, which augments neutrophil-mediated killing as part of the innate response.24 Thus, NLR is often characterized by an increase in neutrophils and a decline in lymphocytes. While in our study, we found that the level of NLR in MDR-PA patients was not as high as in non-MDR group. One possible reason for this phenomenon is that the respective lower level of NLR may be attributed to less virulence of MDR-PA strain. Gómez-Zorrilla et al evaluated the relationship between pathogenicity and the resistance profile of different PA strains. The data indicated that MDR profiles were involved in an elimination of virulence in a murine model.25 Though the virulence of MDR-PA decreases, because the patients with MDR-PA were usually in bad condition, the prognoses of these patients were still poor. Anyway, decline of NLR still could be considered as a convenient predictor for clinical physicians to determine whether patients are infected with MDR strain or not.
Other than NLR, we also found that initial IAT could contribute to the prevalence of MDR-PA infection. Similar conclusion has also been reported by several studies.26–28 Although the development of resistance can occur naturally over time, but this course could be accelerated by the abuse of antibiotics.29 MDR is like a vicious cycle, the more resistance exists, the more antibiotics will be used. Therefore, the usage of antibiotic should be more accurately targeted. After all, the development of new antibiotics is much slower than the process of drug resistance.
Apart from initial IAT, treatment department change is also common in China, while there were few studies focusing on the relationship between treatment department change and MDR-PA. In this study, most patients with MDR-PA had an experience of treatment department change before the date of MDR-PA diagnosis. It is probably because PA carried by the patients from the previous department was activated by the patients’ deteriorated condition or IAT. Furthermore, patients changing to PA colonized or contaminated environment such as ICUs have more risks to become infected. Therefore, MDR-PA should be considered as the most possible pathogen for those new patients with treatment department record.
In previous studies, MDR-PA was reported to be associated with severe clinical outcomes, including 30-day mortality and length of hospital stay.30,31 In this study, we analyzed effective rate of antibiotic treatment and found that the MDR-PA group had worse result. It may owe to MDR-PA virulence, while some authors believed it was because of a higher rate of initial inappropriate therapy.32
Conclusions
IAT, treatment department change in previous 30 days, and decline of NLR levels were significantly associated with HAP caused by MDR-PA. The antibiotic treatment had better effect on non-MDR-PA group than MDR-PA group based on in vitro susceptibility tests.
Acknowledgments
We appreciate Dr Anthony Yii Chau Ang for his helpful suggestions on the design of this study and we thank Dr Yan Yan for carefully proofing our manuscript.
This study was supported by Science and Technology Projects Foundation of Guangzhou City (Number 201709010040).
Author contributions
Y-QZ and D-YF designed the study and participated in the drafting of manuscript. W-JL and H-LY participated in the data collection. Z-NW participated in revisions of the draft. T-TZ designed the study and participated in the critical revision of the draft. Z-GC drafted the manuscript and participated in the critical revisions of the draft. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Barbier F, Andremont A, Wolff M, Bouadma L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med. 2013;19(3):216–228. | ||
American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. | ||
Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427. | ||
Pop-Vicas A, Mitchell SL, Kandel R, Schreiber R, D’Agata EM. Multidrug-resistant gram-negative bacteria in a long-term care facility: prevalence and risk factors. J Am Geriatr Soc. 2008;56(7):1276–1280. | ||
Denkinger CM, Grant AD, Denkinger M, Gautam S, D’Agata EM. Increased multi-drug resistance among the elderly on admission to the hospital – a 12-year surveillance study. Arch Gerontol Geriatr. 2013;56(1):227–230. | ||
Zarb P, Coignard B, Griskeviciene J, et al; National Contact Points for the ECDC pilot point prevalence survey; Hospital Contact Points for the ECDC pilot point prevalence survey. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill. 2012;17(46):20316. | ||
Kollef MH, Chastre J, Fagon JY, et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit Care Med. 2014;42(10):2178–2187. | ||
Sievert DM, Ricks P, Edwards JR, et al; National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34(1):1–14. | ||
Wen XM, Ren N, Ah W, et al. Distribution of pathogens causing nosocomial infection monitored by national nosocomial infection surveillance system and changing trend. Chinese Journal of Nosocomiology. 2011;21:350–355. | ||
Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. | ||
Micek ST, Wunderink RG, Kollef MH, et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Crit Care. 2015;19(19):219. | ||
Cerceo E, Deitelzweig SB, Sherman BM, Amin AN. Multidrug-resistant gram-negative bacterial infections in the hospital setting: overview, implications for clinical practice, and emerging treatment options. Microb Drug Resist. 2016;22(5):412–431. | ||
Tacconelli E, Tumbarello M, Bertagnolio S, et al. Multidrug-resistant Pseudomonas aeruginosa bloodstream infections: analysis of trends in prevalence and epidemiology. Emerg Infect Dis. 2002;8(2):220–221. | ||
Watkins RR, Lemonovich TL. Diagnosis and management of community-acquired pneumonia in adults. Am Fam Physician. 2011;83(11):1299–1306. | ||
C.L.S.I. Performance Standards for Antimicrobial Susceptibility Testing: Twenty Second Informational Supplement. Wayne: CLSI; 2012. | ||
National Clinical Guideline Centre (UK). Pneumonia: Diagnosis and Management of Community- and Hospital-Acquired Pneumonia in Adults. London: National Institute for Health and Care Excellence (UK): Clinical Guidelines; 2014 Dec. | ||
Brewer SC, Wunderink RG, Jones CB, Leeper KV. Ventilator-associated pneumonia due to PA. Chest. 1996;1996(109):1019–1029. | ||
Sligl WI, Dragan T, Smith SW. Nosocomial Gram-negative bacteremia in intensive care: epidemiology, antimicrobial susceptibilities, and outcomes. Int J Infect Dis. 2015;37:129–134. | ||
Berthelot P, Grattard F, Mahul P, et al. Prospective study of nosocomial colonization and infection due to Pseudomonas aeruginosa in mechanically ventilated patients. Intensive Care Med. 2001;27(3):503–512. | ||
Lowsby R, Gomes C, Jarman I, et al. Neutrophil to lymphocyte count ratio as an early indicator of blood stream infection in the emergency department. Emerg Med J. 2015;32(7):531–534. | ||
Terradas R, Grau S, Blanch J, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: a retrospective cohort study. PLoS One. 2012;7(8):e42860. | ||
Riché F, Gayat E, Barthélémy R, et al. Reversal of neutrophil-to-lymphocyte count ratio in early versus late death from septic shock. Crit Care. 2015;19:439. | ||
Alkan Ozdemir S, Arun Ozer E, Ilhan O, Sutcuoglu S. Can neutrophil to lymphocyte ratio predict late-onset sepsis in preterm infants? J Clin Lab Anal. 2018;32(4):e22338. | ||
Jimenez MF, Watson RW, Parodo J, et al. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch Surg. 1997;132(12):1263–1270. | ||
Gómez-Zorrilla S, Juan C, Cabot G, et al. Impact of multidrug resistance on the pathogenicity of Pseudomonas aeruginosa: in vitro and in vivo studies. Int J Antimicrob Agents. 2016;47(5):368–374. | ||
Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. | ||
Zhang JF, Zhu HY, Sun YW, et al. Pseudomonas aeruginosa infection after pancreatoduodenectomy: risk factors and clinic impacts. Surg Infect. 2015;16(6):769–774. | ||
Lee SO, Kim NJ, Choi SH, et al. Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii: a case-control study. Antimicrob Agents Chemother. 2004;48(1):224–228. | ||
Threat Report 2013: Antibiotic/Antimicrobial Resistance. Centers for Disease Control and Prevention. Available from: http://www.cdc.gov/drugresistance/threat-report-2013/. Accessed January 12, 2015. | ||
Tam VH, Rogers CA, Chang KT, et al. Impact of multidrug-resistant Pseudomonas aeruginosa bacteremia on patient outcomes. Antimicrob Agents Chemother. 2010;54(9):3717–3722. | ||
Morata L, Cobos-Trigueros N, Martínez JA, et al. Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2012;56(9):4833–4837. | ||
Qu H, Sun GR, Zhou SQ, He QS. Clinical risk factors of delayed gastric emptying in patients after pancreaticoduodenectomy: a systematic review and meta-analysis. Eur J Surg Oncol. 2013;39(3):213–223. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




