Back to Journals » International Journal of Women's Health » Volume 7
Low-birth-weight babies among hospital deliveries in Nepal: a hospital-based study
Authors Koirala AK, Bhatta DN
Received 13 March 2015
Accepted for publication 21 April 2015
Published 8 June 2015 Volume 2015:7 Pages 581—585
DOI https://doi.org/10.2147/IJWH.S84559
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Arun K Koirala,1 Dharma N Bhatta2,3
1Administrative Department, Helping Hands Community Hospital, Chabahil, Kathmandu, 2Department of Public Health, Nobel College, Pokhara University, Kathmandu, Nepal; 3Epidemiology Unit, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand
Background: Birth weight is an important indicator of a population’s health and is associated with numerous interrelated factors in the infant, mother, and physical environment. The objective of this study was to assess the proportion of low birth weight and identify the associated factors for low birth weight in a liveborn infant among the women in Morang, Nepal.
Methods: A cross-sectional survey was carried out from December 2010 to March 2011 among 255 mothers who gave birth during the study period at the Koshi Zonal Hospital, Nepal. Data were collected using a structured questionnaire with face-to-face interviews. Data were analyzed through logistic regression and presented with crude and adjusted odds ratios (AORs) with 95% confidence intervals (CIs).
Results: The study showed that the prevalence of low-birth-weight babies was 23.1% (95% CI: 17.9–28.1). The mean (standard deviation) age of mothers was 23.23 (4.18) years. The proportion of low birth weight of previous baby was 3.9% (95% CI: 0.1–7.9), and 15.7% (95% CI: 11.5–20.5) of the respondents had preterm delivery. Nearly one-third (36.1%; 95% CI: 26.4–45.6) of the respondents had >2 years’ gap after the previous delivery. Nonformal employment (AOR: 2.14; 95% CI: 0.523–8.74), vegetarian diet (AOR: 1.47; 95% CI: 0.23–9.36), and no rest during pregnancy (AOR: 1.38; 95% CI: 0.41–4.39) were factors more likely to determine low birth weight. However, none of the variables showed a significant association between low birth weight and other dependent variables.
Conclusion: Low birth weight is an important factor for perinatal morbidity and mortality and is a common problem in the developing world. The proportion of low-birth-weight babies was high in hospital delivery, and ethnicities, Hindu religion, education, nonformal employment, food habit, rest during pregnancy, and type of delivery were found to influence the birth weight. Hence, it is important to strengthen health education services at the basic level of a community to solve this problem.
Keywords: low birth weight, hospital, delivery, Nepal, fetal program, LBW, newborn, parity, pregnancy, preterm delivery
Background
Low birth weight (LBW) is defined as a weight of <5 pounds 8 ounces (2,500 g) at birth irrespective of the gestational age.1 LBW infants are more likely to experience developmental deficits. Intrauterine growth retardation (IUGR) has a serious adverse impact and is crucial for work productivity and income-generating potential.2 The risk of diseases such as hypertension, coronary heart disease, stroke, and non-insulin-dependent diabetes (together called “syndrome X”) is associated with size, wasting, and stunting at birth.3 Being of low weight at birth has a profoundly adverse effect on the health and development of the neonate. It is a risk factor for stunting, which starts in utero and becomes worse if the diet or health status is inadequate during postnatal development.4 LBW is also emerging as a public health problem because some studies have shown its negative impact on morbidity and mortality in the infant, child, and at later stages of an individual.5 LBW and prematurity are the principal determinants of perinatal mortality, and LBW infants are more vulnerable to the impact of environmental and social conditions.6
Birth weight is an important indicator of a population’s health and is associated with numerous interrelated factors in the infant, mother, and physical environment.6,7 The main causes of LBW include infection, maternal malnutrition, and smoking, as well as prematurity, multiple pregnancy, high parity, and complications of pregnancy such as preeclampsia.8 Evidence also shows that other less-known environmental factors can affect fetal growth.6 In 2010, a total of 10.9 million births were preterm and appropriate-for-gestational age, 29.7 million births were full term and small-for-gestational age (SGA), and 2.8 million births were preterm and SGA among the total 135 million births in developing countries.9 Seventy-two percent of LBW infants in developing countries are born in Asia.4 In developing countries, IUGR has been estimated to affect between 14 million and 20 million infants per year, which is equivalent to 11% of all births in developing countries.10 In Nepal, LBW infants comprised 21% of live births in 2001.11 The percentage of premature births (<37 weeks gestation) in Nepal suggests a figure of at least 14%.12
This study was carried out to assess the proportion of LBW among babies and its associated factors among women in Morang, Nepal. Multi gravidae, early pregnancy, poverty, and poor diet are the major factors for LBW. In Nepal, there are different sociocultural factors that lead to LBW. LBW is an important indicator for the quality of health and health services. The findings of this study will be taken for structural intervention and development in government and nongovernmental areas.
Materials and methods
A cross-sectional study was carried out at the Koshi Zonal Government Hospital of Morang District, which is in the eastern part of Nepal. The study was conducted between December 2010 and March 2011. The study inclusion criteria were as follows: a delivered mother who gave live birth to a baby at the Koshi Zonal Hospital, and her willingness to participate in the study.
Purposive sampling technique was applied to sample the delivered mothers. All the mothers who met the inclusion criteria were included purposively during the study period. Hence, the total sample size was estimated to be 255 mothers based on the 21% prevalence.13
Data were collected using a structured questionnaire administered by trained public health personnel. The questionnaires were first developed in English and then translated into the Nepali language. The questionnaires were pretested among delivered mothers in a nonselected study area and later refined as required.
The survey assessed different items that pertained to sociodemographic (Table 1), behavioral (Table 2), and parity variables. In addition to this, the respondents were also asked about the food habits during the pregnancy period. Immediately after birth, a trained nurse noted the weight of the baby using Docbel–Braun Baby Classic Weighing Scale, which was verified before each weighing against standard weight, and recorded it on the form. Verbal consent was obtained from the respondents before they were enrolled in the study. Consent form was written in the local language stating the study’s objectives, nature of respondent’s involvement, risk and benefits, and confidentiality of the data. They were given clear options on voluntary participation. Confidentiality of information was ensured by removing personal identifiers from the completed questionnaires. Approval was obtained from the Koshi Zonal Hospital. The study was approved by the institutional review committee of the State University of Bangladesh and Pokhara University, Nobel College, Kathmandu. We have followed the national ethical guidelines related to research on human subject developed by Nepal Health Research Council, World Health Organization and the World Medical Association.
  | Table 1 Demographic distribution of mother (n=255) |
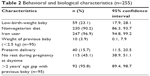  | Table 2 Behavioral and biological characteristics (n=255) |
The coded data were entered into SPSS version 16 for further processing and analysis. Descriptive and inferential statistics were analyzed. Both bivariate and multivariate techniques were applied to identify the factors associated with the likelihood of having an LBW baby. The variables were also examined by the univariate and multivariate binary logistic regression with enter method to identify the predictors. During analysis, multicollinearity among the variables was assessed, and the least important variables were removed from the logistic model. Results were presented as both crude odds and adjusted odds ratios (AORs) with 95% confidence intervals (CIs), using bivariate and multivariate logistic regression (Table 3). In the bivariate and multivariate analyses, a P-value ≤0.05 was considered to be significant. The data were cleaned and cross-checked daily before and after data entry for completeness and accuracy.
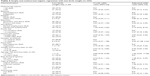  | Table 3 Simple and multivariate logistic regression with low birth weight (n=255) |
Results
A total of 255 respondents were included in the study, in which 63.5% were aged between 20 years and 34 years, 34.5% were aged <20 years, and 2.0% were older than 35 years. The mean ± standard deviation in age was 23.23±4.18 years. Most of the respondents (91.4%) belonged to the Hindu religion and about two-thirds of all respondents were from indigenous ethnicities. Among the total respondents, nearly half (48.2%) had either primary level of education or were uneducated. Though about one-fifth of the respondents (20.4%) had formal employment, 56.5% of respondents had >5,000 NRS (Nepalese Rupees) monthly income. Two-thirds (65.5%) of the respondents had more than four family members, whereas 91.8% of the respondents had ≤2 children. Among the total children born to the respondents in the hospital during the study period, three-fifths (60%) were male and two-fifths (40%) were female (Table 1).
Prevalence of LBW was 23.1% (95% CI: 17.9–28.1). Most of the respondents (90.2%, 95% CI: 86.3–93.7) were nonvegetarian and 96.9% (95% CI: 94.8–99.2) had taken iron tablets during pregnancy. The proportion of LBW of previous baby was 3.9% (95% CI: 0.1–7.9) and 15.7% (95% CI: 11.5–20.5) of the respondents had preterm delivery. Nearly one-third (36.1%, 95% CI: 26.4–45.6) of the respondents had >2 years’ gap after the previous delivery (Table 2).
Table 3 shows the predictor variables for LBW babies. Mothers who were of indigenous ethnicities were less likely (AOR: 0.69; 95% CI: 0.18–2.58) to have LBW babies than those of nonindigenous ethnicities. Mothers who followed a religion other than Hinduism were less likely (AOR: 0.80; 95% CI: 0.10–6.06) to have LBW babies than those who followed the Hindu religion. Mothers who had nonformal employment were two times (AOR: 2.14; 95% CI: 0.52–8.74) more likely to have LBW babies than those in formal employment. Mothers who were vegetarian were more likely (AOR: 1.47; 95% CI: 0.23–9.36) to have LBW babies than nonvegetarians. Mothers who did not take rest at daytime during pregnancy were more likely (AOR: 1.38; 95% CI: 0.41–4.39) to have LBW babies than those who had taken rest. However, no single variable was significantly associated with LBW.
Discussion
This study revealed that LBW was nearly one-fourth of all live births, which is less than what was observed in a hospital-based study (29.8%) in the Western Region Hospital, Pokhara, Nepal, and in Kathmandu.14,15 The estimated LBW in Nepal is 21%, in comparison with the regional estimates of LBW of 25% in South Asia in 2001.11 The proportion of LBW is high in Nepal and is recognized as a national health problem. The main cause of LBW in Nepal is the prevalence of undernourished women, exacerbated by low dietary intake during pregnancy. Iron supplementation may improve maternal appetite,16 thereby increasing energy consumption during pregnancy, with resultant increased intrauterine growth. In this study, almost all mothers had taken iron tablets during pregnancy; however, this variable was not fitted for the logistic regression model.
This study showed that the proportion of LBW of previous baby was very low. Previous literature showed that preterm delivery influenced the LBW of the baby. According to annual vital statistics in the USA, the percentage of preterm delivery was continuously rising from 11% in 1998 to 12.3% in 2003.17 Studies revealed that the incidence is higher in developing countries than in developed countries. The highest rates of preterm birth were in Africa and North America (11.9% and 10.6% of all births, respectively), and the lowest was in Europe (6.2%).18 Our study revealed that the prevalence of preterm delivery was 15.7% which was comparatively higher than that in Europe, Africa, and North America and lower than that in India (20.9%).19
In this study, the prevalence of LBW in the maternal age group of ≤20 years was found to be >50% but it was not found to be a significant and predictable factor in multivariate analysis. Nearly three-fourths of the LBW babies were born among indigenous ethnicities; however, indigenous ethnicities were less likely to have LBW in multivariate analysis. More than half of the LBW babies were born to uneducated and primary-level-educated mothers. Multivariate analysis revealed that uneducated and primary-level-educated mothers were less likely to have LBW babies; however, this finding is not generalized to other studies. Mothers having nonformal employment were more likely to have LBW babies than mothers having formal employment; however, this variable was not found to be statistically significant. The proportion of LBW was maximum (76.3%) among mothers who were not formally employed by occupation; a previous study also indicated the same result as this study.20,21 The proportion of LBW babies decreased with increase in the monthly income of the family. These findings are in accordance with other studies.22,23
Two-thirds of babies born in families having >4 members were found to be of LBW. It may be due to scarcity of nutritious food and lack of care by the pregnant mother; however, multivariate analysis did not show family size to be a predictable variable. Findings of multivariate analysis showed that vegetarian mothers were more likely to have LBW babies than nonvegetarian mothers. Though it is accepted that rest during pregnancy is significant for the birth weight of the baby, the study showed that there is no such difference between the mother who takes rest and the mother who did not. However, the findings of multivariate analysis showed that rest was a protectable variable for LBW. This study showed that two-fifths of cesarean section cases had LBW, but multivariate analysis findings showed no such difference between vaginal delivery and cesarean section.
The limitations of our study are as follows: it was a hospital-based study that cannot be generalized to the community or population. Purposive sampling and small sample size may not develop a valid conclusion. Information was collected within the 1st day after delivery, which can help to reduce information bias.
Conclusion
The proportion of LBW babies was high in hospital delivery, and ethnicities, Hindu religion, education, nonformal employment, food habit, rest during pregnancy, and type of delivery were found to influence the birth weight of a baby. Hence, it is important to strengthen health education, along with conducting nutrition programs for mothers and developing fetal programming in Nepal.
Disclosure
The authors report no conflicts of interest in this work.
References
UNICEF WHO. Low Birthweight: Country, Regional and Global Estimates. New York: UNICEF; 2004. | ||
Wu LA, Katz J, Mullany LC, et al. The association of preterm birth and small birthweight for gestational age on childhood disability screening using the Ten Questions Plus tool in rural Sarlahi district, southern Nepal. Child Care Health Dev. 2012;38:332–340. | ||
Barker DJP. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(sup 6):588S–595S. | ||
UN Administrative Committee on Coordination/Subcommittee on Nutrition (ACC/SCN) and International Food Policy Research Institute. Fourth Report on the World Nutrition Situation. Geneva: ACC/SCN; 2000. | ||
UNICEF. The State of the World’s Children 2012: Children in an Urban World. Mumbai: eSocialSciences; 2012. | ||
Farley TA, Mason K, Rice J, Habel JD, Scribner R, Cohen DA. The relationship between the neighbourhood environment and adverse birth outcomes. Paediatr Perinat Epidemiol. 2006;20(3):188–200. | ||
WHO. Towards The Development of A Strategy for Promoting Optimal Fetal Growth. Geneva: World Health Organization; 2004. | ||
de Bernabé JV, Soriano T, Albaladejo R, et al. Risk factors for low birth weight: a review. Eur J Obstet Gynecol Reprod Biol. 2004;116(1):3–15. | ||
Lee ACC, Katz J, Blencowe H. Born too small: national and regional estimates of term and preterm small-for-gestational-age in 138 low-income and middle-income countries in 2010. Lancet Global Health. 2013;1:e26–e36. | ||
De Onis M, Blossner M, Villar J. Levels and patterns of intrauterine growth retardation in developing countries. Eur J Clin Nutr. 1998;52:S5–S15. | ||
Wardlaw TM. Low Birthweight: Country, Regional and Global Estimates. New York: UNICEF; 2004. | ||
Howson CP, Kinney MV, Lawn JE. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization; 2012. | ||
Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. WHO Bulletin. 1987;65:663–737. | ||
Acharya PP, Alpass F. Birth outcomes across ethnic groups of women in Nepal. Health Care Women Int. 2004;25(1):40–54. | ||
Gurubacharya RL, Karki C. Two years experience of neonatal services in KUTH, B and B hospital. Nepal J Obst Gynae. 2006;1:42–44. | ||
Lawless JW, Latham MC, Stephenson LS, Kinoti SN, Pertet AM. Iron supplementation improves appetite and growth in anemic Kenyan primary school children. J Nutr. 1994;124:645–645. | ||
Marin JA, Kochanek KD, Strobino DM, Guyer B. Annual summary of vital-statistics-2003. Pediatrics. 2005;115:619–634. | ||
McCall EM, Alderdice FA, Halliday HL, Jenkins JG, Vohra S. The world wide incidence of preterm birth: a systematic review of maternal morbidity and mortality. Bull World Health Organization. 2010;88(1):31–38. | ||
Singh U, Singh N, Seth S. A prospective analysis etiology and outcome of preterm labour. J Obs Gynae India. 2007;57(1):48–52. | ||
Mondal B. Risk factors for low birth weight in Nepali infants. Indian J Pediatr. 2000;67(7):477–482. | ||
Anand K, Garg BS. A study of factors affecting LBW. Indian J Community Med. 2000;25(2):57–61. | ||
Spencer N. The effect of income inequality and macro-level social policy on infant mortality and low birthweight in developed countries – a preliminary systematic review. Child Care Health Dev. 2004;30(6):699–709. | ||
Olson ME, Diekema D, Elliott BA, Renier CM. Impact of income and income inequality on infant health outcomes in the United States. Pediatrics. 2010;126(6):1165–1173. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
