Back to Journals » International Journal of General Medicine » Volume 13
Low Yield of Thyroid-Function Tests in Adult Hospitalized Patients — A Retrospective Analysis
Authors Dogra P, Paudel R, Panthi S, Cassity E, Tannock LR
Received 8 April 2020
Accepted for publication 11 June 2020
Published 6 July 2020 Volume 2020:13 Pages 343—349
DOI https://doi.org/10.2147/IJGM.S256868
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Prerna Dogra,1 Robin Paudel,2 Sujata Panthi,3 Evan Cassity,2 Lisa R Tannock4
1Division of Hospital Medicine, Department of Internal Medicine, University of Kentucky, Lexington, KY, USA; 2Division of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine, University of Kentucky, Lexington, KY, USA; 3Division of Hospital Medicine, Department of Internal Medicine, Baptist Memorial Hospital-DeSoto, Southaven, MS, USA; 4Division of Endocrinology and Molecular Medicine, Department of Internal Medicine, University of Kentucky, Lexington, KY, USA
Correspondence: Prerna Dogra Email [email protected]
Background: In the US, serum thyroid-stimulating hormone (TSH) and thyroxine measurements are the fourth- and tenth-commonest laboratory tests ordered, respectively. Diagnosis of thyroid disorder requires clinical suspicion supported by laboratory values. However, in the setting of acute illness, both the clinical and laboratory pictures can be confounded.
Objective: To study clinical outcomes and derangement patterns of inpatient thyroid-function tests.
Design: This retrospective study was conducted at an academic center on admissions aged ≥ 18 years and TSH tests performed over a 1-year period. Admissions with active pregnancy and/or prior thyroid-related diagnosis were excluded.
Main Outcomes: Clinical outcomes were divided based on new diagnosis of thyroid-related disorder, newly prescribed thyroxine replacement, or antithyroid drugs/ endocrinology referrals, or both. In order to analyze the derangement patterns of abnormal TSH, only the results of the first test ordered were considered (as some admissions had multiple TSH tests ordered).
Results: A total of 7,204 admissions aged ≥ 18 years had TSH tests done. Of these, 1,912 were excluded. Of the 5,292 admissions with no prior thyroid disorder or active pregnancy, 183 (3.46%) were assigned a new diagnosis of thyroid-related disorder, 54 (1.02%) received treatment/referral, and 46 (0.87%) received both a new diagnosis and treatment/referral. Based on the TSH results (reference range 0.42– 4.0 mIU/L) of the 5,292 admissions, 4,312 (81.5%) and 980 (18.5%) admissions were flagged normal and abnormal, respectively. Of the 980 admissions with one or more abnormal TSH results, 21 (2.14%) had first ordered TSH < 0.05 mIU/L, 855 (87.25%) admissions had first TSH result between 0.05– 10 mIU/L, and lastly 104 (10.61%) were > 10 mIU/L.
Conclusion: There is low value in testing inpatients for thyroid disorders, and testing comes at significant expense to the health-care system.
Keywords: inpatient thyroid-function test, non–thyroidal illness syndrome, sick euthyroid syndrome, low yield, high-value care
Plain Language Summary
- Serum thyroid-stimulating hormone (TSH) and thyroxine measurements are the fourth- and tenth-commonest laboratory tests ordered, respectively. In the setting of acute illness, both the clinical and lab pictures for diagnosing thyroid disorder can be confounded.
- This retrospective study primarily investigated the number of adult hospitalized admissions that received a new thyroid-related diagnosis and subsequent management based on thyroid-function tests ordered in the inpatient setting.
- Notably, in the 1-year period of this study, only 46 (0.87%) of the 5,292 admissions received a new thyroid-related diagnosis resulting in treatment or specialist referral.
- Additionally, 81.5% of admissions had TSH values within the reference range. In the 18.5% of admissions with TSH values outside the reference range, 2.14% had TSH <0.05 mIU/L and 10.6% >10 mIU/L.
Introduction
The 21st century has seen an alarming increase in health-care costs,1 and one of the major components driving this expenditure is unnecessary services.2,3 In the US, Medicare alone spent a total of $4.29 billion on the top 25 lab tests in 2016. Among these, thyroid-stimulating hormone (TSH) was the fourth-commonest lab test, at a cost of $482 million for 21.5 million ordered tests, and thyroxine measurement the tenth-common, at a cost of $85 million for 7.1 million ordered tests.4
Clinical features of thyroid dysfunction are non-specific, and include fatigue, constipation/diarrhea, weight changes, palpitations, hot/cold intolerance, and brady/tachycardia. In the normal physiological state, the hypothalamic–pituitary–thyroid (HPT) axis, via its tightly controlled feedback mechanism, maintains the level of serum thyroid hormones within a narrow range.5,6 Intrinsic dysfunction of the HPT axis results in predictable changes in serum TSH and thyroid hormones.
In patients with critical or non-critical illness, multiple extrinsic factors (stress, cytokines, interleukins) impact the HPT axis, resulting in deranged thyroid-function tests (TFTs), termed non–thyroidal illness syndrome (NTIS) or sick euthyroid syndrome.7–9 The biochemical disturbances in NTIS are variable and unpredictable, due to a continuum of changes the human body goes through during the process of illness and recovery. Furthermore, certain medications commonly used in inpatient settings (eg, glucocorticoids, dopamine agonists, somatostatins) alter TFTs by either impacting the HPT axis or modulating the secretion or absorption of thyroid hormones.10 The non-specific clinical features and biochemical disturbances shared by acute illness and thyroid disorders make diagnosis of true thyroid dysfunction challenging, particularly in the inpatient setting.
Subclinical thyroid disease is an increasingly diagnosed entity nowadays.11 Subclinical disease occurs when TSH is deranged but thyroid hormones are within normal reference range, provided thyroid function has been stable for 2–3 months, the HPT axis is normal, and there is an absence of recent or ongoing severe illness. Based on current evidence, it is unclear whether treatment is beneficial or harmful in asymptomatic, nonpregnant adults.12,13
The primary objective of this study was to examine the clinical outcomes of inpatient TFTs. Secondary objective included an analysis of the pattern of derangement of the TFTs - TSH and free T4 (FT4) in those with abnormal TSH levels.
Methods
After approval by the institutional review board, we conducted a retrospective study at a tertiary-care center on patients aged ≥18 years who had been admitted to medicine, neurology, and psychiatry services between October 26, 2017 and October 25, 2018. Exclusion criteria were active pregnancy and/or prior thyroid-related disorders. Admissions with a history of thyroid-related disorder were identified when home medications included either thyroxine-replacement or antithyroid drugs (Appendix 1) or the admission history mentioned prior thyroid-related diagnosis (Appendix 2). Next, admissions with no history of thyroid disorder were divided based on serum-TSH results, in relation to our lab’s normal reference range (0.4–4.2 mIU/L), into two groups — normal and abnormal. For admissions with multiple TSH tests, all values had to fall within the reference range to qualify for the normal-TSH group. Even if one test result was outside the reference range, the admission counted as part of the abnormal-TSH group. Following this, in both the normal- and abnormal-TSH groups, we searched for admissions that included either a new diagnosis of thyroid-related disorder based on ICD10 codes (Appendix 3) or new thyroxine replacement or antithyroid drugs prescribed at discharge (Appendix 1). A detailed chart review was performed on all admissions that met one or both criteria. Final outcomes in both abnormal- and normal-TSH groups were subgrouped into four categories: no documented thyroid-related diagnosis or prescribed treatment with thyroxine-replacement or antithyroid drugs; documented thyroid-related diagnosis present, but no treatment or endocrinology referral; presence of treatment with thyroxine-replacement or antithyroid drugs, but no documented diagnosis; and presence of both documented thyroid-related diagnosis and treatment with thyroxine-replacement or antithyroid drugs/endocrinology referral present.
In the cohort with abnormal TSH and no prior thyroid-related disorder or active pregnancy, we studied the pattern of derangement of TFTs (TSH and FT4). Based on the results of the first TSH test ordered, admissions in the abnormal-TSH group were divided into five subgroups: I — <0.05 mIU/L, II — 0.05–0.39 mIU/L, III — 0.40–4.20 mIU/L, IV — 4.21–10 mIU/L, and V — >10 mIU/L. Subgroup III in the abnormal-TSH group comprised admissions that had had first ordered TSH results in the normal reference range (0.40–4.20 mIU/L), but subsequent TSH values outside the reference age (leading these admissions to qualify for the abnormal-TSH group). In each subgroup, the first ordered FT4 level was reviewed and classified as normal and abnormal, based on our lab’s reference range (0.8–1.7 ng/mL), and each admission was sorted by presence or absence of a new diagnosis and treatment or referral.
Results
During the 1-year study period, there were 7,204 admissions and 7,867 TSH tests ordered. A total of 1,912 admissions and 2,127 TSH tests were excluded from the initial cohort: 1,887 with prior thyroid-related diagnosis and 25 with active pregnancy. As such, the final cohort comprised 5,292 admissions with 5,740 TSH tests. Of note, 422 of these 5,292 admissions had multiple TSH tests ordered. The demographic distribution of admissions comprised 2,852 males and 2,440 females (53.89% vs 46.11%), 4,663 Caucasians, 579 African–Americans, 25 Asians, and 25 other ethnicities, including unknowns (88.11% vs 10.94% vs 0.47% vs 0.47%), with an age range of 18–103 years (mean 55.96±17.4 years). Based on admitting department, 3,380 (63.87%) admissions were to medicine, 1,290 (24.38%) to neurology, and 622 (11.75%) to psychiatry.
There were 4,312 admissions with normal-TSH tests (4,617 TSH tests were obtained in this group; Figure 1). Of these, 4,260 (98.79%) admissions had 4,561 TSH tests and 926 FT4 tests, and received neither a diagnosis nor any treatment. However, 49 (1.14%) admissions had 50 TSH tests and 12 FT4 tests obtained, and were given a diagnosis but no treatment or referral was ordered. All of these were diagnosed with non-toxic thyroid nodules (31 single nodules, 12 multinodular goiters, six simple goiters). Finally, three (0.07%) admissions in the normal-TSH group had six TSH tests and seven FT4 tests, and received both a diagnosis and treatment/referral. These three diagnoses comprised two cases of central hypothyroidism and one case of primary hypothyroidism (Figure 1).
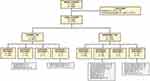 |
Figure 1 Methodology with results.Notes: Treatment includes medications and/or endocrinology referrals. a, admissions; t, TSH tests; +, present; –, absent. |
There were 980 admissions with abnormal-TSH tests (1,123 TSH tests were obtained in this group; Figure 1). Of these, 841 (85.82%) had 949 TSH tests and 522 FT4 tests, but received neither a diagnosis nor any treatment. There were 88 (8.98%) admissions with 100 TSH tests and 84 FT4 tests in whom a new diagnosis of thyroid-related disorder but no treatment or referral was provided. There were eight (0.82%) admissions with nine TSH tests and seven FT4 tests that received thyroid-specific treatment, but with no documented diagnosis provided. Finally, there were 43 (4.39%) admissions with 65 TSH tests and 54 FT4 tests that received both a diagnosis and treatment or referral (Figure 1).
For the 131 admissions in the abnormal-TSH group that had a new thyroid-related diagnosis provided, 49 (37.40%) were diagnosed with hypothyroidism, 12 (9.16%) hyperthyroidism, one (0.76%) autoimmune thyroiditis, 46 (35.11%) subclinical disease, 15 (11.45%) non-toxic thyroid nodules (single nodule or multinodular/simple goiter), and eight (6.11%) with sick euthyroid syndrome or elevated TSH. Of the 49 admissions with a new diagnosis of hypothyroidism, treatment or endocrinology referral was prescribed to only 26 (53.60%). Similarly, for 12 admissions with new diagnosis of hyperthyroidism, only four (33.33%) were prescribed treatment or endocrinology referral (Figure 1).
Therefore, of the 5,292 admissions with no history of thyroid diagnosis or pregnancy, 183 (3.46%) received a new diagnosis of thyroid-related disorder, 54 (1.02%) treatment or referral, and 46 (0.87%) both a new diagnosis and treatment or referral. In other words, 5,510 (95.99%) of the 5,740 TSH tests, 1,448 (89.83%) of the 1,612 FT4 tests, 161 (87.98%) of the 183 total T3 (TT3) tests, and 35 (76.09%) of the 46 free T3 (FT3) tests resulted in no new diagnosis or treatment/referral (Tables S1 and S2).
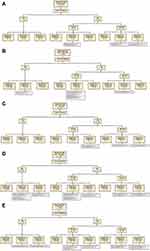
We next sought to examine the pattern of derangement of TFTs (TSH and FT4). The 980 admissions with abnormal TSH were divided into five subgroups based on the results of the first ordered TSH. There were 21 admissions (2.14% of abnormal-TSH group) in subgroup I (TSH <0.05 mIU/L). Of these, 18 (85.71% of admissions in subgroup I) had FT4 levels measured, with ten results outside the reference range (or abnormal). In total, five admissions received a new diagnosis followed by treatment or referral (Figure 2A).
In subgroup II (TSH 0.05–0.39 mIU/L), there were 255 admissions (26.02% of abnormal-TSH group). Of these, 157 (61.57% of admissions in subgroup II) had FT4 levels measured, with 18 results outside the reference range. In this subgroup, there was no new diagnosis followed by treatment or referral (Figure 2B). In subgroup III, there were 36 admissions with normal TSH (first ordered value). Of these, 26 (72.22% of admissions in subgroup III) had FT4 levels measured, with four showing abnormal. Overall, three admissions received both a new diagnosis and treatment or referral (Figure 2C).
In subgroup IV (TSH 4.21–10 mIU/L), there were 564 admissions (57.55% of abnormal-TSH group). Of these, 313 (55.49% of admissions in subgroup IV) had FT4 levels measured, with 27 results outside the reference range. In total, only 13 of these 564 admissions received both a new diagnosis and treatment/referral (Figure 2D). Lastly, in subgroup V (TSH >10 mIU/L), there were 104 admissions (10.61% of abnormal-TSH group). Of these, 85 (81.73% of admissions in subgroup V) had FT4 levels measured, with 26 showing abnormal. In total, only 23 of 104 admissions with TSH >10 mIU/L received a new diagnosis and treatment or referral (Figure 2E).
Discussion
In summary, we found that at a single academic medical center in the US, over a 1-year period 5,740 TSH tests had been ordered for 5,292 admissions. TSH tests were abnormal in 980 (18.5%) admissions and led to a new diagnosis and/or treatment in 139. Notably, a new thyroid diagnosis and/or treatment was made in 52 admissions with normal TSH. Of these, 49 were diagnosed with nodular thyroid disease and two with central hypothyroidism, and TSH measurement is considered appropriate in these scenarios, but one was diagnosed with primary hypothyroidism.
This study highlights multiple points. Large proportion (73.46%) of TSH tests were ordered on admissions with no known thyroid-related diagnosis. The majority (81.48%) of the TSH tests yielded results within the normal reference range. For abnormal TSH, 87.25% were 0.05–10 mIU/L, followed by 90% of FT4 values within the normal reference range. An overwhelming proportion (96.39%) received neither a diagnosis nor treatment. Only a small proportion of admissions (3.46%) had a new thyroid-related diagnosis, and an even smaller proportion received treatment/referral (1.02%). Finally, only 0.87% admissions received both a diagnosis and treatment/ referral. It is interesting to note that 26.54% of admissions in our initial cohort had a prior thyroid-related diagnosis, suggesting that thyroid issues are important comorbidities in patients requiring hospitalization. Nonetheless, our results emphasize that inpatient TFTs provide a low yield of clinical outcomes in patients with no known thyroid disorder.14–16
Based on recommendations by American Thyroid Association and American Association of Clinical Endocrinologists, in hospitalized patients TSH measurement should be done only if there is an index of suspicion for thyroid dysfunction.17 Laboratory Medicine Practice Guidelines suggest use of a widened TSH reference interval (0.05–10 mIU/L) in hospitalized patients, due to complex effects of acute or chronic NTIS on TFTs.18 There are a number of potential explanations for both the number of tests ordered and the outcomes followed by ordering providers in the finding of an abnormal TSH. Thyroid disease is common, and its symptoms can be vague and unspecific. Excluding thyroid disease is a common step in many diagnostic protocols for many different disorders. However, interpretation of TSH results is challenging in the inpatient setting, as TSH is affected by various medications and clinical scenarios. In many cases, abnormal TSH found in the inpatient setting is not addressed immediately, but the patient may be advised to seek repeat lab measurement after discharge and recovery from the illness that led to the admission. As such, abnormal test results may appear to be ignored by ordering providers. In other cases, an ordering provider may be unaware of the potential limitations in interpreting abnormal-TSH and/or -TFT results (eg, due to NTIS and/or medication effects), which risks overdiagnosis with unnecessary treatment and/or generating unnecessary consultations. Assessment of thyroid tests obtained in the inpatient setting is challenging, and likely is an explanation for the outcomes we observed. However, the limitations mentioned should not preclude providers from discreetly ordering inpatient thyroid assays in scenarios with high clinical suspicion of thyroid dysfunction, including subjects with large goiters or before initiating chronic treatment with such medications as amiodarone and lithium.
Overall, 87.25% of total admissions in the abnormal-TSH group had the first ordered TSH value 0.05–10 mIU/L, followed by 90% of first measured FT4 values within the normal reference range. In this subgroup, there was no new diagnosis of hyperthyroidism that received treatment or appropriate endocrinology referral, and three of the eight new diagnoses of primary hypothyroidism that received treatment had normal FT4, raising concerns of potential overdiagnosis and unnecessary treatment. One strategy to limit unnecessary thyroid assays in hospitalized patients with no prior thyroid dysfunction would be to implement reflex testing (automatically generated FT4 for abnormal TSH results) on a widened TSH reference interval (0.05–10 mIU/L).19–22 Although this strategy would curtail unneeded FT4 tests, it may not limit inessential TSH tests. Additionally, if used indiscriminately, any reflex test risks missing cases, such as central hypothyroidism. Interestingly, in their recent meta-analysis of 58 studies, Fitzgerald et al demonstrated thyroid-hormone levels (FT4, TT3/FT3) had a stronger correlation with clinical parameters than TSH levels, and recommended reconsidering the current practice of a TSH-based approach to defining thyroid function.23
Our study has a few limitations: its retrospective design; the fact that it was a single-center study conducted at an academic center; the predominantly Caucasian study population; inpatient services, such as pediatrics, obstetrics–gynecology, and surgery, were not included; we did not research clinical features linked to the ordering of TFTs; and lastly we did not analyze providers’ reasoning behind the ordering of the thyroid assays and arrival at a diagnosis. However, our study adds to the growing literature demonstrating low yield of thyroid labs in the inpatient setting.
Conclusion
Inpatient TFTs for diagnosing thyroid disorder have low overall clinical utility. Lab testing is an indispensable component of current medical practice; however, efforts should be made to minimize unnecessary testing and expenditure.
Ethics and Consent Statement
The study protocol was approved by the institutional review board of the University of Kentucky, Lexington, KY, USA. Patient consent to review medical records was not required by the board, due to the retrospective nature of the study. Patient data were maintained with confidentiality, in accordance with the ethical standards laid down in the Declaration of Helsinki.
Disclosure
All authors of this manuscript declare no conflict of interest in this work.
References
1. Centers of Medicare and Medicaid Services. National Health Expenditure Projections 2017–2026; 2017. doi:10.1377/hlthaff.2016.1627
2. Limb M. A fifth of healthcare spending is wasted, says OECD report. BMJ. 2017. doi:10.1136/bmj.j215
3. Young PL, Leigh AO; Medicine R on E-BMI of. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summar; 2010. doi:10.17226/12750
4. Murrin S Office of inspector general HHS OIG DATA BRIEF medicare payments for clinical diagnostic laboratory tests in 2016: year 3 of baseline data; 2017. Available from: http://oig.hhs.gov/oei/reports/oei-09-17-00140.pdf.
5. Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE. Hypothalamus-pituitary-thyroid axis. Compr Physiol. 2016. doi:10.1002/cphy.c150027
6. Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35(2):159–194. doi:10.1210/er.2013-1087
7. Pappa TA, Vagenakis AG, Alevizaki M. The nonthyroidal illness syndrome in the non-critically ill patient. Eur J Clin Invest. 2011;41(2):212–220. doi:10.1111/j.1365-2362.2010.02395.x
8. Fliers E, Bianco AC, Langouche L, Boelen A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 2015;3(10):816–825. doi:10.1016/S2213-8587(15)00225-9
9. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745–762. doi:10.1016/j.beem.2013.10.003
10. Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab. 2009;23(6):793–800. doi:10.1016/j.beem.2009.08.003
11. Taylor PN, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels - Balancing benefits and risks evidence from a large community-based study. JAMA Intern Med. 2014;174(1):32. doi:10.1001/jamainternmed.2013.11312
12. LeFevre ML, Siu AL, Bibbins-Domingo K, et al. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015. doi:10.7326/M15-0483
13. Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the U.S. preventive services task force. Ann Intern Med. 2015;162(1):35. doi:10.7326/M14-1456
14. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2009;7(4):567–572. doi:10.1016/j.beem.2009.08.003
15. Premawardhana LD. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med. 2017;27(300):300–307. doi:10.11613/BM.2017.033
16. Garnier KA, Ismail KA, Moylan S, Harvey R. Thyroid function testing in an inpatient mental health unit. Australas Psychiatry. 2016;24(3):256–260. doi:10.1177/1039856215618522
17. Gharib H, Guttler RB, Tourtelot JB, et al. American association of clinical endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2016. doi:10.4158/1934-2403-8.6.457
18. Demers LM, Spencer CA. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Clin Endocrinol (Oxf). 2003;58(2):138–140. doi:10.1046/j.1365-2265.2003.01681.x
19. Gilmour JA, Weisman A, Orlov S, et al. Promoting resource stewardship: reducing inappropriate free thyroid hormone testing. J Eval Clin Pract. 2017;23(3):670–675. doi:10.1111/jep.12698
20. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):
21. Notas G, Kampa M, Malliaraki N, Petrodaskalaki M, Papavasileiou S, Castanas E. Implementation of thyroid function tests algorithms by clinical laboratories: a four-year experience of good clinical and diagnostic practice in a tertiary hospital in Greece. Eur J Intern Med. 2018;54:81–86. doi:10.1016/j.ejim.2018.03.012
22. Orgiazzi J. Are Wider TSH cutoffs for reflex testing of free T4 feasible, safe and cost-effective? Clin Thyroidol. 2017;29(11):422–425. doi:10.1089/ct.2017;29.422-425
23. Fitzgerald SP, Bean NG, Falhammar H, Tuke J. Clinical parameters are more likely to be associated with thyroid hormone levels than with Tsh levels: a systematic review and meta-analysis. Thyroid. 2020. doi:10.1089/thy.2019.0535
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.