Back to Journals » Cancer Management and Research » Volume 11
Low-dose of olanzapine has ameliorating effects on cancer-related anorexia
Authors Okamoto H , Shono K, Nozaki-Taguchi N
Received 18 October 2018
Accepted for publication 30 January 2019
Published 19 March 2019 Volume 2019:11 Pages 2233—2239
DOI https://doi.org/10.2147/CMAR.S191330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Hideki Okamoto,1 Koyo Shono,2 Natsuko Nozaki-Taguchi2
1Department of Kampo Medicine (Japanese Traditional Medicine), School of Medicine, International University of Health and Welfare, Tokyo, Japan; 2Palliative Care Center, Chiba University Hospital, Chiba, Japan
Background: Olanzapine (OLZ) has become well-known for its antiemetic effects on chemotherapy-induced nausea and vomiting. However, it remains unclear whether OLZ also has efficacy for treating cancer-related anorexia. This study, therefore, retrospectively examined whether or not OLZ administration affects the food intake in anorexic cancer patients who exhibit neither nausea nor vomiting.
Methods: Eighty patients prescribed OLZ were extracted from 951 inpatients who consulted with our palliative care team at Chiba University Hospital from April 2008 to March 2016. Their food intake described on a nursing record was compared before and after OLZ administration. The observation period was 3 days before and after the start of OLZ treatment, because most inpatients whose food intake increased were discharged in 3 days.
Results: In those 80 patients, the average dose of OLZ for 3 days was 2.28±0.87 (mean±SD) mg/day. First, the food intake in 80 patients was significantly higher after than before starting OLZ, and the relative change in food intake was 149% on average (P<0.0001, Student’s paired t-test). Second, OLZ increased the food intake even in 40 out of 80 patients without nausea or vomiting, and the relative change in food intake was 143% on average (P<0.001, Student’s paired t-test). Third, the average food intake increased in 13 out of 40 patients who were prescribed 1.5 mg/day of OLZ, and the relative change in food intake was 124% on average (P<0.01, Student’s paired t-test). There was no significant difference in food intake between a dose of 1.5 mg/day and a dose of >1.5 mg/day of OLZ (P=0.18, Welch’s unpaired t-test).
Conclusion: We have herein reported OLZ’s ameliorating efficacy in cancer-related anorexia at the low dose of 1.5 mg/day. Although our study has many limitations, low-dose OLZ can be a promising treatment for cancer-related anorexia.
Keywords: chemotherapy, nausea, cachexia, appetite, end-of-life care, tranquilizer
Introduction
Olanzapine (OLZ) has long been known to have antiemetic effects in chemotherapy-induced nausea and vomiting (CINV). Fourteen randomized controlled trials (RCTs) including six phase II1–6 and two phase III7,8 trials and lots of other clinical studies, as well as two systematic reviews,9,10 abundantly proved OLZ’s effectiveness in treating CINV. OLZ was also reported to be effective for nausea and vomiting in advanced cancer patients and a palliative care population not undergoing chemotherapy.11–14
It, however, remains unclear whether OLZ alone has an ameliorating effect on cancer patients’ anorexia, even when those patients exhibit no nausea or vomiting. As a matter of fact, one study suggested that combination treatment with OLZ and megestrol acetate has a beneficial effect on cancer-related anorexia, while one study reported that OLZ failed to increase the body weight of cancer cachexia patients.15,16
Our current study was, therefore, performed to examine whether OLZ alone is effective in treating cancer-related anorexia by comparing food intake before and after OLZ administration in cancer patients.
Methods
Patients
A total of 951 patients, who were consulting with the palliative care team at Chiba University Hospital from April 2008 to March 2016, were identified by searching our inpatient database. Eighty patients out of 951 patients identified met the following inclusion and exclusion criteria and were eligible for further investigation of OLZ’s effectiveness. Those patients were hospitalized and could not be discharged from the hospital because of anorexia, which did not improve even after the chemotherapy was suspended and appropriate management of the other symptoms was done such as pain, dyspnea, constipation, electrolyte abnormalities, and dehydration.
Comparison of food intake before and after OLZ administration in all patients
The inclusion criteria were as follows:
- Patients were diagnosed with cancer.
- OLZ administration started after consulting the palliative care team.
- The severity of appetite loss was greater than Grade 1 in the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
The exclusion criteria were as follows:
- The severity of somnolence was higher than Grade 1 in the CTCAE and/or patients were diagnosed with delirium, which may affect the food intake.
- Patients fasted and/or had an intestinal obstruction.
- Corticosteroid treatment was newly started or the dose of corticosteroid was increased between 7 days before and 3 days after OLZ administration.
Comparison of food intake before and after OLZ administration in patients without nausea or the addition of antiemetics
The exclusion criteria were as follows:
- The severity of vomiting and/or nausea was higher than or equal to Grade 1 in the CTCAE.
- Antiemetic medication was newly started or the dose of antiemetics was increased between 7 days before and 3 days after OLZ administration.
Dosage of OLZ
OLZ was administered once daily at night (after 6 p.m. and before midnight) in all cases. The dosage of the administered OLZ was averaged for 3 days in each case.
Comparison of food intake before and after OLZ administration
OLZ’s effect on cancer-related anorexia was evaluated by comparing the average food intake before and after starting OLZ. The observation period was 3 days before and after the start of OLZ treatment, respectively, because the patients whose food intake increased were discharged in 3 days after OLZ administration. The food intake at every meal was described on a nursing record by nurses without knowing whether OLZ affected the food intake or not. The average total food intake over 3 days was determined for the periods just before and after OLZ administration and compared. Even when a patient could not eat any main dishes or side dishes or even when a patient’s food intake was described as “one bite” or “one sip” on the nursing record, the food intake was counted as 5% rather than 0%. Usually, the food intake was separately quantified as principal food and other food components in Japan. Table 1 shows an example of a nursing record and how to quantify the average food intake in this study. For example, if a patient ate 100% of principal food and 90% of the other foods at breakfast and 70% of principal food and 90% of the other foods at lunch and he skipped supper due to loss of appetite 3 days before starting OLZ, his average food intake of the day should be {(100+90)/2 + (70+90)/2 + (5+5)/2}/3 = (95+80+5)/3=60 (%). When the average food intake for 3 days before starting OLZ was 50% and the average food intake for 3 days after starting OLZ was 70%, the relative change in food intake was expressed as 140%.
  | Table 1 An example of nursing record and quantification of food intake Note: All numbers in columns show quantification of food intake (%). Abbreviation: OLZ, olanzapine. |
Data analysis
The relative change in average food intake was expressed as mean (%)±standard error of the mean (SEM) (Figures 1–4), and the average dose of administered OLZ was expressed as mean (mg/day)±SD in all results as appropriate. Student’s paired t-test was used for the analyses (Figures 1–3), and Welch’s unpaired t-test was used (Figure 4). The statistical significance levels of the two-tailed P-values were considered to be <0.05 in all analyses. Microsoft Excel 2016 software was used for all statistical analyses. This study was conducted in accordance with the Declaration of Helsinki and with the approval of the Human Research Ethics Committee of Chiba University Graduate School of Medicine (Identification number: 2529). Most patients are deceased, and patient consent was not required under country-specific regulations because this was a retrospective study where all patient data were anonymized and no direct access to source data by the clinical research organization was allowed.
Results
Patient characteristics
Eighty patients who started OLZ treatment during our palliative care team’s intervention were extracted from 951 patients who met the inclusion and exclusion criteria as described previously. The average age in these 80 cases, consisting of 45 females and 35 males, was 60.4±15.7 years. Table 2 shows the primary cancer sites in these 80 patients. For example, the primary cancer sites in order of descending prevalence were the uterus in 12 patients, lung in 10 patients, pancreas in 9 patients, and stomach in 7 patients out of 80 patients. There was no significant difference in the average age and dose of OLZ between genders and in response rate depending on the primary cancer sites (data not shown).
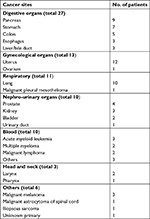  | Table 2 Primary cancer sites in 80 patients |
Comparison of food intake between before and after OLZ administration in all patients
First, we checked whether or not OLZ increased the food intake in all 80 cancer-related anorexia patients who started OLZ treatment during our palliative care team’s intervention. The average food intake over 3 days was compared before and after the start of OLZ in these 80 patients. The average dose of OLZ for 3 days was 2.28±0.87 (mean±SD) mg/day. The maximum average dose was 5 mg/day, the minimum dose was 1.5 mg/day prescribed in powder forms, and the median dose was 2.5 mg/day. There was no patient with nausea and/or vomiting who was administered 1.5 mg/day of OLZ through 3 days because the dose of OLZ was increased during the observational time period expecting further antiemetic effects, even if the starting dose was 1.5 mg/day. In these 80 patients who were prescribed OLZ, the food intake was significantly higher after than before starting OLZ, and the relative change in food intake was 149% on average (P<0.0001, Student’s paired t-test) (Figure 1).
Comparison of food intake before and after OLZ administration in patients without nausea or the addition of antiemetics
Second, we examined whether OLZ increases the food intake regardless of its antiemetic effects. Forty out of 80 patients met the exclusion criteria of having no nausea or vomiting, and not having newly started or increased antiemetics between 7 days before and 3 days after OLZ administration. Twenty-four out of 40 patients were under the best supportive care, 7 patients received oral palliative chemotherapy (molecular-targeted agents), 5 patients were under cytotoxic chemotherapy where the elapsed time since the last chemotherapy was 15.4±8.0 (mean±SD) days. Two out of 40 patients were suffering from acute graft vs host disease (GVHD) after the transplants, 1 patient suffered from chronic GVHD, and 1 patient had been anorexic before her cancer was found and was hospitalized to receive surgery. In the 40 nausea-free patients, the average food intake was significantly higher after starting OLZ than before starting OLZ, and the relative change in food intake was 143%, on average (P<0.001, Student’s paired t-test) (Figure 2).
Effect of low-dose OLZ administration on food intake in patients without nausea or the addition of antiemetics
Third, we confirmed whether or not 1.5 mg/day of OLZ increased the food intake in nausea-free cancer-related anorexia patients. In 13 nausea-free patients who were prescribed with 1.5 mg/day of OLZ, the average food intake for 3 days after starting OLZ was significantly higher after than before, and the relative change in food intake was 124% on average (P<0.01, Student’s paired t-test) (Figure 3). Figure 5 shows the time course of each patient’s average daily food intake through the 3 days before and after starting treatment with 1.5 mg of OLZ.
  | Figure 5 Change of average daily food intake before and after low-dose OLZ administration in 13 patients (A–M) without nausea or the addition of antiemetics. Abbreviation: OLZ, olanzapine. |
Correlation between OLZ dose and effect of OLZ in increasing food intake
Finally, we validated whether a dose of >1.5 mg/day of OLZ was superior to 1.5 mg/day, the minimum dose, in increasing the food intake. As a result, there was no significant difference in food intake between a dose of 1.5 mg/day and a dose of >1.5 mg/day, ranging from 1.83 to 5.0 (2.9±0.91; mean±SD) mg/day of OLZ among nausea-free cancer-related anorexia patients (P=0.18, Welch’s unpaired t-test) (Figure 4).
Adverse effects
The adverse effects caused by OLZ treatment were described in the CTCAE. No patient showed extrapyramidal symptoms during the observational period. The major adverse effect was somnolence, which was shown by 18 out of 40 patients without nausea or the addition of antiemetics (Table 3). Although the lower dose of OLZ tended to be chosen in patients who had already been somnolent or had a history of drowsiness due to medication, 1 patient taking 1.5 mg/day of OLZ showed Grade 2 somnolence.
Discussion
Our current study was performed by comparing the average food intake before and after the start of OLZ treatment in order to validate that OLZ has an ameliorating effect on cancer anorexia. Although it may be uncertain whether the average food intake directly reflects the appetite, the significant increase of food intake strongly implies that there is some improvement in appetite. In addition, good quality and well-portioned meals containing appropriate food types are served three times a day at all hospitals in Japan, and are adjusted according to each patient’s condition. Each patient’s food intake, including between-meal intake, is tracked in nursing reports in order to monitor the patient’s condition in detail, and all nurses in Japan are trained to quantify the food intake ensuring inter-rater reliability. Our current study was retrospective, but was, therefore, performed more accurately and objectively based on the food intake already cited in nursing reports.
In our study, the average of all patients’ food intake significantly increased in the 3 days after starting OLZ treatment, even in cancer patients without nausea or the addition of antiemetics, which strongly supports our hypothesis that OLZ has an ameliorating effect on cancer-related anorexia. In addition, our study revealed that the effective dose of OLZ in cancer anorexia was very low (1.5 mg/day). Our result is consistent with the previous report showing that low-dose OLZ monotherapy (mean dose: 4.13 mg/day) is effective in girls with anorexia nervosa, suggesting that OLZ at a lower dose can be a possible therapeutic agent against cancer-related anorexia, while causing fewer adverse effects such as extrapyramidal symptoms and somnolence.17
Considering the time course of the average daily food intake in 13 patients treated with OLZ at 1.5 mg/day (Figure 5), there were responders and nonresponders. Interestingly, the responders’ average food intake for 3 days before OLZ treatment was between >25% and <75%, whereas the nonresponders’ average food intake for 3 days before OLZ treatment was <25% (Patients I, J, and K) or >75% (Patients A and B). Together with the report that OLZ had no significant effect on weight loss in patients with cachexia due to advanced cancer, our report suggests that OLZ may ameliorate cancer-related anorexia prior to the cachectic stage.16
A previous report suggested the presence of a dose effect for OLZ (2.5, 5, and 10 mg/day) in the treatment of nausea in advanced cancer patients,18 but, although there may be a tendency, we could not find an obvious dose-dependent difference between a dose of 1.5 mg/day and doses of >1.5 mg/day in terms of the efficacy of OLZ in treating cancer-related anorexia. In fact, the dose of OLZ was increased in 2 cases (1.5→2.5, 2.5→5, respectively), but neither resulted in an increase in food intake (data not shown). These results further support our hypothesis that OLZ ameliorates cancer-related anorexia at a low-dose, and also imply that there is no need to increase the dose of OLZ even when a low-dose of OLZ is not effective.
Our current study has many limitations due to the small study population and the retrospective methodology. For example, the observation period might be too short to evaluate whether OLZ’s efficacy is sustained or not. However, patients were discharged soon from our hospital when their appetite was increased, which made our observation period limited. Another concern is that patients in this study have so many differences in their age, the primary cancer sites, their chemotherapy regimens, and receiving treatments. However, despite these limitations, this study shows that low-dose OLZ can be an effective and safe solution in cases of cancer-related anorexia. This is a preliminary retrospective study that we expect will contribute to the design of a future clinical trial to investigate the ameliorating efficacy of OLZ in cancer-related anorexia.
Conclusion
We have herein reported our single-institution 8-year experience treating cancer-related anorexia with low-dose OLZ. The food intake increased in the 3 days after the start of OLZ in cancer patients without nausea, strongly suggesting that OLZ has an ameliorating effect on cancer-related anorexia that is independent of its antiemetic efficacy. The effective dose of OLZ in cancer-related anorexia was 1.5 mg/day, and there was no dose-dependent difference in effect between a dose of 1.5 mg/day and a dose of >1.5 mg/day. Although more clinical studies are required to confirm the effectiveness of OLZ, low-dose OLZ is a promising treatment for cancer-related anorexia.
Acknowledgment
The authors sincerely thank Ms. Fujisawa, a palliative care nurse and a member of our palliative care team at Chiba University Hospital, for her excellent work on creating a database of inpatients who consulted with our palliative care team at the hospital.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Navari RM, Einhorn LH, Passik SD, et al. A phase II trial of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier Oncology Group study. Support Care Cancer. 2005;13(7):529–534. | ||
Navari RM, Einhorn LH, Loehrer PJ, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier Oncology Group study. Support Care Cancer. 2007;15(11):1285. | ||
Abe M, Hirashima Y, Kasamatsu Y, et al. Efficacy and safety of olanzapine combined with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy in gynecological cancer: KCOG-G1301 phase II trial. Support Care Cancer. 2016;24(2):675–682. | ||
Maeda A, Ura T, Asano C, et al. A phase II trial of prophylactic olanzapine combined with palonosetron and dexamethasone for preventing nausea and vomiting induced by cisplatin. Asia Pac J Clin Oncol. 2016;12(3):254–258. | ||
Nakashima K, Murakami H, Yokoyama K, et al. A phase II study of palonosetron, aprepitant, dexamethasone and olanzapine for the prevention of cisplatin-based chemotherapy-induced nausea and vomiting in patients with thoracic malignancy. Jpn J Clin Oncol. 2017;47(9):840–843. | ||
Yanai T, Iwasa S, Hashimoto H, et al. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol. 2018;23(2):382–388. | ||
Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10(2):115–124. | ||
Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9(5):188–195. | ||
Chow R, Chiu L, Navari R, et al. Efficacy and safety of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV) as reported in phase I and II studies: a systematic review. Support Care Cancer. 2016;24(2):1001–1008. | ||
Chelkeba L, Gidey K, Mamo A, Yohannes B, Matso T, Melaku T. Olanzapine for chemotherapy-induced nausea and vomiting: systematic review and meta-analysis. Pharm Pract. 2017;15(1):877. | ||
Suzuki M, Komuro K, Ohara K. Olanzapine and betamethasone are effective for the treatment of nausea and vomiting due to metastatic brain tumors of rectal cancer. Case Rep Gastroenterol. 2014;8(1):13–17. | ||
Kaneishi K, Kawabata M, Morita T. Olanzapine for the relief of nausea in patients with advanced cancer and incomplete bowel obstruction. J Pain Symptom Manage. 2012;44(4):604–607. | ||
Kaneishi K, Nishimura K, Sakurai N, et al. Use of olanzapine for the relief of nausea and vomiting in patients with advanced cancer: a multicenter survey in Japan. Support Care Cancer. 2016;24(6):2393–2395. | ||
Mackintosh D. Olanzapine in the management of difficult to control nausea and vomiting in a palliative care population: a case series. J Palliat Med. 2016;19(1):87–90. | ||
Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer. 2010;18(8):951–956. | ||
Naing A, Dalal S, Abdelrahim M, et al. Olanzapine for cachexia in patients with advanced cancer: an exploratory study of effects on weight and metabolic cytokines. Support Care Cancer. 2015;23(9):2649–2654. | ||
Leggero C, Masi G, Brunori E, et al. Low-dose olanzapine monotherapy in girls with anorexia nervosa, restricting subtype: focus on hyperactivity. J Child Adolesc Psychopharmacol. 2010;20(2):127–133. | ||
Passik SD, Lundberg J, Kirsh KL, et al. A pilot exploration of the antiemetic activity of olanzapine for the relief of nausea in patients with advanced cancer and pain. J Pain Symptom Manage. 2002;23(6):526–532. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





