Back to Journals » Cancer Management and Research » Volume 14
Low MARCO Expression is Associated with Poor Survival in Patients with Hepatocellular Carcinoma Following Liver Transplantation
Authors Zhang Q, Wei Y, Li Y, Jiao X
Received 19 February 2022
Accepted for publication 9 May 2022
Published 11 June 2022 Volume 2022:14 Pages 1935—1944
DOI https://doi.org/10.2147/CMAR.S363219
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Qi Zhang,* Yuxuan Wei,* Yihu Li, Xingyuan Jiao
Organ Transplant Center, the First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, 510080, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xingyuan Jiao, Organ Transplant Center, the First Affiliated Hospital, Sun Yat-sen University, Guangzhou, 510080, People’s Republic of China, Email [email protected]
Background: Macrophage receptor with collagenous structure (MARCO) reportedly plays a crucial role in the occurrence and development of several cancers. However, the association between MARCO and the prognosis of hepatocellular carcinoma (HCC) post-liver transplantation remains poorly elucidated.
Methods: We examined MARCO expression at mRNA and protein level in 145 HCC samples and adjacent nontumor tissues using quantitative reverse transcription PCR, Western blot and immunohistochemistry. Furthermore, we analyzed the correlation of MARCO expression with clinicopathologic features and prognosis.
Results: We assessed the association between MARCO expression and clinicopathologic features and used the Cox proportional hazards regression model to explore the association between MARCO expression and clinical prognosis of patients with HCC post-liver transplantation. We observed that the expression of MARCO at mRNA and protein level in adjacent nontumor tissues was higher than that in the HCC tissues. Low MARCO expression in HCC tissues was correlated with higher alpha-fetoprotein levels, higher incidence of microvascular invasion, and a higher number of patients beyond Milan criteria. Kaplan–Meier survival curves showed that patients with HCC with low MARCO expression exhibited poor overall survival (OS) and disease-free survival (DFS). Univariate and multivariate analysis revealed that MARCO expression was an independent prognostic factor for OS (hazard ratio [HR] 2.696, 95% confidence interval [CI] 1.335– 5.444, P=0.006) and DFS (HR 2.867, 95% CI 1.665– 4.936, P< 0.001) in patients with HCC post-liver transplantation. Based on immunofluorescence analysis, MARCO expression was primarily localized to macrophages and might be associated with M2-like macrophage polarization during HCC.
Conclusion: MARCO expression was downregulated in HCC and associated with poor prognosis of patients with HCC post-liver transplantation. Moreover, it could be a potential prognostic marker and therapeutic target in post-liver transplantation HCC.
Keywords: MARCO, hepatocellular carcinoma, liver transplantation, prognosis
Introduction
Hepatocellular carcinoma (HCC) is one of the malignant tumors with a growing incidence and is reportedly the third highest cause of cancer-related mortality worldwide.1 Primary therapeutic strategies for HCC include tumor resection, radiofrequency ablation or transcatheter arterial chemoembolization (TACE), and liver transplantation.2 Liver transplantation is the most effective treatment strategy for early-stage HCC.3,4 However, given the limited availability of organ donors, physicians need to appropriately select patients with considerable survival benefits post-liver transplantation and optimally utilize a scarce source of liver grafts. Several criteria have been established to help select patients most likely to benefit from an LT, including the Milan criteria (the diameter of a single tumor was less than 5 cm, and the maximum diameter of multiple tumors was less than 3 cm). Despite careful selection, the incidence of HCC recurrence after liver transplantation (LT) is estimated to range between 8 and 18%,5 and only 75% of patients achieve the 5-year disease-free survival (DFS) of HCC post-LT.6 Therefore, it is critical to comprehensively elucidate molecular mechanisms underlying the progression of HCC post-LT and identify relevant biomarkers for early HCC diagnosis or as HCC treatment targets to improve patient prognosis.
Accumulating evidence suggests that the deficiency of host immunosurveillance against tumor cells plays an important role in cancer initiation, progression, and metastasis.7 Macrophages exert crucial effects during immune defense against tumor cells.8 Macrophage receptor with collagenous structure (MARCO), a class A scavenger receptor, is best characterized in macrophages. Systemic bacterial sepsis or bacterial lipopolysaccharide were shown to upregulate MARCO expression.9,10 In addition, MARCO is considered to play a crucial role in the formation of dendritic processes and some macrophage-related immune responses by mediating binding and phagocytosis. A recent study has shown that MARCO expression can enhance the trafficking and anti-tumor efficacy of tumor lysate-pulsed dendritic cells.11 In human breast cancer, MARCO overexpression was detected in the tumor microenvironment and associated with a poor prognosis.12 However, few studies have evaluated the expression of MARCO in solid tumor progression, and the association between MARCO and the prognosis of HCC post-LT has not been comprehensively elucidated.
In the present study, we examined the expression of MARCO and further analyzed its potential value as a prognostic biomarker in patients with HCC post-LT. We observed that MARCO expression was downregulated during HCC and associated with patient survival and tumor recurrence post-LT. Our results may provide critical clues for the treatment and prevention of HCC.
Materials and Methods
Patients and Tissue Samples
We enrolled two independent cohorts of patients with HCC. For cohort 1, we collected 30 fresh HCC samples and adjacent nontumor tissues from 30 patients who underwent LT between January 2017 and May 2017. For cohort 2, we collected formalin-fixed, paraffin-embedded HCC tissues from 115 patients with HCC who underwent LT at the same hospital from January 2014 to December 2016 and were followed until June 2018. The study procedure was approved by the research ethics committee of the First Affiliated Hospital, Sun Yat-sen University. All organs were donated voluntarily with written informed consent, and this study was conducted in accordance with the Declaration of Istanbul. Table 1 presents the clinicopathologic variables of patients.
 |
Table 1 Relationship Between the Expression of MARCO and Clinicopathological Characteristics |
Immunohistochemistry (IHC)
After deparaffinization with dimethylbenzene, tissue sections were sequentially rehydrated using graded alcohol. Antigen retrieval was achieved by boiling sections in citrate–disodium hydrogen phosphate buffer (pH 6.0) under high pressure for 5 min. Endogenous peroxidase was inactivated by incubating with 0.3% hydrogen peroxide for 30 min. Subsequently, slides were overnight incubated with primary antibodies (diluted 1:100) against MARCO (Abcam, MA, USA) at 4°C. Sections were incubated in biotinylated secondary antibody and streptavidin peroxidase (Invitrogen, NE, USA) and then developed with 3,3′-diaminobenzidine solution (Genetech, Shanghai, China) before counter-staining with hematoxylin. The intensity and extent of MARCO immunostaining were evaluated for all samples under double-blinded conditions. The percentage of positive staining was scored as 0 (0–9%), 1 (10–25%), 2 (26–50%), 3 (51–75%), or 4 (76–100%), with the intensity scored as 0 (no staining), 1 (weak staining), 2 (moderate staining), or 3 (dark staining). The total score was calculated as the product of intensity and extent, ranging from 0 to 12. A total score of ˂6 was defined as low MARCO expression, while ≥6 was defined as high MARCO expression. Two pathologists independently assessed the specimens. Images were obtained using an Olympus BX63 microscope (Olympus, Tokyo, Japan).
RNA Extraction and Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
For cohort 1, the total RNA of 30 fresh HCC samples and adjacent nontumor tissues was prepared using Trizol reagent (Invitrogen, Karlsruhe, Germany) following the manufacturer’s instructions. qRT-PCR was performed using the SYBR green detection system RT-PCR system (Takara, Dalian, China). All experiments were performed in quadruplicate. MARCO primer sequences used were as follows:
Forward primer: 5′-CTGGTGGTCCAAGTTCTGAATCT-3′ Reverse primer: 5′-TCAGCCGCCAGAGTGTCA-3′;
Western Blot Analysis
The antibodies used were as follows: rabbit-anti-human MARCO (Abcam, MA, USA) as primary antibody and anti-rabbit-IgG (H+L) (CST, MA, US) as secondary antibody.Western blot analyses were performed as below. In brief, total protein was isolated from tissue samples using RIPA buffer (CST, MA, US) with PMSF (GenStar, Beijing, China) and phosphatase inhibitor tablets (CWBIO, Jiangsu, China) and quantified using a Pierce BCA Protein Assay Kit (KeyGEN BioTECH, Jiangsu, China). The total protein samples were loaded and separated on SDS-PAGE gels and transferred to PVDF membranes (Roche, Basel, Switzerland). The membranes were blocked with 5% skim milk and incubated with the indicated primary antibodies overnight at 4°C, which was followed by incubation with the corresponding secondary antibodies for 2 h at room temperature. Signals were visualized by Immobilon Western HRP Substrate (Millipore, MA, USA) and captured by a ChemiDocTM Touch Imaging System (Bio-Rad, MA, USA). GAPDH was used as a loading control.
Immunofluorescence Analysis
For immunofluorescence analysis, primary antibodies were incubated overnight at 4°C, followed by staining of secondary antibodies for 1 h at room temperature. The antibodies used were as follows: rabbit-anti-human MARCO (Abcam, MA, USA) and mouse-anti-human CD68 (Abcam, MA, USA), or rabbit anti-human MARCO and monoclonal mouse anti-human CD163 (Abcam, MA, USA), the secondary goat-anti-rabbit IgG and goat anti-mouse IgG. All slides were stained with DAPI (Santa Cruz Biotechnology, Dallas, USA) for 1 min and mounted with Prolong Diamond mounting medium (Invitrogen, NE, USA). Images were obtained using a confocal microscope and analyzed using ImageJ software (National Institutes of Health, MD, USA).
Statistical Analysis
Data analyses were performed using SPSS version 23.0 statistical software (SPSS, IL, USA). MARCO expression in HCC tissues was divided into high or low by the median immunostaining score for further analysis. The χ2 test was used to analyze the association between MARCO expression and the clinicopathological parameters. Furthermore, we assessed the impact of various prognostic factors on overall survival (OS) and DFS of patients with HCC post-LT using Cox regression and Kaplan–Meier analyses. A p-value<0.05 was considered statistically significant.
Results
Downregulation of MARCO Expression in HCC Tissues
To examine the expression and significance of MARCO in HCC tissues, we first detected the expression of MARCO at mRNA and protein level in 30 fresh HCC specimens and adjacent nontumor tissues using qRT-PCR (Figure 1A) and Western blot (Figure 1B). Compared with matched adjacent nontumor tissues, expression of MARCO was significantly reduced at mRNA (P<0.0001) and protein level in HCC tissues.
Correlation of MARCO Expression with Clinicopathologic Features
To further confirm the qRT-PCR results, we performed an IHC analysis of MARCO protein expression in 115 paired HCC and matched nontumor tissue samples. We found that MARCO was highly expressed in adjacent nontumor tissues, whereas HCC tissues showed low levels of MARCO expression (Figure 2). Subsequently, we sorted HCC samples into high and low MARCO expression groups to estimate the relationship between MARCO expression and clinicopathologic variables. The MARCO immunostaining signals revealed that 82 (71.3%) of 115 patients with HCC exhibited high MARCO expression, while 33 (28.7%) patients showed low expression levels. Low MARCO expression in HCC tissues was correlated with a higher alpha-fetoprotein (AFP) level (P<0.001), higher incidence of microvascular invasion (P=0.042) and a higher number of patients beyond Milan criteria (P=0.001) (Table 1). No statistical correlation was observed between MARCO expression and other clinicopathological characteristics (Table 1).
Decreased MARCO Expression Predicted Poor Prognosis in Patients with HCC Post-LT
Kaplan–Meier survival curves revealed that patients with HCC exhibiting low MARCO expression had poor OS (Figure 3A, P<0.001) and DFS than those with high MARCO expression (Figure 3B, P<0.001). Only 15 of 33 patients (45.5%) in the MARCO low expression group were alive at last follow-up when compared with 65 of 82 (75.6%) in the MARCO high-expression group. To determine whether MARCO has potential as an independent prognostic factor in patients with HCC post-LT, Cox regression analysis was performed to examine the OS and DFS. Univariate analysis indicated that MARCO expression (hazard ratio [HR] 3.378, 95% confidence interval [CI] 1.734–6.581, P<0.001), microvascular invasion (HR 3.192, 95% CI 1.407–7.242, P=0.005), Milan criteria (HR 2.196, 95% CI 1.083–4.455, P=0.029), AFP (HR 3.438, 95% CI 1.742–6.786, P<0.001) and gender (HR 3.099, 95% CI 1.349–7.116, P=0.008) were associated with OS. Furthermore, multivariate analysis revealed that MARCO expression (HR 2.696, 95% CI 1.335–5.444, P=0.006), microvascular invasion (HR 2.429, 95% CI 1.034–5.709, P=0.042) and gender (HR 2.854, 95% CI 1.222–6.664, P=0.015) were independent prognostic factors for OS in patients with HCC post-LT (Table 2). As shown in Table 3, univariate analysis indicated that MARCO expression (HR 3.276, 95% CI 1.913–5.581, P<0.001), microvascular invasion (HR 3.524, 95% CI 1.852–6.706, P<0.001), Edmonson Grading (HR 2.220, 95% CI 1.299–3.795, P= 0.004), AFP (HR 3.276, 95% CI 1.912–5.613, P<0.001) and Milan criteria (HR 2.096, 95% CI 1.203–3.652, P=0.009) were associated with DFS. Furthermore, multivariate analysis demonstrated that MARCO expression (HR 2.867, 95% CI 1.665–4.936, P<0.001), microvascular invasion (HR 2.765, 95% CI 1.433–5.334, P=0.002), and Edmonson Grading (HR 2.319, 95% CI 1.355–3.968, P=0.002) were independent prognostic factors for DFS in patients with HCC post-LT (Table 3). Collectively, MARCO expression could serve as an independent prognostic factor in patients with HCC post-LT.
 |
Table 2 Univariate and Multivariate Analysis of Risk Factors Associated with OS |
 |
Table 3 Univariate and Multivariate Analysis of Risk Factors Associated with DFS |
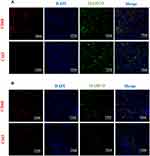
MARCO Expression Was Primarily Localized to Macrophages and Might Be Associated with M2-Like Macrophage Polarization in HCC
Immunofluorescence analysis was performed to confirm the expression of MARCO, CD68 and CD163 in HCC tissues (Figure 4A) and matched adjacent nontumor tissues (Figure 4B). The results suggested that the expression of MARCO was downregulated in HCC tissues compared with matched adjacent nontumor tissues. And the colocalization of MARCO and CD68 suggested that MARCO was predominantly expressed by macrophages. We further investigated whether MARCO can affect macrophage polarization and found CD163+ cells showed lower expression level of MARCO, suggesting that MARCO expression in HCC tissues might be associated with M2-like macrophage polarization.
Discussion
In the present study, we, for the first time, report the clinical significance of MARCO expression in patients with HCC post-LT. We observed that MARCO expression was significantly decreased in HCC tissues when compared with adjacent nontumor tissues. Low MARCO expression was an independent prognostic factor for OS and DFS in patients with HCC post-LT. Based on the immunofluorescent analysis, we confirmed that MARCO expression was primarily localized to macrophages and might be associated with M2-like macrophage polarization in HCC.
Studies have demonstrated that MARCO plays a crucial role in the occurrence and development of several cancers. Bergamaschi et al12 have found that MARCO expression levels can be associated with breast cancer survival and the risk of recurrence. In non-small cell lung cancer, La Fleur L et al13 have reported that MARCO-expressing tumor-associated macrophages could block cytotoxic T-cell and natural killer-cell activation, inhibiting their proliferation, cytokine production, and tumor-killing capacity. In addition, MARCO could be a potential therapeutic target for lung cancer. Therefore, we hypothesized that MARCO could be a valuable biomarker for HCC post-LT. First, we detected MARCO expression in 30 pairs of HCC tissues and adjacent nontumor tissues using qRT-PCR. The results indicated that MARCO expression levels are lower in HCC tissues than in adjacent nontumor tissues. In addition, we performed IHC to detect MARCO expression in 115 pairs of HCC samples. We evaluated the relationship between MARCO expression and clinical characteristics of patients with HCC and found that the MARCO expression level in HCC tissues could be associated with the AFP level, microvascular invasion, and Milan criteria. Accordingly, these findings suggest that HCC tumorigenesis could be associated with decreased MARCO expression.
Furthermore, the post-LT survival of patients with HCC exhibiting low MARCO expression was significantly reduced when compared with patients with HCC exhibiting high MARCO expression. Kaplan–Meier analyses revealed that patients with low MARCO expression exhibited poorer OS and DFS than those with high MARCO expression. In addition, univariate and multivariate analyses revealed that MARCO expression was an independent prognostic factor for OS and DFS in patients with HCC post-LT. Collectively, these results suggest that MARCO expression could be a potential prognostic indicator of HCC prognosis.
Kupffer cells are liver-resident macrophages associated with proinflammatory reactions, ischemia-reperfusion, and immune reactions.14,15 MARCO is typically expressed on restricted subpopulations of macrophages in peripheral immune organs.16 We verified that MARCO expression was predominantly localized to Kupffer cells in HCC tissues, as determined by the colocalization of MARCO and CD68 using immunofluorescence. Macrophages can be sorted into classically (M1) and alternatively (M2) activated phenotypes based on surface receptors and functional characteristics.17 Accumulating evidence indicates that intratumoral macrophages exhibit M2 phenotypes and are associated with poor prognosis in numerous malignancies.18,19 In addition, we observed that MARCO was primarily colocalized in situ with M2 marker CD163+ cells, indicating that MARCO expression in HCC tissues might be associated with M2-like macrophage polarization. In mouse melanoma models, treatment with MARCO-targeting antibodies could significantly suppress melanoma progression and metastasis.20 Herein, we present a novel perspective on a potential treatment targeting MARCO expression, representing a therapeutic strategy for patients with HCC patients post-LT.
Conclusions
Herein, we report, for the first time, that MARCO expression can be significantly downregulated in HCC, and low MARCO expression could be associated with poor OS and DFS in patients with HCC post-LT. MARCO could be used as a valuable biomarker to predict the prognosis of patients with HCC post-LT. In addition, MARCO expression in HCC can be associated with M2 macrophages, warranting in-depth investigations to determine underlying molecular mechanisms.
Acknowledgments
This study was supported by the Science and Technology Project of Guangdong Province, China (2017B090901066) and the Science and Technology Project of Guangzhou, China (20180310002).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Vibert E, Schwartz M, Olthoff KM, et al. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol. 2020;72(2):262–276. doi:10.1016/j.jhep.2019.11.017
3. Bruix J, Reig M, Sherman M, et al. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.041
4. Fan R, Papatheodoridis G, Sun J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. 2020;73(6):1368–1378. doi:10.1016/j.jhep.2020.07.025
5. Bhoori S, Mazzaferro V. Current challenges in liver transplantation for hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2014;28(5):867–879. doi:10.1016/j.bpg.2014.08.001
6. Pinna AD, Yang T, Mazzaferro V, et al. Liver transplantation and hepatic resection can achieve cure for hepatocellular carcinoma. Ann Surg. 2018;268(5):868–875. doi:10.1097/SLA.0000000000002889
7. Finn OJ, Mazzaferro V. Cancer immunology. N Engl J Med. 2008;358(25):2704–2715. doi:10.1056/NEJMra072739
8. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–382. doi:10.1038/s41577-019-0127-6
9. van der Laan LJ, Döpp EA, Haworth R, et al. Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. J Immunol. 1999;162:939–947.
10. Novakowski KE, Yap NVL, Yin C, et al. l. Human-Specific Mutations and Positively Selected Sites in MARCO Confer Functional Changes. Mol Biol Evol. 2018;35(2):440–450. doi:10.1093/molbev/msx298
11. Matsushita N, Komine H, Grolleau-Julius A, et al. Targeting MARCO can lead to enhanced dendritic cell motility and anti-melanoma activity. Cancer Immunol Immunother. 2010;59(6):875–884. doi:10.1007/s00262-009-0813-5
12. Bergamaschi A, Tagliabue E, Sørlie T, et al. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J Pathol. 2008;214(3):357–367. doi:10.1002/path.2278
13. La Fleur L, Botling J, He F, et al. Targeting MARCO and IL37R on Immunosuppressive Macrophages in Lung Cancer Blocks Regulatory T Cells and Supports Cytotoxic Lymphocyte Function. Cancer Res. 2021;81(4):956–967. doi:10.1158/0008-5472.CAN-20-1885
14. Song K, Kwon H, Han C, et al. Yes-Associated Protein in Kupffer Cells Enhances the Production of Proinflammatory Cytokines and Promotes the Development of Nonalcoholic Steatohepatitis. Hepatology. 2020;72(1):72–87. doi:10.1002/hep.30990
15. Ni M, Zhang J, Sosa R, et al. T-Cell Immunoglobulin and Mucin Domain-Containing Protein-4 Is Critical for Kupffer Cell Homeostatic Function in the Activation and Resolution of Liver Ischemia Reperfusion Injury. Hepatology. 2021;74(4):2118–2132. doi:10.1002/hep.31906
16. Kangas M, Brännström A, Elomaa O, et al. Structure and chromosomal localization of the human and murine genes for the macrophage MARCO receptor. Genomics. 1999;58(1):82–89. doi:10.1006/geno.1999.5811
17. Oscar WH, Yeung S, Ling -C-C, et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62(3):607–616. doi:10.1016/j.jhep.2014.10.029
18. Wang -T-T, Yuan J-H, Ma J-Z, et al. CTGF secreted by mesenchymal-like hepatocellular carcinoma cells plays a role in the polarization of macrophages in hepatocellular carcinoma progression. Biomed Pharmacother. 2017;95:111–119. doi:10.1016/j.biopha.2017.08.004
19. Yang Q, Guo N, Zhou Y, et al. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B. 2020;10(11):2156–2170. doi:10.1016/j.apsb.2020.04.004
20. Georgoudaki AM, Prokopec K, Boura V, et al. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016;15:2000–2011. doi:10.1016/j.celrep.2016.04.084
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.