Back to Journals » Cancer Management and Research » Volume 12
Low Albumin to Fibrinogen Ratio Predicts Poor Overall Survival in Esophageal Small Cell Carcinoma Patients: A Retrospective Study
Authors Wang Y , Li J, Chang S, Zhou K , Che G
Received 18 February 2020
Accepted for publication 30 March 2020
Published 21 April 2020 Volume 2020:12 Pages 2675—2683
DOI https://doi.org/10.2147/CMAR.S250293
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Yan Wang,* Jialong Li,* Shuai Chang, Kun Zhou, Guowei Che
Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu 610041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guowei Che Email [email protected]
Purpose: To explore the prognostic value of albumin to fibrinogen ratio (AFR) in patients with esophageal small cell carcinoma (ESCC).
Patients and Methods: Patients diagnosed with ESCC in West China Hospital from June 1, 2010, to July 31, 2019, were retrospectively reviewed. The AFR was defined as the ratio between the serum albumin level and fibrinogen level. The receiver operating characteristic curves were conducted to determine optimal cut-off values for survival prediction. Univariate and multivariate cox regression analyses were performed to clarify independent prognostic risk factors.
Results: A total of 88 ESCC patients were enrolled in our study with the median follow-up time of 6 months (range 1– 91 months). In the univariate analysis, the node metastasis, extraesophageal metastasis status, tumor-node-metastasis (TNM) stage and AFR were found to be potentially related with overall survival (OS) of ESCC patients. After the multivariate analysis, AFR [hazard ratio (HR)=3.487, 95% confidence interval (CI): 1.179– 10.312; P=0.024] and TNM stage (HR=6.044, 95% CI: 1.045– 34.974; P=0.045) were testified to be independent prognostic factors and low AFR (≤ 12.36) level was significantly associated with poor OS in ESCC patients.
Conclusion: The current study reported that AFR could serve as a novel prognostic indicator in ESCC and patients with AFR≤ 12.36 were more likely to have poor prognosis.
Keywords: albumin to fibrinogen ratio, esophageal small cell carcinoma, overall survival
Introduction
Primary esophageal small cell carcinoma (ESCC) is one kind of highly malignant cancer, accounting for 0.05% to 3.1% of all esophageal cancers,1,2 and was first reported by Mckeown in 1952.3 It is characterized by aggressive nature with poor prognosis and differs from the squamous cell carcinoma and adenocarcinoma of the esophagus, but it is similar to small cell lung cancer (SCLC). Up to now, no consensus on the standard therapeutic strategy for ESCC has been reached and reliable and valuable prognostic factors for ESCC are still missing now because of few relevant studies.
According to previous studies, the tumor-node-metastasis (TNM) stage, treatment strategy and Ki-67 expression were independent prognostic factors for ESCC patients.4–6 However, no studies have clarified the prognostic value of some laboratory indicators including the albumin to fibrinogen ratio (AFR) in ESCC. Actually, some investigators have reported that pretreatment AFR could serve as a promising biomarker to predict survival in several cancers such as the non-small cell lung cancer (NSCLC), breast cancer, squamous cell carcinoma of esophagus (SCCE) and hepatocellular carcinoma and low AFR predicts poorer survival for cancer patients.7–10 Albumin and fibrinogen are both inflammatory and immunological proteins which involve in the initiation, growth and metastasis of tumors.7 Hence, we hypothesized that circulating AFR could serve as an effective indicator to predict the prognosis and contribute to the formulation of therapeutic strategy for ESCC patients.
In the present study, we retrospectively reviewed patients diagnosed as ESCC in our center during the last decade to explore the prognostic value of AFR and also other laboratory biomarkers such as the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR).
Patients and Methods
Study Design
This is a retrospective study from a single center.
Patients
Patients diagnosed with ESCC in West China Hospital from June 1, 2010 to July 31, 2019 were reviewed. The diagnoses of ESCC were confirmed pathologically by surgically resected specimens or gastroscopic biopsies.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: 1) patients were pathologically diagnosed with ESCC in our hospital; 2) the laboratory indicators including the serum albumin and fibrinogen concentrations were collected before anti-cancer treatment or at the time of diagnosis; 3) patients had normal liver function.
The exclusion criteria were as follows: 1) patients with liver diseases, hematology diseases, inflammatory or infectious diseases; 2) combined with other malignant tumor; 3) recurrent tumors; 4) transfusion of albumin or fibrinogen within 1 week before the detection of albumin and fibrinogen concentrations.
Data Collection
The following information was extracted from the medical records of our hospital: sex, age, comorbidity, treatment strategy, tumor location, pathological type, tumor (T) stage, node (N) stage, extraesophageal metastasis (M), tumor-node-metastasis (TNM) stage based on the 2009 AJCC TNM staging system for esophageal squamous cell carcinoma,11 immunohistochemical indicators, cluster differentiation 56 (CD56), synaptophysin (Syn), cytokeratin 5/6 (CK5/6), chromogranin A (CgA), thyroid transcription factor-1 (TTF-1), Ki-67 and P63, red cell distribution width (RDW), NLR, PLR, LMR, glutamic oxalacetic transaminase/alanine aminotransferase (AST/ALT), albumin to globulin ratio (AGR), lactate dehydrogenase (LDH), hydroxybutyrate dehydrogenase (HBDH) and AFR.
The location of the tumor was determined on the basis of endoscopic findings and was divided into three segments including the upper/cervical (15–25 cm from the incisor teeth), middle (25–30 cm from the incisor teeth) and lower (30–40 cm from the incisor teeth).
Definition of the Endpoint Event
Overall survival (OS) was defined as the interval from the date of diagnosis to the date of death caused by any causes or the date of the last follow-up. Follow-up information was obtained from the out-patient clinic records or telephone contacts.
Statistical Analysis
Statistical analysis was conducted by using SPSS 22.0 version software (IBM Corp, USA). The receiver operating characteristic curve was used to determine the optimal cut-off values for prognosis prediction. Categorical data were presented as number (%). The Chi-square test or Fisher’s exact test was applied for the comparison of categorical data. The Kaplan-Meier analysis was used for the calculation of OS and the Log rank test was used to assess the relation between eligible variables and OS. The univariate and multivariate cox hazard regression model was applied to identify independent prognostic factors for ESCC patients and variables with a P value≤0.10 were enrolled in multivariate regression analysis. All statistical tests were two-sided and a P value<0.05 was considered statistically significant.
Results
Basic Patient Characteristics
A total of 88 ESCC patients were enrolled in our study with a male to female ratio of 3:1. The median age was 61 years old with a range of 42 to 83 years. More than half of enrolled patients (50/88, 56.8%) received surgical resection and the majority of ESCC were located in the middle segment of esophagus (47/88, 53.4%). Fifty-three patients (60.2%) were with TNM III/IV stage. Based on the results of ROC curves, the optimal cut-off values of RDW, NLR, PLR, LMR, AST/ALT, AGR, LDH, HBDH and AFR were 13.15%, 3.75, 93.81, 4.77, 1.265, 1.4, 133.5, 127 and 12.36, respectively. Besides, the sensitivity and specificity for the threshold of AFR was 76.0% and 58.7% separately with the area under the curve (AUC) of 0.628 (Figure 1). The median follow-up time for all included patients was 6 months (ranging 1 to 91 months). During the entire follow-up period, 25 patients died with a median survival time of 12 months (ranging from 2 to 49 months) and the median follow-up time of 63 alive patients was 4 months (ranging from 1 to 91 months) (Figure 2). Other specific information was presented in Table 1.
 | 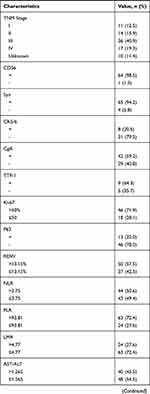 | 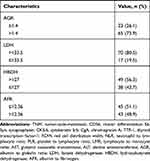 |
Table 1 Basic Characteristics of Enrolled Esophageal Small Cell Carcinoma Patients |
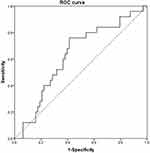 |
Figure 1 ROC curve of the AFR for predicting overall survival. Abbreviation: AFR, albumin to fibrinogen ratio. |
 |
Figure 2 Kaplan–Meier survival curve for overall survival of all included patients. |
Association Between AFR and Clinicopathological Parameters
According to the critical value of AFR, 45 and 43 patients were divided into the low AFR group (AFR≤12.36) and high AFR group (>12.36). The results revealed that patients with low AFR were related with treatment strategy (P=0.007), higher T stage (P=0.026), N stage (P=0.018), TNM stage (P=0.003), CgA (P=0.029), lower NLR (P=0.002), higher PLR (P=0.003) and lower LMR (P=0.047). However, no significant association of AFR with sex, age, comorbidity, location, pathological type, extraesophageal metastasis, CD56, Syn, CK5/6, TTF-1, Ki-67, P63, RDW, AST/ALT, AGR, LDH or HBDH. Detailed data were shown in Table 2.
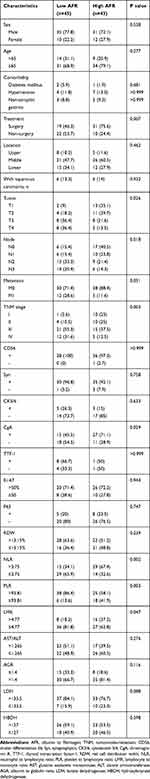 |
Table 2 Associations Between AFR and Clinicopathological Characteristics in Esophageal Small Cell Carcinoma Patients |
Prognostic Factors for OS of ESCC Patients
We explored the prognostic value of the clinicopathological parameters mentioned above using the cox regression model. According to the univariate analysis, we found that the node metastasis (HR=2.004, 95% CI: 1.283–3.132, P=0.002), extraesophageal metastasis (HR=3.239, 95% CI: 1.217–8.622, P=0.019), TNM stage (HR=1.908, 95% CI: 1.046–3.483, P=0.035) and AFR (HR=3.117, 95% CI: 1.241–7.828, P=0.016) were potentially independent prognostic factors for OS of ESCC patients. However, after the multivariate analysis, extraesophageal metastasis (HR=6.044, 95% CI: 1.045–34.974, P=0.045) (Figure 3) and low AFR (HR=3.487, 95% CI: 1.179–10.312, P=0.024) (Figure 4) were independent risk indicators for poor OS in ESCC. (Table 3)
 |
Table 3 Univariate and Multivariate Analyses of Clinicopathological Characteristics Associated with Overall Survival in Esophageal Small Cell Carcinoma |
 |
Figure 3 Kaplan–Meier survival curves showing the association between extraesophageal metastasis and overall survival. |
 |
Figure 4 Kaplan–Meier survival curves showing the association between AFR and overall survival. Abbreviation: AFR, albumin to fibrinogen ratio. |
Discussion
The present study explored the prognostic role of pretreatment AFR in ESCC by retrospectively analyzing 88 ESCC patients who were admitted to our hospital from 2010 to 2019. The results indicated that pretreatment AFR was significantly associated with treatment strategy, T stage, N stage, TNM stage, CgA expression, pretreatment NLR, PLR, LMR and OS and ESCC patients with low AFR were more likely to have a worse survival than patients with high AFR (HR=3.487, 95% CI: 1.179–10.312, P=0.024). Besides, we found that extraesophageal metastasis was also an independent prognostic risk factor for ESCC factor (HR=6.044, 95% CI: 1.045–34.974, P=0.045). However, no significant relation of the TNM stage, Ki-67 expression or therapeutic strategy with OS was observed in our research.
As major indicator of acute-phase proteins and systemic chronic inflammation, albumin is inexpensive and easily obtained testes in clinics. Besides, it is also widely used to reflect the nutrition level of the body. The production of albumin by the liver is
suppressed by the activation of proinflammation cytokines such as the interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrotic factor-α (TNF-α) when inflammation occurs.12,13 Besides, low serum albumin concentration has been well demonstrated to be associated with poor survival in several cancers such as the head, neck, urothelial, epithelial ovarian, bladder and esophageal cancer.14–18
Fibrinogen is predominantly produced in the liver and plays an important role in the composition of hemostatic proteins and acute phase proteins.19,20 In addition, fibrinogen and its degradation products can regulate the proinflammatory activity, which stimulates the endothelium to secrete and release von Willebrand factor indirectly, resulting in the activation of platelets accompanying neoplastic disorders.21,22 Furthermore, it could also play an important role in the cellular adhesion, proliferation and migration during the process of angiogenesis and tumor cell growth.23 In 2015, the meta-analysis conducted by Perisanidis et al manifested that fibrinogen was an independent prognostic biomarker in cancer patients and could make a contribution to the tumor growth and metastasis.24,25 Since low serum albumin and high fibrinogen concentrations both indicate poor survival for cancer patients, it is believed that the combination of them could show higher prognostic value than each single marker. However, no studies have explored the prognostic role of AFR in ESCC patients up to now due to the very low incidence of ESCC.
Few studies reported the clinicopathological and prognostic characteristics of ESCC. Ishida et al revealed that the ulcerative or diffusely infiltrative microscopic features (P=0.017), high T stage (P=0.034), N stage (P=0.022) and location of tumor (middle segment) (P=0.035) seemed to be favorable prognostic factors after reviewing 29 ESCC patients.26
Song et al demonstrated that high TNM stage (P=0.001) and vessel involvement (P=0.019) predicted worse survival by retrospectively analyzing 151 patients.4 In 2019, Chen et al enrolled 42 ESCC patients who received radiotherapies and found that ESCC patients could benefit from radiotherapy and a higher dose of radiotherapy predicted better OS in limited stage ESCC.5 Deng et al indicated that the low TNM stage, adjuvant therapy and high Ki-67 expression were independent favorable prognostic factors for ESCC patients who underwent esophagectomy with lymphadenectomy.6 However, in our study, we found that only extraesophageal metastasis (HR=6.044, 95% CI: 1.045–34.974, P=0.045) and low AFR (HR=3.487, 95% CI: 1.179–10.312, P=0.024) were significantly associated with poor OS in ESCC. No significant relationship of the treatment strategy, location, T stage, N stage, TNM stage or Ki-67 expression level with OS of ESCC patients, which is different from previous researches. In overall, many fields about ESCC remains unclear or controversial, more investigations are urgently needed.
There are some limitations in this study. First, only 88 patients were enrolled and analyzed. Second, a small amount of information in our study such as the T stage and N stage was missing due to the incomplete medical records. Third, we were unable to verify our findings using external data because of the low incidence of ESCC. Four, the median follow-up time of alive included patients is 4 months which is a little short. Five, we were unable to explore the association of pretreatment AFR with other endpoint events such as the postoperative complications or disease-free survival due to the lack of some relevant information. Six, the serum albumin level may have an interaction with body-mass index (BMI); however, the BMI values at diagnose of one-third of included patients was unobtained.
In conclusion, we demonstrated that a high pretreatment AFR could serve as a favorable prognostic factor of ESCC. However, more prospective studies with bigger sample sizes are still needed to verify our findings.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of West China Hospital, Sichuan University and national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Because of the nature of retrospective design, the Ethics Committee of West China Hospital approved the current study and also determined that the informed consent was not required.
Informed Consent
Written informed consent for the information to be stored and used in the hospital database was obtained from each patient.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang SY, Mao WM, Du XH, et al. The 2002 AJCC TNM classification is a better predictor of primary small cell esophageal carcinoma outcome than the VALSG staging system. Chin J Cancer. 2013;32:342–352. doi:10.5732/cjc.012.10161
2. Feng JF, Huang Y, Zhao Q, et al. Clinical significance of preoperative neutrophil lymphocyte ratio versus platelet lymphocyte ratio in patients with small cell carcinoma of the esophagus. Sci World J. 2013;2013:1–7.
3. McKeown F. Oat-cell carcinoma of the oesophagus. J Pathol Bacteriol. 1952;64(4):889–891. doi:10.1002/path.1700640420
4. Song Y, Wang L, He J, et al. Treatment and prognosis of primary esophageal small cell carcinoma: a report of 151 cases. Chin J Cancer. 2009;28(3):303–307.
5. Chen B, Yang H, Ma H, et al. Radiotherapy for small cell carcinoma of the esophagus: outcomes and prognostic factors from a retrospective study. Radiat Oncol. 2019;14(1):210. doi:10.1186/s13014-019-1415-9
6. Deng H, Chen Z, Wang Z, et al. High expression of Ki-67 is an independent favorable prognostic factor for esophageal small cell carcinoma. Oncotarget. 2017;8(33):55298–55307. doi:10.18632/oncotarget.19426
7. Li SQ, Jiang YH, Lin J, et al. Albumin-to-fibrinogen ratio as a promising biomarker to predict clinical outcome of non-small cell lung cancer individuals. Cancer Med. 2018;7(4):1221–1231. doi:10.1002/cam4.1428
8. Hwang KT, Chung JK, Roh EY, et al. Prognostic influence of preoperative fibrinogen to albumin ratio for breast cancer. J Breast Cancer. 2017;20(3):254–263. doi:10.4048/jbc.2017.20.3.254
9. Li XH, Gu WS, Wang XP, et al. Low preoperative albumin-to-globulin ratio predict poor survival and negatively correlated with fibrinogen in resectable esophageal squamous cell carcinoma. J Cancer. 2017;8(10):1833–1842. doi:10.7150/jca.19062
10. Xu Q, Yan Y, Gu S, et al. A novel inflammation-based prognostic score: the fibrinogen/albumin ratio predicts prognoses of patients after curative resection for hepatocellular carcinoma. J Immunol Res. 2018;22(2018):4925498.
11. Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union against cancer cancer staging manuals. Cancer. 2010;116(16):3763–3773. doi:10.1002/cncr.25146
12. McMillan DC, Watson WS, O’Gorman P, et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–213. doi:10.1207/S15327914nc392_8
13. Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol. 2005;39(Supplement 2):S143–S146. doi:10.1097/01.mcg.0000155514.17715.39
14. Danan D, Shonka DC
15. Liu J, Wang F, Li S, et al. The prognostic significance of preoperative serum albumin in urothelial carcinoma: a systematic review and meta-analysis. Biosci Rep. 2018;38(4):BSR20180214. doi:10.1042/BSR20180214
16. Ge LN, Wang F. Prognostic significance of preoperative serum albumin in epithelial ovarian cancer patients: a systematic review and dose-response meta-analysis of observational studies. Cancer Manag Res. 2018;10:815–825. doi:10.2147/CMAR.S161876
17. Li J, Cheng Y, Liu G, et al. The association of pretreatment serum albumin with outcomes in bladder cancer: a meta-analysis. Onco Targets Ther. 2018;11:3449–3459. doi:10.2147/OTT.S162066
18. Matsuda S, Niihara M, Tsubosa Y, et al. Clinical significance of postoperative recovery of serum albumin levels in patients with esophageal cancer who underwent transthoracic esophagectomy. Surg Today. 2016;46(10):1138–1145. doi:10.1007/s00595-015-1300-6
19. Pulanic´ D, Rudan I. The past decade: fibrinogen. Coll Antropol. 2005;29(1):341–349.
20. Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43–62. doi:10.1007/s00281-011-0290-8
21. Kołodziejczyk J, Ponczek MB. The role of fibrinogen, fibrin and fibrin(ogen) degradation products (FDPs) in tumor progression. Contemp Oncol (Pozn). 2013;17(2):113–119. doi:10.5114/wo.2013.34611
22. Falanga A, Panova-Noeva M, Russo L. Procoagulant mechanisms in tumour cells. Best Pract Res Clin Haematol. 2009;22(1):49–60. doi:10.1016/j.beha.2008.12.009
23. Simpson-Haidaris PJ, Rybarczyk B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann N Y Acad Sci. 2001;936:406–425. doi:10.1111/j.1749-6632.2001.tb03525.x
24. Perisanidis C, Psyrri A, Cohen EE, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):960–970. doi:10.1016/j.ctrv.2015.10.002
25. Lv GY, Yu Y, An L, et al. Preoperative plasma fibrinogen is associated with poor prognosis in esophageal carcinoma: a meta-analysis. Clin Transl Oncol. 2018;20(7):853–861. doi:10.1007/s12094-017-1794-z
26. Ishida H, Kasajima A, Onodera Y, et al. A comparative analysis of clinicopathological factors between esophageal small cell and basaloid squamous cell carcinoma. Medicine (Baltimore). 2019;98(8):e14363. doi:10.1097/MD.0000000000014363
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
