Back to Journals » OncoTargets and Therapy » Volume 8
Low claudin-6 expression correlates with poor prognosis in patients with non-small cell lung cancer
Authors Wang Q, Zhang Y, Zhang T , Han Z, Shan L
Received 26 March 2015
Accepted for publication 18 June 2015
Published 31 July 2015 Volume 2015:8 Pages 1971—1977
DOI https://doi.org/10.2147/OTT.S85478
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Faris Farassati
Qiang Wang,1 Yan Zhang,1 Tao Zhang,2 Zhi-Gang Han,1 Li Shan1
1Department of Thoracic Oncology, The Affiliated Tumor Hospital of Xinjiang Medical University, Urumqi, Xinjiang Province, 2Department of Oncology, First Hospital of Lanzhou University, Lanzhou, Gansu Province, People’s Republic of China
Objective: Claudins are found in junctional complexes mediating cell adhesion and are involved in the attachment of tight junctions to the underlying cytoskeleton. Abnormal claudin-6 expression has been observed for a variety of malignant solid tumors, but the expression of claudin-6 in non-small cell lung cancer (NSCLC) has not yet been characterized.
Methods: Immunohistochemistry, reverse transcription-polymerase chain reaction (RT-PCR), and western blot analysis were used to quantify claudin-6 expression in 123 cases of NSCLC and non-cancerous adjacent tissue. We analyzed the relationship between claudin-6 expression and clinicopathological features of NSCLC. The Kaplan–Meier method was used to analyze postoperative survival rates, and the log-rank test was used to assess differences in survival rates. The Cox regression model was used to perform multivariate analysis.
Results: Claudin-6 expression was low for 61 of 123 (49.6%) NSCLC tissue samples and for 33 of 123 (26.8%) normal adjacent tissue samples. RT-PCR and western blot analyses confirmed the immunohistochemistry results. Claudin-6 expression was associated with lymph node metastasis (P<0.001) and TNM stage (P=0.007). Kaplan–Meier analysis indicated that patients with low claudin-6 expression had significantly lower survival rates than those with high claudin-6 expression. Multivariate analysis suggested that low claudin-6 expression was an independent indicator of prognosis in NSCLC patients.
Conclusion: Low claudin-6 expression is an independent prognostic biomarker that indicates a worse prognosis in patients with NSCLC.
Keywords: NSCLC, claudin-6, immunohistochemistry, RT-PCR, western blot
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer mortality in the world, and its incidence and mortality are increasing annually.1 The 5-year survival rate for lung cancer patients in the United States is only 15%, and in the People’s Republic of China, the survival rate is even lower.2 The initiation and progression of NSCLC comprise a complicated process that depends on multiple factors and steps, including proto-oncogene activation, tumor-suppressor gene inactivation, and abnormal expression of various proteins.
Tight junctions (TJs) provide extracellular adhesive contacts between cells; destabilization of junctional complexes directly affects nutrient intake, cell proliferation, and migration.3 Claudins are a large family of 24 proteins and critical components of TJs and help to attach junctional complexes to the underlying cell cytoskeleton.4 As an important component of TJs, claudin-6 is closely related to a variety of biological habits of cells. For example, it is possible to regulate cell proliferation and gene transcription. Numerous studies have demonstrated an association between abnormal claudin expression and multiple forms of cancer.5–8 However, the study of claudin-6 expression in NSCLC was rarely reported. Here, immunohistochemistry, reverse transcription-polymerase chain reaction (RT-PCR), and western blot analyses were together used to quantify claudin-6 expression in NSCLC and to better define the relationship between claudin-6 expression and prognosis in NSCLC patients.
Materials and methods
Patients and tumor specimens
NSCLC and adjacent non-cancerous tissue samples were surgically removed and collected from 123 patients of Xinjiang Medical University Affiliated Tumor Hospital from March 2005 to December 2010. All tissues were routinely fixed in 10% buffered formalin and embedded in paraffin blocks. Complete clinical data were available for all patients, and a pathologist confirmed the NSCLC diagnosis postoperatively. For adjacent non-cancerous tissue samples, tissue was obtained at a distance of more than 5 cm from the cancerous tissue; pathological analysis confirmed that the adjacent tissue samples were normal lung tissues. A total of 63 patients were male and 60 were female, with an age range of 32–75 years and an average age of 67.3±10.5 years. The histologic grade and clinical stage of the tumors were defined according to the seventh edition of the TNM classification of the International Union Against Cancer. None of the 123 NSCLC patients received chemotherapy or radiation therapy before surgery. All patients or their family members provided signed consent forms for the use of patient samples. The Ethics Committee of Subsidiary Tumor Hospital of Xinjiang Medical University approved the study.
Immunohistochemistry
Two-step method was used to stain the samples for immunohistochemical analysis. Paraffin-embedded samples were sectioned with a thickness of 4 μm, incubated overnight at 60°C, and then dewaxed. Sections were incubated at room temperature for 10 minutes in 3% hydrogen peroxide prepared fresh in deionized water in order to block endogenous peroxidase. After high-pressure retrieval in 0.01 mol/L citrate buffer solution (pH =6.0), the sections were incubated with rabbit antihuman claudin-6 monoclonal antibody (1:200; Santa Cruz Biotechnology Inc., Dallas, TX, USA) overnight at 4°C. Following three washes in phosphate-buffered saline (PBS), the sections were incubated with secondary antibody at 37°C for 10 minutes. After the sections were washed three times in PBS, they were incubated with horseradish peroxidase-conjugated streptavidin at 37°C for 10 minutes. Following three washes in PBS, the sections were incubated with diaminobenzidine to initiate the colorimetric reaction, which was monitored using microscopy and then terminated with distilled water.
The pathologists were blinded with respect to clinical data associated with patients. At medium magnification (200×), five visual fields were randomly selected, with each visual field containing 200 tumor cells, for a total of 1,000 cells. Cells were rated according to staining intensity on the following scale: 0 point for no staining, 1 point for pale yellow staining, 2 points for yellowish brown staining, and 3 points for brown staining. Cells were also rated according to the proportion of positive cells, ranging from 0% to 100%. If the product of the staining intensity rating and the percentage of positive cell nuclei was greater than zero, we defined the sample as claudin-6 positive; otherwise, the sample was considered negative for claudin-6 expression. For positive samples, low claudin-6 expression was defined by staining intensities of 0-1 scores, whereas staining intensities of 2-3 scores defined high expression. In addition, we performed immunohistochemistry without the primary antibody as negative control.
Reverse transcription-polymerase chain reaction
RNA was extracted from samples in a sterile, RNAse-free environment by using TRIzol in strict accordance with manufacturer instructions. cDNA was produced from the purified mRNA transcripts by using reverse transcriptase. PCR was used to amplify and quantify claudin-6 cDNA using the following primer sequences: sense, 5′-CACTGCCACTTCTGGATGG-3′ and antisense, 5′-CAGTGCAGCTCCTTCAACCT-3′. The PCR program was as follows: initial denaturation at 94°C for 3 minutes; 35 cycles each of denaturation at 94°C for 30 seconds, annealing at 56.2°C for 30 seconds (annealing temperature of the internal reference GAPDH, 65°C), and extension at 72°C for 45 seconds and extension at 72°C for 2 minutes. Target gene and reference gene PCR products were visualized on a 1.5%–2.0% Sepharose gel using the JS-780 Automatic Gel Imaging Analysis System (Hangzhou Chincan Trading Co., Ltd., Zhejiang, People’s Republic of China) and gray-scale analysis. The internal reference for each sample was set to one, and the claudin-6 mRNA level was defined as (claudin-6 mRNA gray value)/(internal reference mRNA gray value).
Western blotting
Cryopreserved tissue samples were weighed and then lysed in lysis buffer (Beyotime Institute of Biotechnology, Shanghai, People’s Republic of China). In all, 50 μg of protein were resolved by 8%–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Bio-Rad Laboratories Inc., Hercules, CA, USA), followed by western blotting using standard procedures. The blot was incubated in Tris-buffered saline (TBST) containing 5% skimmed milk powder to block nonspecific binding, followed by incubation with claudin-6 rabbit antihuman monoclonal antibody (1:1,000) overnight at 4°C. The blot was washed three times with TBST (10 minutes per wash). Finally, the blot was incubated with goat antirabbit IgG (1:2,000; Santa Cruz Biotechnology Inc.) at 37°C for 1 hour and washed three times with TBST (10 minutes per wash). Electrogenerated chemiluminescence was used for autoradiography. The relative amount of Raf kinase inhibitor protein was represented by the claudin-6/GAPDH gray scale ratio, which was analyzed using the Quantity One software (Bio-Rad Laboratories Inc.).
Statistical analysis
The Mann–Whitney test was used to compare claudin-6 expression in NSCLC to that in normal tissues and lymph nodes. Categorical data comparisons were made using the χ2 test, and quantitative comparisons were made using the Student’s t-test. The primary outcomes were disease-free survival (DFS) and overall survival (OS). The Kaplan–Meier method was used to analyze postoperative survival rates, and the log-rank test was used to assess differences in survival rates. The Cox regression model was used to perform multivariate analysis. All statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Two-sided P-values were calculated, and P<0.05 was considered to be statistically significant.
Results
Low claudin-6 expression in primary NSCLC
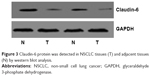
Claudin-6 protein was mainly localized to the nucleus of NSCLC tissues and adjacent tissues, as detected by immunohistochemistry. Claudin-6 was expressed at low levels in 61 of 123 (49.6%) NSCLC tissue samples and in 33 of 123 (26.8%) normal adjacent tissue samples (P<0.05, Figure 1). RT-PCR and western blot analyses were used to quantify mRNA and protein levels, respectively; we found that claudin-6 mRNA expression and protein levels were significantly higher in adjacent tissues than in NSCLC tissues (P<0.05, Figures 2 and 3). Thus, these data suggest that claudin-6 protein and mRNA levels are low in NSCLC tissues.
Expression of claudin-6 and clinicopathological factors of NSCLC
We analyzed the relationship between claudin-6 expression and clinicopathological factors of NSCLC (Table 1). Low claudin-6 expression was associated with lymph node metastasis (P<0.001) and TNM stage (P=0.007). Low claudin-6 expression had no significant correlation with other clinical parameters, including age, sex, smoking status, pathological type, differentiation, or tumor size (P>0.05).
  | Table 1 Correlation between claudin-6 expression and clinicopathological factors of NSCLC |
Claudin-6 expression and prognosis in NSCLC patients
Tissue samples from 123 NSCLC patients were classified according to the claudin-6 expression level. The Kaplan–Meier analysis suggested that patients with low claudin-6 expression levels had significantly shorter survival rates (DFS and OS) than patients with high claudin-6 expression levels (Table 2 and Figure 4). Univariate analysis showed that lymph node metastasis, TNM stage, and low claudin-6 expression levels were correlated with OS (Table 3). Multivariate analysis using the Cox regression model suggested that low claudin-6 expression was an independent indicator of poor patient prognosis (Table 4).
  | Figure 4 Kaplan–Meier survival curves of patients based on claudin-6 expression. |
  | Table 3 Univariate analysis of clinicopathological factors for overall survival |
  | Table 4 Multivariate analysis of clinicopathological factors for overall survival |
Discussion
TJs between epithelial and endothelial cells form a tight barrier that allows the passage of water and solute through the paracellular pathway.9 Junctional complexes are critical in maintaining adhesive contacts between cells, establishing cell polarity, controlling permeability, and regulating cell proliferation.10 Claudins are a large family of 24 proteins that are critical components of TJs.11,12 Claudins range in molecular mass from 17 kDa to 27 kDa, but all contain four transmembrane domains and two extracellular annular areas.13 Claudins are also involved in intercellular signal transduction.11–13 Abnormal claudin expression plays an important role in the occurrence and development of multiple cancers; low claudin expression is associated with tumors.14–16
Claudin-6 is located at chromosome 16p13.3 and encodes a 219-amino-acid protein with a molecular mass of 23 kDa.17 As an important component of TJs, claudin is not only capable of regulating intercellular material flow and maintaining epithelial cell polarity but also involved in cell proliferation, gene transcription, and tumor suppression.18 Claudin downregulation can influence the initiation and progression of cancer by causing a decrease in cell adhesiveness and cohesion, variation in differentiation, and an increase in cell invasiveness.19,20 The study by Micke et al was to assess which fraction of patients with NSCLC express claudin-6 by immunohistochemistry and gene expression microarray data. The results suggested that high claudin-6 protein expression was associated with worse prognosis in lung adenocarcinoma.21 This finding encourages further clinical exploration of targeting ectopically activated claudin-6 expression as a valuable treatment concept in NSCLC. We do not know whether there is a similar phenomenon in Chinese patients and other pathological types with NSCLC. Here, we have quantified claudin-6 expression levels in NSCLC and have uncovered a correlation between low claudin-6 expression and decrease in survival rates of NSCLC patients.
In this study, we examined the expression levels of claudin-6 in NSCLC and corresponding adjacent tissues by immunohistochemistry. The results suggest that the rate of claudin-6 low expression was significantly higher in NSCLC tissues than in the surrounding non-tumor tissues. At the mRNA level, we have verified the above results using RT-PCR and western blot analysis, and we reached the same conclusion. In addition, we investigated the relationship between claudin-6 expression levels and clinicopathological features of NSCLC patients and found that claudin-6 expression was significantly correlated with lymph node metastasis and TNM stage, but not with age, sex, smoking status, pathological type, differentiation, and tumor size of patients with NSCLC. For patients with NSCLC, carrying out survival and prognostic analysis is necessary. The Kaplan–Meier analysis suggested that patients with low claudin-6 expression levels had significantly shorter survival rates (DFS and OS) than patients with high claudin-6 expression levels. Moreover, multivariate survival analysis demonstrated that low claudin-6 expression could be a significantly independent hazard factor for OS of patients with NSCLC, along with lymph node metastasis and TNM stage.
In conclusion, low claudin-6 expression is an independent prognostic biomarker that indicates a worse prognosis in patients with NSCLC.
Acknowledgment
This study was funded by Wu Jie-ping Medical Foundation of the People’s Republic of China (320.6799.1130). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Jänne PA, Ou SH, Kim DW, et al. Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: a multicentre, open-label, phase 2 trial. Lancet Oncol. 2014;15(13):1433–1441. | ||
Wu YL, Lu S, Cheng Y, et al. Efficacy and safety of pemetrexed/cisplatin versus gemcitabine/cisplatin as first-line treatment in Chinese patients with advanced nonsquamous non-small cell lung cancer. Lung Cancer. 2014;85(3):401–407. | ||
Steed E, Elbediwy A, Vacca B, et al. MarvelD3 couples tight junctions to the MEKK1-JNK pathway to regulate cell behavior and survival.J Cell Biol. 2014;204(5):821–838. | ||
Harrell JC, Pfefferle AD, Zalles N, et al. Endothelial-like properties of claudin-low breast cancer cells promote tumor vascular permeability and metastasis. Clin Exp Metastasis. 2014;31(1):33–45. | ||
Philip R, Heiler S, Mu W, Büchler MW, Zöller M, Thuma F. Claudin-7 promotes the epithelial-mesenchymal transition in human colorectal cancer. Oncotarget. 2015;6(4):2046–2063. | ||
Agarwal R, Mori Y, Cheng Y, et al. Silencing of claudin-11 is associated with increased invasiveness of gastric cancer cells. PLoS One. 2009;4(11):e8002. | ||
Jakab CS, Rusvai M, Demeter Z, Gálfi P, Szabó Z, Kulka J. Expression of claudin-4 molecule in canine exocrine pancreatic acinar cell carcinomas. Histol Histopathol. 2011;26(9):1121–1126. | ||
Huang GW, Ding X, Chen SL, Zeng L. Expression of claudin 10 protein in hepatocellular carcinoma: impact on survival. J Cancer Res Clin Oncol. 2011;137(8):1213–1218. | ||
Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124(1):119–131. | ||
Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286(6):C1213–C1228. | ||
Evans MJ, von Hahn T, Tscherne DM, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007; 446(7137):801–805. | ||
Nagase S, Doyama R, Yagi K, Kondoh M. Recent advances in claudin-targeting technology. Biol Pharm Bull. 2013;36(5):708–714. | ||
Kotler BM, Kerstetter JE, Insogna KL. Claudins, dietary milk proteins, and intestinal barrier regulation. Nutr Rev. 2013;71(1):60–65. | ||
Sabatier R, Finetti P, Guille A, et al. Claudin-low breast cancers: clinical, pathological, molecular and prognostic characterization. Mol Cancer. 2014;13:228. | ||
Izraely S, Sagi-Assif O, Klein A, et al. The metastatic microenvironment: claudin-1 suppresses the malignant phenotype of melanoma brain metastasis. Int J Cancer. 2015;136(6):1296–1307. | ||
Sung CO, Han SY, Kim SH. Low expression of claudin-4 is associated with poor prognosis in esophageal squamous cell carcinoma. Ann Surg Oncol. 2011;18(1):273–281. | ||
Wang L, Jin X, Lin D, et al. Clinicopathologic significance of claudin-6, occludin, and matrix metalloproteinases-2 expression in ovarian carcinoma. Diagn Pathol. 2013;8:190. | ||
Ushiku T, Shinozaki-Ushiku A, Maeda D, Morita S, Fukayama M. Distinct expression pattern of claudin-6, a primitive phenotypic tight junction molecule, in germ cell tumours and visceral carcinomas. Histopathology. 2012;61(6):1043–1056. | ||
Wu Q, Liu Y, Ren Y, et al. Tight junction protein, claudin-6, downregulates the malignant phenotype of breast carcinoma. Eur J Cancer Prev. 2010;19(3):186–194. | ||
Birks DK, Kleinschmidt-DeMasters BK, Donson AM, et al. Claudin 6 is a positive marker for atypical teratoid/rhabdoid tumors. Brain Pathol. 2010;20(1):140–150. | ||
Micke P, Mattsson JS, Edlund K, et al. Aberrantly activated claudin 6 and 18.2 as potential therapy targets in non-small-cell lung cancer.Int J Cancer. 2014;135(9):2206–2214. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.