Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Loss of OTUD6B Stimulates Angiogenesis and Promotes Diabetic Atherosclerosis
Authors Wang Z , Zhang L, Li L, Zhou M
Received 8 July 2022
Accepted for publication 21 September 2022
Published 28 September 2022 Volume 2022:15 Pages 3027—3038
DOI https://doi.org/10.2147/DMSO.S380986
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Zhongqun Wang,1,* Lili Zhang,1,* Lihua Li,2 Mengxue Zhou1
1Department of Cardiology, Affiliated Hospital of Jiangsu University, Zhenjiang, 212001, People’s Republic of China; 2Department of Pathology, Affiliated Hospital of Jiangsu University, Zhenjiang, 212001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mengxue Zhou, Department of Cardiology, Affiliated Hospital of Jiangsu University, 438 Jiefang Road, Zhenjiang, 212001, People’s Republic of China, Tel +86 511 85030586, Email [email protected]
Purpose: Angiogenesis is an essential promoter of atherosclerotic plaque rupture. However, the mechanism of its regulation is not understood. OTUD6B regulates cell proliferation, migration, and angiogenesis. We investigated the role of OTUD6B in angiogenesis in diabetic atherosclerotic plaques.
Patients and Methods: The expression of OTUD6B was analyzed by single cell RNA sequencing (scRNA-seq) and RNA sequencing (RNA-seq) and evaluated by Immunofluorescence in human anterior tibial arteries from diabetic amputees and ApoE−/− mice. Furthermore, we constructed a mouce model of diabetic atherosclerosis and used the mice to study the effect of OTUD6B downregulation in vivo by injecting them with AAV-shOTUD6B. Mouse brain microvascular endothelial cells (MBVECs) were treated with normal glucose and high lipid (NG/HL) or high glucose and high lipid (HG/HL), and siOTUD6B was used to investigate the effect of OTUD6B on proliferation, migration, and lumen formation of endothelial cells.
Results: We found that OTUD6B expression was markedly downregulated in human anterior tibial arteries from diabetic amputees and ApoE−/− mice. The silencing of OTUD6B resulted in diabetic atherosclerotic mice plaque instability and increased angiogenesis. In addition, the silencing of OTUD6B expression enhanced the proliferation, migration, and lumen formation of endothelial cells.
Conclusion: OTUD6B can reduce angiogenesis in atherosclerotic plaques, enhance plaque stability and delay the progression of atherosclerosis by regulating the proliferation, migration, and lumen formation of endothelial cells.
Keywords: OTUD6B, angiogenesis, atherosclerosis, diabetes
Introduction
Diabetes mellitus (DM) is one of the most common silent killers threatening human life.1 Currently, China has become the country with the highest incidence of diabetes, and the number of people with diabetes has increased exponentially.2 From 2011 to 2021, the number of people with diabetes in China rose from 90 million to 140 million, and it is estimated that it will soar to 174 million by 2045.3 Compared with non-diabetic patients with atherosclerotic cardiovascular disease (ASCVD), diabetic patients have atherosclerotic lesions with similar vascular pathological features, but diabetes enhances the progression of the necrotic core in atherosclerotic plaques and leads to more advanced lesions, such as vascular calcification.4 Relevant data show that ASCVD is the leading cause of death in patients with diabetes.5 Furthermore, the rupture of vulnerable atherosclerotic plaques is the primary cause of acute cardiovascular and cerebrovascular events such as myocardial infarction and cerebral stroke.6,7 It was initially thought that the prominent features of vulnerable atherosclerotic plaques were thin fibrous caps and large necrotic cores.8 However, with in-depth research, it was found that angiogenesis in plaques was closely related to the formation of vulnerable atherosclerotic plaques.9,10
Angiogenesis refers to the activation, proliferation, differentiation, and migration of endothelial cells to develop a vascular network based on the original capillaries or venules to generate new blood vessels and participate in various physiological and pathological processes.11–13 Under physiological conditions, neovascularization in atherosclerotic plaques can transport oxygen and nutrients, relieve hypoxia, and compensate for the ischemic vessels, but under pathological conditions, the generated pathological neovascularization will continuously transport lipids, inflammatory cells and activated proteases into the atherosclerotic lesions, resulting in the increase of apoptotic cells in the plaque, the increase of the necrotic core and the thinning of the fibrous cap, thereby accelerating the malignant evolution of atherosclerotic plaque vulnerability.14 Besides, neovascularization is composed of only one layer of endothelial cells, which is immature and has structural defects such as high permeability and brittleness, eventually leading to atherosclerotic plaque rupture.14,15 Ultrasonography has shown that plaque neovascularization is more likely to cause plaque rupture.16,17 Plaque neovascularization is a crucial histological feature of unstable plaques, but its molecular mechanism remains unclear.
Deubiquitinating enzymes can inhibit the translation of target protein by removing ubiquitin, thereby maintaining the normal function of the cell.18 OTU deubiquitinase 6B (OTUD6B), an essential member of the ovarian tumor domain (OTU)-containing subfamily of deubiquitinating enzymes, affects cell proliferation through a binding protein initiation complex.18 In addition, overexpression of OTUD6B in Ba/F3 cells led to the decrease of cyclin D2 levels and significantly retarded cell growth.19 A previous study revealed that OTUD6B regulates angiogenesis by enhancing the stability of von Hippel-Lindau tumor suppressor (pVHL) and inducing the inactivation of the Hypoxia-Inducible Factor (HIF) pathway.20 In addition, OTUD6B can promote ubiquitination-mediated degradation of HIF-1α in the tumor, thereby inhibiting tumor angiogenesis.20 However, there is no research on the role of OTUD6B in diabetic atherosclerotic plaque neovascularization.
In this study, we comprehensively investigated the role of OTUD6B in clinical samples, mouse brain microvascular endothelial cells (MBVECs), and a diabetic atherosclerotic mouse model to determine the effect of OTUD6B on diabetic atherosclerotic plaque neovascularization. We innovatively confirmed that OTUD6B was associated with the stability of diabetic atherosclerotic plaques.
Materials and Methods
Materials
Oil red O was obtained from Solaibao Technology Co. Ltd. (Beijing, China). Fluorescein isothiocyanate (FITC)-lectin was obtained from Sigma–Aldrich (Saint Louis, MO, USA). MBVECs were obtained from the cell bank of the Chinese Academy of Sciences (Beijing, China). The primary antibodies against OTUD6B were purchased from BIOSS Antibodies (Beijing, China). The primary antibodies against CD31, CD34, LYVE-1, and β-actin were purchased from Abcam (Cambridge, UK). The immunohistochemistry kit was obtained from Kang Wei Century Biotechnology Co. Ltd. (Beijing, China). AAV-shOTUD6B was purchased from Jikai Company (Shanghai, China).
Human Participants
The specimens of the anterior tibial artery were obtained from 3 patients undergoing amputation due to diabetic gangrene at the Affiliated Hospital of Jiangsu University (Zhenjiang, China) (Table 1). All patients gave written informed consent, and this study was approved by the Ethics Committee of the Affiliated Hospital of Jiangsu University and conducted according to its institutional guidelines. Patients with the following criteria were included in the study: patients aged ≥40 years and <70 years old; patients diagnosed with diabetes according to the diagnostic criteria of the Guidelines for the Prevention and Treatment of Type 2 Diabetes in China (2020); patients undergoing diabetic foot surgery according to the criteria of the Guidelines for the Prevention and Treatment of Diabetic Foot in China (2019). Patients with the following criteria were excluded from the study: patients with severe immunodeficiency disease or post-organ transplant; severe car accident trauma; history of malignancy.
 |
Table 1 Characteristics of Patients |
Animal Experiments
All animals in this study were reared, housed, and maintained at the laboratory animal center of Jiangsu university following the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No.5–23, revised 1996; NIH Bethesda, MD, USA). Ethics approval for this study was obtained from the Animal Care and Use Committee of Jiangsu University. Six-week-old ApoE−/− mice were injected intraperitoneally with 40 mg/kg/d streptozotocin (STZ) for five consecutive days and fed with a high-fat diet (HFD). Mice were divided into four groups as followed: Negative control (NC) group (HFD) (n = 5), diabetes mellitus (DM) group (HFD + STZ) (n = 5), AAV-shscramble group (HFD + STZ + AAV-shscramble) (n = 5), and AAV-shOTUD6B group (HFD + STZ + AAV-shOTUD6B) (n = 5). After four months, the aortas of the mice were harvested and stored for subsequent experiments.
Cell Culture
MBVECs were divided into three groups: Normal glucose and high lipid (NG/HL) group, high glucose and high lipid (HG/HL) group, and high glucose and high lipid + siOTUD6B (siOTUD6B) group. Then, the cells were maintained in DMEM with 10% fetal bovine serum (FBS) and penicillin and streptomycin in a humidified atmosphere incubator at 37 °C with a 5% CO2.
RNA Sequencing Data Collection and Analysis
The GSE169332 dataset, from the Gene Expression Omnibus (GEO) database, containing single cell RNA sequencing (scRNA-seq) data enriched with endothelial cells from the heart and aorta of Ldlr null (Ldlr−/−) mice was selected for the analysis of endothelial cells in diabetic atherosclerosis and processed and analyzed by the Seurat R package (version 4.0.2). The published RNA-sequencing (RNA-seq) data shown in this study include the GSE173669 and GSE193781 datasets, which were also downloaded from the GEO database and analyzed by BioLadder. The GSE173669 data set contained RNA-seq data from HUVEC cells (2 Mannitol+nLDL and 2 HG+oxLDL). The GSE193781 dataset contained RNA-seq data from 4 control peripheral blood serums of pigs and 16 diabetic peripheral blood serums of pigs (4 HC+STZ, 3 STZ+HC, 4 HL+STZ, and 5 STZ+HL). Then, we defined the normal glucose sample as the control group and the high glucose sample as the DM group. Additionally, we combined the GSE173669 and GSE193781 datasets to form a single dataset. In addition, the “limma” package in R language was used to perform batch correction and to determine the differences in expression levels of OTUD6B between the control group and DM group. This can increase the number of samples to ensure the accuracy of diagnostic genes.
Immunofluorescence and Immunohistochemistry
For immunofluorescence analysis, frozen tissue sections were fixed in 4% paraformaldehyde for 20 mins at room temperature and washed with PBS. Then, tissue sections were followed incubated with 1% bovine serum albumin and 0.2% Triton X-100 for 1 h at 37 °C. The tissue sections were then incubated overnight at 4°C with diluted (1:200) OTUD6B antibody. Subsequently, the sections were incubated in the dark for 1 h at 37 °C with the appropriate diluted (1:200) secondary fluorescent antibodies (Alexa Fluor 594). Images were obtained with an IX51Olympus microscope (Olympus Corporation, Tokyo, Japan).
For immunohistochemistry, Paraffin embedded tissue sections were dewaxed, hydrated and antigen retrieved. The steps were performed using the SP Rabbit & Mouse Horseradish Peroxidase (HRP) Kit (Kang Wei Century Biotechnology Co. Ltd.). Ultimately, the stained tissue sections were photographed under an Olympus microscope (Olympus Corporation).
Oil Red O Staining
Frozen tissue sections were fixed in 4% paraformaldehyde for 20 mins at room temperature and washed with PBS, followed by dip washing in isopropanol. Then, the tissue sections were placed in the dark and stained with an Oil Red O working solution for 30 min at room temperature and subsequently washed with isopropanol. The sections were eventually evaluated under an Olympus microscope (Olympus Corporation).
Masson Staining
Paraffin embedded tissue sections were dewaxed, hydrated and antigen retrieved. The procedure was performed using Masson trichrome staining Kit. Finally, the sections were photographed under an Olympus microscope (Olympus Corporation).
Western Blotting
Protein samples were extracted from aortic tissue by centrifugation in RIPA lysis buffer. The protein samples were separated by SDS-polyacrylamide gel and then transferred onto PVDF membranes. Subsequently, membranes were incubated with overnight at 4°C in the dark with anti-OTUD6B and anti-β-actin primary antibodies. Afterward, the membranes were washed and incubated for 1 h at room temperature with the appropriate HRP-conjugated secondary antibodies. Ultmately, the bands were visualized using electrochemiluminescence ECL detection reagents (GE Healthcare, Chicago, IL, USA).
Aortic Ring Budding Test
The aorta of mice was collected and digested with type II collagenase in an incubator at 37 °C for 15 minutes. Then, the aorta without its adventitia was cut into several arterial rings of about 0.5mm and starved in medium with Opti-MEM overnight in an incubator at 37 °C in with a 5%CO2. The n, the aortic rings were incubated with rat tail type I collagen. Ultimately, the growth state of arterial rings and the number and branches of new microvascular were observed under an inverted microscope.
Cell Counting Kit-8 (CCK-8) Assay
The cell counting kit-8 (CCK-8) assay was used to assess the proliferation of MBVECs cultured with or without HG/HL and siOTUD6B. The CCK-8 solution (10 μL) was added to every well in the plate after stimulation for 2–4 h at 37°C. The absorbance (OD value) of cells in each well was measured with a microplate reader.
Wound Healing Assay
MBVECs were seeded in 12 well plates and allowed to grow to confluence, then the cell monolayer was scratched using a sterile micropipette tip. The cells were observed and images were captured at 0, 24, and 48 h. Then,3 sites on a scratch were selected to measure the scratch width and the average value was calculated to minimize the experimental error. The ImageJ software (NIH) was used to measure the scratch area of cells and perform statistical analysis.
Lumen Formation Assay
Matrix glue (80 μL) was added to each well of a 96-well plate and the plate was incubated in a humidified incubator for 30 minutes. Then, 100 μL of MBVECs suspension was added to each well and cultured in a humidified incubator. The formation of the lumen was observed under a microscope every hour. The ImageJ software was used to count the number of tubes formed and the branches of tiny tubes.
Statistical Analysis
The SPSS 22.0 software (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. The data conforming to the normal distribution are presented as the mean ± S.D. (x ̅±s). Unpaired Student’s t-test was used to compare two variables. P<0.05 was considered statistically significant.
Results
OUTD6B Expression is Downregulated in Diabetic Atherosclerosis
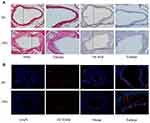
The framework and workflow of this study are described (Figure 1). To elucidate the expression of OTUD6B in diabetic atherosclerosis, we analyzed the scRNA-seq data (GSE169332) and RNA-seq data (GSE173669 and GSE193781) comprising the DM and control samples (Table 2). We found that OTUD6B was expressed in endothelial cells in diabetic atherosclerosis (Figure 2A–C). Furthermore, OTUD6B expression was significantly downregulated in the DM group compared with the control group (Figure 2D). We also determined the OTUD6B expression profile in human anterior tibial arteries from diabetic amputees and detected OTUD6B and lectin (a marker of endothelial cells) expression (Figure 2E).
 |
Table 2 Summary of Data Sets Used in This Research |
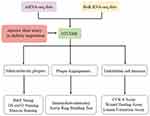 |
Figure 1 Framework and workflow of the present study. |
Diabetes Decreases the Expression of OUTD6B in ApoE−/− Mice
ApoE−/− mice were injected with STZ and fed a HFD diet for 12 weeks to establish a diabetic atherosclerosis model. H&E staining revealed that the atherosclerotic lesion area and the necrotic core area were increased in the DM group compared with the NC group (Figure 3A). Additionally, oil red O staining revealed increased lipid accumulation in the lesions of the DM group compared to that in the NC group (Figure 3A). Overall, these results indicated that diabetes exacerbates atherosclerosis in ApoE−/− mice. Also, further analysis of OTUD6B expression in diabetic atherosclerosis ApoE−/− mice by immunofluorescence staining revealed that OTUD6B expression was reduced in the DM group (Figure 3B). These findings indicate that OTUD6B is reduced during the development of diabetic atherosclerosis in ApoE−/− mice.
OUTD6B Deficiency Enhances the Progression of Diabetic Atherosclerosis
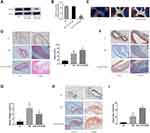
To investigate the functional role of OUTD6B in diabetic atherosclerosis, ApoE−/− mice were injected with an AAV-shOUTD6B. Then, measurement of the protein level of OUTD6B showed that while the expression of OUTD6B did not change significantly after the injection with AAV-shscramble and AAV-shOUTD6B significantly downregulated its expression (Figure 4A and B). Moreover, the overall perfusion of ApoE−/− mice showed that the atherosclerotic lesion area was increased in the DM+ AAV-shOUTD6B group compared with those from the DM group and NC group (Figure 4C). H&E staining revealed that after silencing of OTUD6B, more cholesterol crystals were deposited inside the plaque, the intima was rough, and the atherosclerotic lesion area was aggravated (Figure 4D and E). Masson staining revealed reduced collagen content in the DM+ AAV-shOUTD6B group compared to that in the DM group and NC group (Figure 4F and G). In addition, oil red O staining showed a significant increase in lipid accumulation in the lesions of the DM+ AAV-shOUTD6B group compared to that in the DM group and NC group (Figure 4H and I). According to the above results, OTUD6B can increase the collagen content in atherosclerotic plaques, reduce the lipid composition, and stabilize atherosclerotic plaques.
OUTD6B Deficiency Enhances Diabetic Plaque Angiogenesis
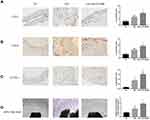
To further investigate the effect of OTUD6B on diabetic plaque angiogenesis, we measured the expression of CD31(a specific biomarker of endothelial cells), CD34(a specific marker of microvascular endothelial cells), and LYVE-1(a specific marker of lymphatic endothelial cells) by immunohistochemistry analysis. As shown in Figure 5A–C, OTUD6B affects microvascular endothelial cells and lymphatic endothelial cells, but it mainly regulates microvascular endothelial cells in diabetic plaques, with few positive lymphatic endothelial cells. The sprouting experiments of isolated mouse aortic rings showed that the number of sprouting cells and new microvascular were further increased in the aortic rings of mice in the DM+ AAV-shOUTD6B group, indicating that OTUD6B could attenuate the sprouting ability of aortic ring and new microvascular (Figure 5D). The above results clearly indicate that OTUD6B can inhibit diabetic plaque angiogenesis and delay the progression of diabetic atherosclerosis.
OUTD6B Deficiency Enhances Proliferation, Migration, and Lumen Formation in Endothelial Cell
To further verify the effect of OTUD6B on angiogenesis, endothelial cells were transfected with siRNA targeting OTUD6B. The CCK-8 cell proliferation assay showed increased cell viability, and proliferation ability was more pronounced in the siOTUD6B group than in the HG/HL group and NG/HL group (Figure 6A). In addition, wound healing assay revealed that the cell migration ability increased significantly in the siOTUD6B group (Figure 6B and C). The lumen formation assay found that the length of the lumen and the number of branches were increased in the siOTUD6B group compared with those in the HG/HL group and NG/HL group (Figure 6D–F). These findings indicate that OTUD6B inhibits endothelial cell proliferation, migration, and lumen formation, which is consistent with the results of the in vivo animal experiments.
Discussion
OTUD6B, the gene encoding a crucial member of the ovarian tumor-domain containing subfamily of functional deubiquitinating proteases, is located on chromosome 8q21.3 and consists of 7 exons encoding 323 amino acids.21 In this study, we found that OTUD6B was expressed in endothelial cells in diabetic atherosclerosis. Furthermore, OTUD6B expression was significantly downregulated in diabetic atherosclerosis, indicating that OTUD6B may be a protective factor in diabetic atherosclerosis. Previous studies have suggested that OTUD6B is essential in regulating cell proliferation, migration, and other cellular processes.22 In a subcutaneous transplanted tumor model in nude mice, the sciencing of OTUD6B by lentivirus injection increased the expression of CD31 positive endothelial cells, the number of new blood vessels, and the tumor load. In this study, we have demonstrated that OTUD6B and lectin expression in the human anterior tibial artery and diabetes decrease the expression of OUTD6B in ApoE−/− mice.
The protease family of ovarian tumor-domain containing deubiquitinating enzymes plays an essential role in the progression of atherosclerosis. Accordingly, OUTD6B may be related to diabetic atherosclerosis. In this study, using a diabetic atherosclerosis model, we confirmed that OUTD6B inhibits plaque formation. The internal structure of plaques containing lipid, collagen, neovascularization, and other components, is diverse and complex, which leads to unstable plaques. As an essential component of the extracellular matrix, collagen is mainly involved in the formation of the fibrous cap of plaque to prevent plaque rupture. Thus, it can be said that the higher than collagen content, the stronger the stability of the plaque,23 which is consistent with our results of this study suggesting that OUTD6B increases plaque stability. In addition, the accumulation of lipids in the plaque reduces the hardness of the plaque, and the larger lipid core damages the strength of the plaque and makes it easier to rupture.24,25 We also found that OUTD6B inhibits lipid accumulation. In general, OUTD6B promotes the stability of diabetic atherosclerotic plaque.
Current studies have shown that angiogenesis is closely related to the stability of atherosclerotic plaque. Consistent with this finding, we observed that OTUD6B promotes angiogenesis in diabetic atherosclerosis. Endothelial cell proliferation, migration, and tubulation are vital signs of angiogenesis. In vitro study found that OTUD6B promotes endothelial cell proliferation, migration, and lumen formation, which is consistent with the animal study.
The amount of neovascularization in atherosclerotic plaques is stimulated by hypoxia, reactive oxygen species and HIF signaling, which increases with atherisclerotic progression.26 Studies have shown that SIRT6 promotes the expression of HIF-1α by inhibiting the deubiquitination of HIF-1α at K37 and K532, thereby promoting the invasion, migration, proliferation and tubulation of HUVECs.27 Furthermore, loss of endogenous OTUD stabilizes HIF-1α, while ectopic overexpression of OTUD promotes ubiquitination of HIF-1α.28 In summary, it is hypothesized that OTUD6B may participate in the progression of diabetic atherosclerosis and angiogenesis by regulating the ubiquitination of HIF-1α.
The limitations of this study are as follows: 1) Besides the inhibition of OTUD6B by the adeno-associated virus, overexpression of OTUD6B can be used to investigate its effect. 2) The specific mechanism by which OTUD6B regulates angiogenesis in plaques needs to be further explored. 3) The role of OTUD6B in non-diabetic atherosclerotic plaques and angiogenesis need further confirmation in vivo and in vitro.
Conclusion
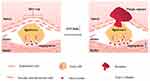
In conclusion, our study demonstrated that OTUD6B inhibited angiogenesis in diabetic atherosclerotic plaques as well as the proliferation, migration, and lumen formation of endothelial cells (Figure 7). All the effects of OTUD6B ultimately promoted the stability of diabetic atherosclerotic plaques.
Data Sharing Statement
All datasets supporting the conclusions of this article are included in the article.
Ethics Approval and Consent to Participate
The ethical review number of the study is ChiCTR1900025122 and was conducted in accordance with the principles of the “Helsinki Declaration”. Written informed consent was obtained from all patients. All animal experiments in this study were conducted in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No.5–23, revised 1996) and approved by the Animal Care and Use Committee of Jiangsu University, China.
Funding
This work was supported by the National Natural Science Foundation of China (82070455); the related Foundation of Jiangsu Province (BK20201225, M2020016); Medical Innovation Team Project of Jiangsu Province (CXTDA2017010); the Open Project Program of Guangxi Key Laboratory of Centre of Diabetic Systems Medicine (GKLCDSM-20210101-02).
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
1. Wojciechowska J, Krajewski W, Bolanowski M, et al. Diabetes and cancer: a review of current knowledge. Exp Clin Endocrinol Diabetes. 2016;124(5):263–275. doi:10.1055/s-0042-100910
2. Wang Z. Focus on the pathogenesis, mechanism, evaluation, prevention and treatment of diabetic macrovascular complications. Medical Science Journal of Central South China. 2022;50(1):1–6 doi:10.15972/j.cnki.43-1509/r.2022.01.001.
3. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
4. Eckel RH, Bornfeldt KE, Goldberg IJ. Cardiovascular disease in diabetes, beyond glucose. Cell Metab. 2021;33(8):1519–1545. doi:10.1016/j.cmet.2021.07.001
5. Schmidt AM. Diabetes mellitus and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2019;39(4):558–568. doi:10.1161/ATVBAHA.119.310961
6. Chen R, Fu Y, Wang W. Intraplaque Neovascularization and Its Influence on Stability of Atherosclerosis Plaque. Chin J Arterioscler. 2016;24(3):311–315.
7. The Joint Task Force for Guideline on the Assessment and Management of Cardiovascular Risk in China. Guideline on the Assessment and Management of Cardiovascular Risk in China. Chinese Circulation Journal. 2019;34(1):4–28.
8. Dawson LP, Lum M, Nerleker N, Nicholls SJ, Layland J. Coronary atherosclerotic plaque regression: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(1):66–82. doi:10.1016/j.jacc.2021.10.035
9. Kwon TG, Lerman LO, Lerman A. The vasa vasorum in atherosclerosis: the vessel within the vascular wall. J Am Coll Cardiol. 2015;65(23):2478–2480. doi:10.1016/j.jacc.2015.04.032
10. Taruya A, Tanaka A, Nishiguchi T, et al. Vasa vasorum restructuring in human atherosclerotic plaque vulnerability: a clinical optical coherence tomography study. J Am Coll Cardiol. 2015;65(23):2469–2477. doi:10.1016/j.jacc.2015.04.020
11. Perrotta P, Emini Veseli B, Van der Veken B, et al. Pharmacological strategies to inhibit intra-plaque angiogenesis in atherosclerosis. Vascul Pharmacol. 2019;112:72–78. doi:10.1016/j.vph.2018.06.014
12. Bikfalvi A. History and conceptual developments in vascular biology and angiogenesis research: a personal view. Angiogenesis. 2017;20(4):463–478. doi:10.1007/s10456-017-9569-2
13. Wang Y, Yan X, Liu C, et al. Research progress of angiogenesis in atherosclerotic plaque. J Chin Arteriosclerosis. 2021;29(8):5. doi:10.1016/j.atherosclerosis.2021.04.009
14. Wang Y, Yong Q. Progress of carotid plaque neovascularization. Journal of Vascular and Endovascular Surgery. 2017;3 2 :672–673.
15. Chistiakov DA, Orekhov AN, Bobryshev YV. Contribution of neovascularization and intraplaque haemorrhage to atherosclerotic plaque progression and instability. Acta Physiol. 2015;213(3):539–553. doi:10.1111/apha.12438
16. Yuan J, Zhu Y. Research progress in detection of neovascularization in plaque. Journal of Vascular and Endovascular Surgery. 2015;1(1):57–61. doi:10.19418/j.cnki.issn2096-0646.2015.01.019
17. Zhang M, Lin L, Chen H, et al. Study on the enhanced character of contrast-enhanced ultrasonography of different parts of carotid atherosclerotic plaques. Med J West China. 2018;30(4):582–585. doi:10.3969/j,issn.1672-3511.2018.04.025
18. Sobol A, Askonas C, Alani S, et al. Deubiquitinase OTUD6B isoforms are important regulators of growth and proliferation. Mol Cancer Res. 2017;15(2):117–127. doi:10.1158/1541-7786.MCR-16-0281-T
19. Xu Z, Zheng Y, Zhu Y, et al. Evidence for OTUD-6B participation in B lymphocytes cell cycle after cytokine stimulation. PLoS One. 2011;6(1):e14514. doi:10.1371/journal.pone.0014514
20. Liu X, Zhang X, Peng Z, et al. Deubiquitylase OTUD6B governs pVHL stability in an enzyme-independent manner and suppresses hepatocellular carcinoma metastasis. Adv Sci. 2020;7(8):1902040. doi:10.1002/advs.201902040
21. Wang T, Yu B, Zhang X, et al. Construction of a deubiquitinase OTUD6B truncation mouse model and analysis of phenotype. Mil Med Sci. 2022;46(5):355–384. doi:10.7644/j.issn.1674-9960.2022.05.007
22. Guo K, Wei Y, Wang Z, et al. Deubiquitylase OTUD6B stabilizes the mutated pVHL and suppresses cell migration in clear cell renal cell carcinoma. Cell Death Dis. 2022;13(2):97. doi:10.1038/s41419-021-04135-3
23. Shami A, Gonçalves I, Hultgårdh-Nilsson A. Collagen and related extracellular matrix proteins in atherosclerotic plaque development. Curr Opin Lipidol. 2014;25(5):394–399. doi:10.1097/MOL.0000000000000112
24. Visscher M, Moerman AM, Burgers PC, et al. data processing pipeline for lipid profiling of carotid atherosclerotic plaque with mass spectrometry imaging. J Am Soc Mass Spectrom. 2019;30(9):1790–1800. doi:10.1007/s13361-019-02254-y
25. Guo M, Cai Y, He C, et al. Coupled modeling of lipid deposition, inflammatory response and intraplaque angiogenesis in atherosclerotic plaque. Ann Biomed Eng. 2019;47(2):439–452. doi:10.1007/s10439-018-02173-1
26. Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol. 2009;218(1):7–29. doi:10.1002/path.2518
27. Yang Z, Huang Y, Zhu L, et al. SIRT6 promotes angiogenesis and hemorrhage of carotid plaque via regulating HIF-1α and reactive oxygen species. Cell Death Dis. 2021;12(1):77. doi:10.1038/s41419-020-03372-2
28. Bremm A, Moniz S, Mader J, et al. Cezanne (OTUD 7B) regulates HIF −1α homeostasis in a proteasome-independent manner. EMBO Rep. 2015;15(12):1268–1277. doi:10.15252/embr.201438850
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.