Back to Journals » International Journal of General Medicine » Volume 15
Low Triiodothyronine Syndrome Increased the Incidence of Acute Kidney Injury After Cardiac Surgery
Authors Lang H , Wan X, Ma M, Peng H, Zhang H, Sun Q, Zhu L, Cao C
Received 18 November 2021
Accepted for publication 21 December 2021
Published 25 January 2022 Volume 2022:15 Pages 867—876
DOI https://doi.org/10.2147/IJGM.S349993
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hong Lang,1 Xin Wan,2 Mengqing Ma,1 Hui Peng,1 Hao Zhang,2 Qing Sun,1 Li Zhu,1 Changchun Cao1
1Department of Nephrology, Sir Run Run Hospital, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 2Department of Nephrology, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China
Correspondence: Changchun Cao, Department of Nephrology, Sir Run Run Hospital, Nanjing Medical University, 109 Longmian Road, Nanjing, 211166, Jiangsu, People’s Republic of China, Email [email protected]
Background: Acute kidney injury (AKI) is a severe complication of cardiac surgery. This study was designed to explore the association between the preoperative low T3 syndrome and cardiac surgery-associated acute kidney injury (CSA-AKI).
Methods: This was a retrospective single-center study. Data on 784 patients undergoing elective coronary artery bypass grafting (CABG) or valve surgery were collected from January 2016 to July 2019. AKI was defined according to Kidney Disease: Improving Global Outcomes guidelines. The effect of preoperative low T3 syndrome (fT3 < 3.5pmol/L) on the risk of the postoperative AKI was analyzed in a logistic regression model.
Results: There were 171 (21.8%) patients developing AKI. Preoperative T3 and FT3 levels were lower in patients with AKI than in those without AKI (P < 0.001). The incidence of postoperative AKI was higher in patients with low T3 syndrome than in those without (31.0% vs 19.8%; P = 0.003). Multivariate logistic regression analysis showed that low T3 syndrome was an independent risk factor for CSA-AKI patients (OR = 1.609, 95% CI: 1.033– 2.504; P = 0.035), after adjusting for confounding factors, such as age, albumin, and uric acid. Subgroup analyses showed that preoperative low T3 syndrome also increased incidence of CSA-AKI in those with high risk factors, such as age ≧60 yrs (OR: 1.891, 95% CI: 1.183– 3.022, P = 0.008), hypertension (OR: 2.104, 95% CI: 1.218– 3.3.635, P = 0.008), and hyperuricemia (OR: 2.052, 95% CI: 1.037– 4.06, P = 0.039).
Conclusion: Low T3 syndrome independently increases the risk of CSA-AKI. Patients with low T3 syndrome should be considered at higher risk and be evaluated before cardiac surgery.
Keywords: acute kidney injury, cardiac surgery, low triiodothyronine syndrome, risk factor
Introduction
Acute kidney injury (AKI) is a common complication with cardiac surgery, the incidence of cardiac surgery-associated AKI (CSA-AKI) varies from 5% to 42%.1 CSA-AKI is the second most common cause of AKI in the intensive care setting (after sepsis) and is independently associated with increased morbidity and mortality.2 At present, there is no effective treatment for AKI, so early identification of high-risk groups and timely intervention are critical for controlling CSA-AKI.
Thyroid hormone levels and kidney function are closely related.3 Many systemic diseases, other than thyroid diseases, also disrupt the secretion of thyroid hormones.4 Abnormal T4-to-T3 conversion leads to the decrease of T3 level, a condition commonly known as low T3 syndrome, also as euthyroid sick syndrome (ESS) or non-thyroidal illness syndrome (NTIS). In severe cases, T4 level also drops, but not accompanied by a usual feedback increase in thyroid-stimulating hormone (TSH).5 Low T3 syndrome increases the severity and worsens the outcomes of many diseases, such as cancer,5,6 heart failure,7 chronic kidney disease8 and acute kidney injury.9 However, few studies have reported the relationship between preoperative low T3 syndrome and CSA-AKI. Therefore, we hypothesized that preoperative low T3 syndrome is a risk of CSA-AKI, and undertook the present study to test this hypothesis.
Materials and Methods
Study Design
This retrospective single-center study was carried out at Nanjing First Hospital (Nanjing, China) over a period between January 2016 and July 2019. The study was performed in accordance with the Declaration of Helsinki and approved by the Regional Human Research Ethics Committee of Nanjing First Hospital (KY20190404-03-KS-01). The requirement to obtain written informed consent from each patient was waived because this was an retrospective study. The patients’ information were anonymous and non identifiable.
Participants
The inclusion criteria: (1) age between 18 and 90 years; (2) patients underwent elective CABG or valve surgery; (3) patients underwent cardiopulmonary bypass; (4) preoperative thyroid hormone levels were measured. Exclusion criteria: (1) patients were diagnosed preoperative acute kidney injury; (2) patients had a history of thyroid diseases, such as hypothyroidism, hyperthyroidism and thyroiditis; (3) patients used drugs affecting thyroid hormone levels, such as amiodarone; (4) patients received dialysis therapy before the operation; (5) patients died within 48 hours; (6) patients had incomplete medical records, such as missed serum creatinine. Ultimately, 784 patients were included.
Data Collection and Relevant Definitions
Demographic, preoperative, intraoperative and postoperative data were obtained from an electronic medical record database. Laboratory data within 24 hr after admission, including preoperative thyroid hormone levels, were collected from the hospital-based laboratory service. The reference values for hospital thyroid hormone levels were as follows: TSH, 0.55–4.78mIU/L; T4 (TT4), 58.1–140.6nmol/L; FT4,11.5–22.7pmol/L; T3 (TT3), 0.92–2.79nmol/L; FT3, 3.5–6.5pmol/L.
The final Scr before surgery was taken as the baseline Scr. eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). AKI was diagnosed and staged according to the 2012 Kidney Disease: Improving Global Outcomes criteria (KDIGO). Stage 1 was considered as mild, and stage 2 or stage 3 as severe. CSA-AKI was defined as AKI occurring within 7 days after surgery.10
Low T3 syndrome was defined as FT3 levels below the lower limit of the reference value (FT3< 3.5pmol/L), accompanied by low or normal serum T4 and TSH levels.5
A cut-off age of 60 years was chosen to distinguish the elder from the young. Anemia was defined as hemoglobin <120g/l in females or <130g/l in males. Hyperuricemia was defined as uric acid >360μmol/l (6mg/dl) in females or >420μmol/l (7mg/dl) in males. According to the American Diabetes Association Practice Guidelines, diabetes mellitus (DM) was defined by fasting blood glucose≥126 mg/dl, or a clinical diagnosis of DM with dietary, oral, or insulin treatment. Hypertension was defined as office systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg.
Study Outcomes
The primary endpoint was defined as CSA-AKI. The secondary endpoints were defined as stay in hospital and ICU, hospital death or need for CRRT.
Statistical Analysis
SPSS 25.0 software was used for all comparisons between groups with and without low T3 syndrome. Continuous variables were compared using the Student’s t-test and expressed as the mean ± standard deviation. Categorical variables were compared using the Chi-square test or Fisher’s exact test and presented as frequencies. Serum T3 and FT3 levels among groups of different degrees of AKI were compared by the one-way analysis of variance analysis (ANOVA). We first performed univariate analysis for significant baseline variables. Furthermore, multivariate logistic regression analysis with the backward stepwise method was employed to determine independent risk factors for CSA-AKI. Included variables were age, history of diabetes and hypertension, use of ACEI/ARB drug, baseline eGFR, preoperative albumin, uric acid, hemoglobin, fibrinogen, low T3 syndrome, cross-clamp time and CPB time. The effect of preoperative low T3 syndrome on CSA-AKI was also evaluated in various subgroups stratified by age, history of diabetes, history of hypertension, use of ACEI/ARB drug, hyperuricemia, anemia and CPB time.
Results
Baseline Clinical Characteristics
A total of 1165 adult patients underwent elective CAGB or valve surgery under CPB in the period of study. Preoperative thyroid hormone levels were not measured in 263 patients. Of the remaining, 118 patients were excluded (n=10, history of thyroid disease; n=37, use of drugs affected thyroid hormone levels; n=39, preoperative acute kidney injury; n=15, preoperative dialysis; n=16, incomplete medical records; n=1, death within 48 hours). Ultimately, 784 eligible patients were enrolled (Figure 1), with a mean age of 61.40±10.15 years. Of them, 613 (78.2%) had no AKI, 147 (18.8%) had stage 1 AKI, 17 (2.2%) had stage 2 AKI, and 7 (0.9%) had stage 3 AKI. At admission, 142 (18.1%) had low T3 syndrome. The participants were divided into two groups based serum thyroid hormone levels: low T3 syndrome group (n=142), without low T3 syndrome group (n=642). All baseline characteristics we comparable between the two groups (Table 1).
 |
Table 1 Clinical Characteristics in Patients with or without Preoperative Low T3 Syndrome |
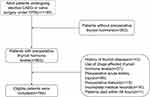 |
Figure 1 Flow chart of inclusion of study participants. Abbreviations: CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass. |
Early Outcome and Complications
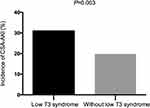
One patient died postoperatively, three patients needed CRRT treatment, and 171 patients presented postoperative AKI. In the low T3 syndrome group, 98 patients had no AKI, and 44 patients had AKI, including 35 with stage 1 AKI and 9 with stage 2 or 3 AKI. Compared with those without low T3 syndrome, the patients with low T3 syndrome had higher incidence of AKI (31.0% vs 19.8%, P=0.003; Figure 2) and longer postoperative ICU stay and hospital stay (54.11±67.42h vs 37.74±29.13h, P <0.001; 18.69±6.63d vs 16.68±5.76d, P <0.001). Numbers of CRRT treatment and hospital death showed no differences (Table 2).
 |
Table 2 Early Outcomes and Complications in Patients with or without Preoperative Low T3 Syndrome |
Association Between Preoperative Low T3 Syndrome and CSA-AKI
Preoperative T3 and FT3 levels were lower in patients with severe AKI than in those with mild and non-AKI (Figure 3), lower in patients with AKI than in those without AKI (1.26±0.25nmol/L vs 1.35±0.29nmol/L, P<0.001; 3.77±0.61pmol/L vs 3.96±0.55pmol/L, P<0.001, respectively). More patients with AKI exhibited low T3 syndrome than did those without AKI (25.7% vs 16.0%, P=0.003). As mentioned above, low T3 syndrome was associated with a higher incidence of CSA-AKI.
In univariate analysis, the occurrence of CSA-AKI was significantly associated with age, presence of diabetes and hypertension, use of ACEI/ARB, LVEF, baseline eGFR, baseline creatinine, albumin, uric acid, hemoglobin, fibrinogen, CPB and cross-clamp time, surgical procedure and preoperative low T3 syndrome (Table 3). Meanwhile, multivariate logistic regression analysis showed that preoperative low T3 syndrome was a major independent risk factor for CSA-AKI (OR:1.609, 95% CI:1.033–2.504, P=0.035; Table 3). Additional independent risk factors included age, diabetes, hypertension, use of ACEI/ARB, uric acid, hemoglobin and CPB time (Table 3). The presence of preoperative low T3 syndrome was associated with an increased risk of CSA-AKI in various subgroups, especially in those with high-risk factors, such as age≧60 yrs (OR:1.891, 95% CI:1.183–3.022, P=0.008), history of hypertension (OR:2.104, 95% CI:1.218–3.3.635, P=0.008), hyperuricemia (OR:2.052, 95% CI:1.037–4.06, P=0.039) (Figure 4).
 |
Table 3 Univariate and Multivariate Predictors of Postoperative AKI |
Discussion
To our knowledge, this is the first study to discuss and analyze the relationship between the preoperative low T3 syndrome and AKI in patients undergoing CABG or valve surgery. Our study demonstrates that the presence of the low T3 syndrome at admission is independently associated with an increased risk of postoperative AKI, especially in those with high-risk factors, such as age≧60 yrs, hypertension, hyperuricemia, even after adjustment for all potential and significant confounders.
Studies have shown that Low T3 syndrome complicated by CKD exhibited a high prevalence.11 Many previous studies have also reported the relationship between low T3 syndrome and the poor prognosis, and greater mortality of chronic kidney disease.12,13 However, limited studies have explored the relationship between the low T3 syndrome and AKI. Liu et al have revealed that the low T3 syndrome at admission increased the risk of AKI after aortic aneurysm surgery, this was consistent with our study.14 Similarly, in an observational study, Chen et al observed that low T3 syndrome was a risk factor for in-hospital death and acute renal failure in patients with acute aortic dissection.15 However, another study has also shown that there was no association between preoperative thyroid function and AKI after thyroidectomy, this may be due to the small sample size, and the association between low T3 syndrome and AKI was not further analyzed.16 In addition, Cerillo et al found that preoperative low T3 syndrome served as an independent risk factor of short-term prognosis after CABG surgery, including low cardiac output and hospital death.17 Meanwhile, our study showed that the low T3 syndrome independently increased the risk of CSA-AKI, which can be complementary to the study by Cerillo et al.
In our study, the incidence of CSA-AKI was consistent with those in previous reports.18 Previous studies have showed that the pathogenesis of CSA-AKI were involved in hemodynamic instability,19 excessive inflammatory response,20 and perioperative release of reactive oxygen species (ROS).21 However, no mechanisms have not yet been uncovered to elucidate the association between low T3 syndrome and CSA-AKI. To our knowledge, thyroid hormone levels directly regulate renal growth and development, glomerular filtration rate and sodium and water homeostasis.3 As mentioned earlier, hemodynamic instability was involved in the development of CSA-AKI. Studies have revealed that thyroid hormones directly regulate the level of renins or partially enhance the activity of beta-adrenergic receptors, thus regulating in this way the renin-angiotensin system.22 Other studies have shown that as serum FT3 and T3 levels fall, myocardial contractility and cardiac output decrease, and peripheral vascular resistance increases, thus reducing renal blood flow and eGFR.23 An animal study also showed that thyroid hormones (eg, T3) can increase in blood flow in the ventricles and kidneys, a possible mechanism that underlies T3-induced improvement of cardiac and renal function.24 Kumar et al have revealed that the patients who received levothyroxine experienced rapid improvement in hemodynamics.25 In addition, histologic studies have documented the effects of T3 on outer and cortical medullary tubular segments, especially the distal convoluted tubules and proximal tubules. Renal tubular epithelial cells bear the brunt of AKI.3 Renal ischemia reperfusion injury was involved in the development of CSA-AKI, and T3 can reverse renal ischemia-reperfusion injury by inhibiting oxidative stress and the apoptosis of renal tubular epithelial cells.26 However, the protective effects of T3 weakens as its level drops, thereby increasing the vulnerability of renal tubular epithelial cells to surgical stress. In animal models, supplement of thyroid hormone can ameliorate or reverse ischemic and toxic kidney injury.27 Therefore, the patients with preoperative low T3 syndrome may be more likely to develop AKI, who are a high-risk group of CSA-AKI. Further research is needed to determine whether the risk of postoperative AKI can be reduced by T3 correction.
This study has certain limitations. First, this is a retrospective single-center study, but not a randomized study, and the data may have deviations. Second, some clinical data are missing, such as postoperative inflammatory biomarkers (C reactive protein) and intraoperative mechanical ventilation parameters and intraoperative laboratory indicators (hemoglobin), which may have an impact on the results. Third, other factors influencing serum thyroid hormone levels might not have been completely excluded. Finally, thyroid hormones are in change, but thyroid function is not a routine test, which cannot be monitored and followed up, we were unable to dynamically analyze the effect of thyroid function on AKI. However, our study is one of the few articles to study the relationship between low T3 and CSA-AKI.
Conclusions
The results of this study suggest that preoperative low T3 syndrome may be an independent risk factor in patients who developed CSA-AKI. For this reason, the patient’s thyroid profile should be evaluated before cardiac surgery. Further studies are needed to elucidate the molecular basis of association.
Data Sharing Statement
Data related to this paper can be made available from the corresponding author upon reasonable request.
Statement of Ethics
The study was performed in accordance with the Declaration of Helsinki and approved by the Regional Human Research Ethics Committee of Nanjing First Hospital (KY20190404-03-KS-01). The requirement to obtain written informed consent from each patient was waived because this was an retrospective study. The patients’ information were anonymous and non identifiable.
Acknowledgments
We thank the surgeons and nursing staff of the department of cardiothoracic surgery and ICU for providing consultation and useful information at Nanjing First Hospital.
Funding
There is no funding to report.
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
1. Wang Y, Rinaldo B. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13(11):697–711. doi:10.1038/nrneph.2017.119
2. Mao H, Katz N, Ariyanon W, et al. Cardiac surgery-associated acute kidney injury. Cardiorenal Med. 2013;3(3):178–199. doi:10.1159/000353134
3. Iglesias P, Bajo MA, Selgas R, et al. Thyroid dysfunction and kidney disease: an update. Rev Endocr Metab Disord. 2017;18(1):131–144. doi:10.1007/s11154-016-9395-7
4. Fliers E, Bianco AC, Langouche L, et al. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 2015;3(10):816–825. doi:10.1016/S2213-8587(15)00225-9
5. Gao R, Chen RZ, Xia Y, et al. Low T3 syndrome as a predictor of poor prognosis in chronic lymphocytic leukemia. Int J Cancer. 2018;143(3):466–477. doi:10.1002/ijc.31327
6. Gao R, Liang JH, Wang L, et al. Low T3 syndrome is a strong prognostic predictor in diffuse large B cell lymphoma. Br J Haematol. 2017;177(1):95–105. doi:10.1111/bjh.14528
7. Sato Y, Yoshihisa A, Kimishima Y, et al. Low T3 syndrome is associated with high mortality in hospitalized patients with heart failure. J Card Fail. 2019;25(3):195–203. doi:10.1016/j.cardfail.2019.01.007
8. Xiong H, Yan P, Huang Q, et al. A prognostic role for non-thyroidal illness syndrome in chronic renal failure: a systematic review and meta-analysis. Int J Surg. 2019;70:44–52. doi:10.1016/j.ijsu.2019.08.019
9. Iglesias P, Olea T, Vega-Cabrera C, et al. Thyroid function tests in acute kidney injury. J Nephrol. 2013;26(1):164–172. doi:10.5301/jn.5000106
10. Himmelfarb J, Chertow GM, McCullough PA, et al. Perioperative THR-184 and AKI after cardiac surgery. J Am Soc Nephrol. 2018;29(2):670–679. doi:10.1681/ASN.2017020217
11. Yuasa R, Ohashi Y, Saito A, et al. Prevalence of hypothyroidism in Japanese chronic kidney disease patients. Ren Fail. 2020;42(1):572–579. doi:10.1080/0886022X.2020.1777162
12. Afsar B, Yilmaz MI, Siriopol D, et al. Thyroid function and cardiovascular events in chronic kidney disease patients. J Nephrol. 2017;30(2):235–242. doi:10.1007/s40620-016-0300-y
13. Fan JX, Yan P, Wang YD, et al. Prevalence and clinical significance of low T3 syndrome in non-dialysis patients with chronic kidney disease. Med Sci Monit. 2016;22:1171–1179. doi:10.12659/MSM.895953
14. Liu J, Xue Y, Jiang W, et al. Thyroid hormone is related to postoperative AKI in acute type A aortic dissection. Front Endocrinol. 2020;11:588149. doi:10.3389/fendo.2020.588149
15. Chen LZ, Zhou H, Huang WJ, et al. Outcome predictors in patients presenting with acute aortic dissection. J Cardiothorac Vasc Anesth. 2016;30(5):1272–1277. doi:10.1053/j.jvca.2016.03.149
16. Joo EY, Kim YJ, Go Y, et al. Relationship between perioperative thyroid function and acute kidney injury after thyroidectomy. Sci Rep. 2018;8(1):13539. doi:10.1038/s41598-018-31946-w
17. Cerillo AG, Storti S, Kallushi E, et al. The low triiodothyronine syndrome: a strong predictor of low cardiac output and death in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2014;97(6):2089–2095. doi:10.1016/j.athoracsur.2014.01.049
18. Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. 2015;10(3):500–514. doi:10.2215/CJN.07830814
19. Haase-Fielitz A, Haase M, Bellomo R, et al. Perioperative hemodynamic instability and fluid overload are associated with increasing acute kidney injury severity and worse outcome after cardiac surgery. Blood Purif. 2017;43(4):298–308. doi:10.1159/000455061
20. Moledina DG, Mansour SG, Jia Y, et al. Association of T cell-derived inflammatory cytokines with acute kidney injury and mortality after cardiac surgery. Kidney Int Rep. 2019;4(12):1689–1697. doi:10.1016/j.ekir.2019.09.003
21. Billings FT, Pretorius M, Schildcrout JS, et al. Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol. 2012;23(7):1221–1228. doi:10.1681/ASN.2011090940
22. Vargas F, Rodriguez-Gomez I, Vargas-Tendero P, et al. The renin-angiotensin system in thyroid disorders and its role in cardiovascular and renal manifestations. J Endocrinol. 2012;213(1):25–36. doi:10.1530/JOE-11-0349
23. Schairer B, Jungreithmayr V, Schuster M, et al. Effect of thyroid hormones on kidney function in patients after kidney transplantation. Sci Rep. 2020;10(1):2156. doi:10.1038/s41598-020-59178-x
24. Kimura K, Shinozaki Y, Jujo S, et al. Triiodothyronine acutely increases blood flow in the ventricles and kidneys of anesthesized rabbits. Thyroid. 2006;16(4):357–360. doi:10.1089/thy.2006.16.357
25. Kumar E, Mccurdy MT, Koch CA, et al. Impairment of thyroid function in critically ill patients in the intensive care units. Am J Med Sci. 2018;355(3):281–285. doi:10.1016/j.amjms.2017.06.026
26. Kim SM, Kim SW, Jung YJ, et al. Preconditioning with thyroid hormone (3,5,3-triiodothyronine) prevents renal ischemia-reperfusion injury in mice. Surgery. 2014;155(3):554–561. doi:10.1016/j.surg.2013.10.005
27. Li F, Lu S, Zhu R, et al. Heme oxygenase-1 is induced by thyroid hormone and involved in thyroid hormone preconditioning-induced protection against renal warm ischemia in rat. Mol Cell Endocrinol. 2011;339(1–2):54–62. doi:10.1016/j.mce.2011.03.019
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.