Back to Journals » OncoTargets and Therapy » Volume 12
Longdaysin inhibits Wnt/β-catenin signaling and exhibits antitumor activity against breast cancer
Authors Xiong Y, Zhou L, Su Z, Song J, Sun Q, Liu SS, Xia Y, Wang Z, Lu D
Received 1 November 2018
Accepted for publication 8 December 2018
Published 5 February 2019 Volume 2019:12 Pages 993—1005
DOI https://doi.org/10.2147/OTT.S193024
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Yanpeng Xiong,* Liang Zhou,* Zijie Su, Jiaxing Song, Qi Sun, Shan-Shan Liu, Yuqing Xia, Zhongyuan Wang, Desheng Lu
Guangdong Key Laboratory for Genome Stability and Disease Prevention, Shenzhen University International Cancer Center, Department of Pharmacology, Shenzhen University Health Science Center, Shenzhen 518060, Guangdong, China
*These authors contributed equally to this work
Background: CK1 is involved in regulating Wnt/β-catenin signaling and represents a promising target for the treatment of breast cancer. A purine derivative longdaysin has recently been identified as a novel modulator of cellular circadian rhythms through targeting the protein kinases CK1δ, CK1α, and ERK2. However, the antitumor activity of longdaysin and its underlying mechanisms remain unclear.
Methods: The inhibitory effect of longdaysin on Wnt/β-catenin signaling was investigated using the SuperTOPFlash reporter system. The levels of phosphorylated LRP6, total LRP6, DVL2, active β-catenin, and total β-catenin were examined by Western blot. The expression of Wnt target genes was determined using real-time PCR. The ability of colony formation of breast cancer cells was measured by colony formation assay. The effects of longdaysin on cancer cell migration and invasion were assessed using transwell assays. The effect of longdaysin on cancer stem cells was tested by sphere formation assay. The in vivo antitumor effect of longdaysin was evaluated using MDA-MB-231 breast cancer xenografts.
Results: Longdaysin suppressed Wnt/β-catenin signaling through inhibition of CK1δ and CK1ε in HEK293T cells. In breast cancer Hs578T and MDA-MB-231 cells, micromolar concentrations of longdaysin attenuated the phosphorylation of LRP6 and DVL2 and reduced the expression of active β-catenin and total β-catenin, leading to the downregulation of Wnt target genes Axin2, DKK1, LEF1, and Survivin. Furthermore, longdaysin inhibited the colony formation, migration, invasion, and sphere formation of breast cancer cells. In MDA-MB-231 breast cancer xenografts, treatment with longdaysin suppressed tumor growth in association with inhibition of Wnt/β-catenin signaling.
Conclusion: Longdaysin is a novel inhibitor of the Wnt/β-catenin signaling pathway. It exerts antitumor effect through blocking CK1δ/ε-dependent Wnt signaling.
Keywords: longdaysin, Wnt, β-catenin signaling, CK1δ, CK1ε, breast cancer
Background
CK1 is a family of serine/threonine-specific protein kinases that is conserved in eukaryotes from yeast to humans.1,2 It regulates diverse cellular processes including Wnt signaling, circadian rhythms, and DNA repair.3,4 The CK1 family consists of six human isoforms (α, δ, ε, γ1, γ2, γ3). The amino-terminal kinase domain is highly conserved in all CK1 family members.5–7
The Wnt/β-catenin signaling pathway plays a pivotal role in embryonic development, tumorigenesis, and stem cell self-renewal.8,9 This pathway is activated by the binding of secreted Wnt proteins to a receptor complex containing a member of the FZD family and LRP5/6.8,10 CK1 family members phosphorylate several important components in the Wnt/β-catenin signaling pathway and act as either negative or positive regulators of the pathway.11,12 In the destruction complex, CK1α phosphorylates β-catenin and subsequently leads to degradation of β-catenin.13 Moreover, CK1δ or CK1ε can phosphorylate the APC protein to enhance the binding of APC to β-catenin for β-catenin degradation.14 However, in the presence of Wnt, CK1δ and CK1ε can phosphorylate LRP6 and DVL to disrupt the formation of the β-catenin degradation complex, resulting in β-catenin accumulation and nuclear translocation, as well as the expression of Wnt target genes, including DKK1, LEF1, Axin2, and Survivin.10,15–17
CK1δ and CK1ε play a critical role in circadian pacemaking because they phosphorylate the core clock protein PER, leading to proteasome-mediated degradation and nuclear translocation.18 The human familial advanced sleep-phase syndrome is associated with a T44A missense mutation in CK1δ.19,20 In Syrian hamsters and mice, the Tau mutation of CK1ε (CK1ε
Methods
Reagents and plasmids
Longdaysin was purchased from Sigma-Aldrich. The construction of SuperTOPFlash reporter and the expression plasmids encoding Wnt1, LRP6, DVL2, CK1δ, CK1ε, and β-gal have been described previously.24
Cell culture
All cell lines were obtained from the American Type Culture Collection (ATCC). HEK293T and Hs578T cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin in a humidified incubator at 37°C with 5% CO2. MDA-MB-231 cells were grown in Leibovitz’s L-15 medium supplemented with 10% FBS and 1% penicillin–streptomycin at 37°C in a humidified incubator without CO2.
Transfection and luciferase reporter analysis
HEK293T cells were transfected with SuperTOPFlash reporter along with control plasmid pCMXβgal, and the indicated expression plasmids, using the X-tremeGENE HP DNA Transfection Reagent (Roche) according to the manufacturer’s instructions. Cells were then incubated with DMSO or the indicated concentrations of longdaysin. Luciferase activity was evaluated using a luciferase assay kit (Promega), and the luciferase values were normalized to β-gal activities.
Western blot analyses
Lysates of cells or tumor tissues were prepared with lysis buffer containing 20 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM ethylene glycol tetraacetic acid, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate, 1 mM sodium orthovanadate, 2 μg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride. Equal amounts of proteins were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes. Immunoblotting was performed with anti-phosphorylated LRP6 (Ser1490) (2568; Cell Signaling), anti-LRP6 (2560; Cell Signaling), DVL2 (3216; Cell Signaling), anti-active β-catenin (8814; Cell Signaling), anti-β-catenin (sc-7963; Santa Cruz), anti-CK1α (108296; Abcam), anti-CK1δ (sc-55554; Santa Cruz), anti-CK1ε (12448; Cell Signaling), anti-CD44 (3570; Cell Signaling), anti-Slug (9585; Cell Signaling), anti-Snail (3879; Cell Signaling), anti-GAPDH (HC-301; TransGen Biotech), and anti-β-actin (HC-201; TransGen Biotech).
Real-time PCR analyses
Total RNA was extracted using RNAiso Plus (TaKaRa) and was then reverse-transcribed into cDNA using the PrimeScript RT Reagent Kit (TaKaRa) according to the manufacturer’s instructions. The prepared cDNA was then subjected to quantitative PCR analysis using GoTaq® qPCR Master Mix (Promega). The primer pairs used for quantitative PCR amplification were as follows: Axin2: sense, 5′-TACACTCCTTATTGGGCGATCA-3′ and antisense, 5′-TTGGCTACTCGTAAAGTTTTGGT-3′; DKK1: sense, 5′-ATAGCACCTTGGATGGGTATTCC-3′ and antisense, 5′-CTGATGACCGGAGACAAACAG-3′; LEF1: sense, 5′-AGGAACATCCCCACACTGAC-3′ and antisense, 5′-AGGTCTTTTTGGCTCCTGCT-3′; Survivin: sense, 5′-AGGACCACCGCATCTCTACAT-3′ and antisense, 5′-AAGTCTGGCTCGTTCTCAGTG-3′; CD44: sense, 5′-CTGCCGCTTTGCAGGTGTA-3′ and antisense, 5′-CATTGTGGGCAAGGTGCTATT-3′; Slug: sense, 5′-CGAACTGGACACACATACAGTG-3′ and antisense, 5′-CTGAGGATCTCTGGTTGTGGT-3′; Snail: sense, 5′-TCGGAAGCCTAACTACAGCGA-3′ and antisense, 5′-AGATGAGCATTGGCAGCGAG-3′; GAPDH: sense, 5′-CCAGAACATCATCCCTGCCTCTACT-3′ and antisense, 5′-GGTTTTTCTAGACGGCAGGTCAGGT-3′.
Lentiviral shRNA
The sequences of CK1 shRNAs were as follows: shCK1δ: sense, GATCCGTGATCAGTCGCATCGAATATTCAAGAGATATTCGATGCGACTGATCATTTTTTGGAAA and antisense, AGCTTTTCCAAAAAATGATCAGTCGCATCGAATATCTCTTGAATATTCGATGCGACTGATCACG; and shCK1ε: sense, GATCCGTATATCCACTCCAAGAACTTCAAGAGAGTTCTTGGAGTGGATATACTTTTTTGGAAA and antisense, AGCTTTTCCAAAAAAGTATATCCACTCCAAGAACTCTCTTGAAGTTCTTGGAGTGGATATACG. For infection with lentivirus, the cells were cultured with lentiviral solution for 24 hours in the presence of 5 μg/mL Polybrene (Sigma).
Colony formation assays
Cells were seeded onto a six-well plate at a density of 1×103 cells per well and then incubated with the indicated concentrations of longdaysin. After incubation for 7 days, cells were stained with crystal violet and photographed.
In vitro migration and invasion analyses
As previously described,24 2×105 cells were suspended in 100 μL serum-free medium containing the indicated concentrations of longdaysin, and then seeded in 24-transwell chambers with 8 μm pore membrane. The lower chamber contained medium with 20% FBS. After incubation at 37°C for 6 hours, the unmigrated cells on the upper side of membrane were removed by a cotton swab, and the migrated cells were stained with crystal violet and stained cells were photomicrographed. For invasion assays, the transwell chambers with 8 μm pore membranes were coated with Matrigel.
Sphere formation assays
Cells were seeded in DMEM/F12 medium containing 2% B-27, 10 ng/mL EGF, 10 ng/mL fibroblast growth factor, and 10 μg/mL insulin with the indicated concentrations of longdaysin in a 24-well plate with an Ultra-Low Attachment surface (250 cells per well). After incubation for 10 days, spheres were photographed.
Animal model study
All animal experiments were performed according to the protocols approved by the Administrative Committee on Animal Research of Shenzhen University, and the Committee also approved all the animal experiments performed in this study. Female BALB/c nude mice were obtained from Beijing Vital River Laboratory Animal Technology Co, Ltd. MDA-MB-231 cells were injected s.c. into the right flank of nude mice (1×107 cells per mouse), and tumor growth was closely observed and measured every 3 days. When the tumors reached approximately 50 mm3, the mice were randomly divided into two groups (eight mice per group) and i.p. injected with the vehicle (0.8% DMSO/12% Cremophor/8% ethanol in normal saline) or 5 mg/kg longdaysin in vehicle every 3 days. This longdaysin dosage was selected based on results from preliminary experiments, and was well tolerated in the mouse model. Subsequently, tumor volumes were measured with a caliper and calculated as follows: 0.523×(length)×(width)2. After treatment for 3 weeks, the mice were sacrificed and the tumor tissues were collected and weighed before being fixed in buffered formalin.
Histologic analyses
As previously described,24 formalin-fixed tumors were embedded with paraffin and sectioned, after which immunocytochemistry and H&E staining were performed. Immunocytochemistry was performed using the following primary antibodies: anti-Ki-67 (350501; BioLegend), anti-active β-catenin (8814; Cell Signaling), and anti-β-catenin (sc-7963; Santa Cruz).
Statistical analyses
Statistical analyses were performed with the GraphPad Prism software (version 5.0). Results are presented as mean ± SD. A P-value <0.05 was considered statistically significant.
Results
CK1δ/ε-dependent suppression of Wnt/β-catenin signaling by longdaysin
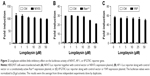
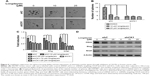
CK1 family members play both positive and negative roles in the Wnt/β-catenin signaling pathway.25 A novel compound longdaysin (Figure 1A) exhibited a drastic effect on the circadian period through inhibition of CK1δ, CK1α, and ERK2.23 To determine the effect of longdaysin on Wnt/β-catenin signaling, HEK293T cells were transfected with a SuperTOPFlash reporter vector together with expression plasmids for Wnt1, LRP6, DVL2, CK1δ, CK1ε, CK1δ/LRP6, CK1ε/LRP6, CK1δ/DVL2, CK1ε/DVL2, and β-catenin. Longdaysin dramatically blocked Wnt signaling activated by Wnt1, LRP6, DVL2, CK1δ, CK1ε, CK1δ/LRP6, CK1ε/LRP6, CK1δ/DVL2, and CK1ε/DVL2 in a dose-dependent manner (Figure 1B–H). Moreover, increased Wnt reporter activity induced by Wnt3A-conditioned medium was also inhibited by longdaysin (Figure 1J). However, this compound had little effect on β-catenin-induced reporter activity (Figure 1I). These results indicate that longdaysin may target the upstream components of Wnt signaling. In control experiments, longdaysin at Wnt-inhibitory concentrations had little effect on the luciferase activity of NFAT reporter (NFAT-Luc), AP-1 reporter (AP-1-Luc), or Hippo reporter (8× GTIIC-Luc) (Figure 2A–C).
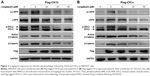
Since longdaysin has been demonstrated to inhibit CK1δ activity,23 we speculate this compound may suppress the Wnt/β-catenin signaling cascade through targeting CK1δ and CK1ε. To investigate whether the Wnt-inhibitory effect of longdaysin is dependent on CK1δ/ε, HEK293T cells were transfected with Flag-tagged CK1δ or CK1ε expression plasmid and then treated with the indicated concentrations of longdaysin. As expected, CK1δ (Figure 3A) or CK1ε (Figure 3B) expression increased the level of phosphorylated LRP6 and DVL2 and activated β-catenin. Treatment with longdaysin significantly reduced the levels of phosphorylated LRP6, total LRP6, phosphorylated DVL2, activated β-catenin, and total β-catenin in a dose-dependent manner (Figure 3), indicating that longdaysin may inhibit the Wnt/β-catenin pathway by suppressing CK1δ/ε. In addition, Western blot analysis was performed to examine the basal expression of CK1 isoforms in HEK293T cells. As shown in Figure S1, the basal expression of CK1α, CK1δ, and CK1ε was detected in HEK293T cells. Longdaysin treatment had no effect on their basal expression (Figure S1).
CK1δ/ε-dependent inhibition of the Wnt/β-catenin signaling cascade in breast cancer cells by longdaysin
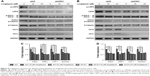
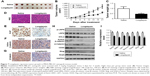
To determine the effect of CK1δ and CK1ε on Wnt/β-catenin signaling in breast cancer cells, lentivirus-mediated shRNAs were used to suppress the expression of CK1δ and CK1ε in Hs578T and MDA-MB-231 cells. Similar to longdaysin, shRNA-mediated silencing of CK1δ and CK1ε resulted in decreased protein levels of phosphorylated LRP6, total LRP6, phosphorylated DVL2, active β-catenin, and total β-catenin (Figure 4A and B). Importantly, depletion of CK1δ and CK1ε abolished the effects of longdaysin on LRP6, DVL2, and β-catenin in both cell lines (Figure 4A and B). Furthermore, knockdown of CK1δ and CK1ε downregulated the mRNA expression of Wnt target genes Axin2, DKK1, LEF1, and Survivin, while longdaysin treatment had no further effects on the expression of these Wnt target genes in shRNA-silenced cells (Figure 4C and D).
Repression of colony formation, migration, and invasion in breast cancer cells by longdaysin via targeting CK1δ/ε
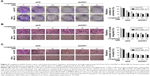
To examine the effect of longdaysin on the colony formation ability of breast cancer cells, a colony formation assay was performed. Longdaysin treatment inhibited colony formation of Hs578T and MDA-MB-231 cells in a dose-dependent manner, while knockdown of CK1δ/ε abrogated the effect of longdaysin (Figure 5A).
Considering the important role of Wnt/β-catenin signaling in migration and invasion of malignant cells, we investigated the effect of longdaysin on migration and invasion in breast cancer cells using transwell assay. As shown in Figure 5B and C, longdaysin treatment dose-dependently suppressed the migration and invasion of Hs578T and MDA-MB-231 cells (Figure 5B and C). However, the effects of longdaysin on the migration and invasion of breast cancer cells were abolished in CK1δ/ε-knockdown cells (Figure 5B and C). These results suggest that longdaysin may repress breast cancer cell colony formation, migration, and invasion through targeting CK1δ/ε.
Blockage of breast cancer cell stemness by longdaysin through inhibition of CK1δ/ε
Since Wnt/β-catenin signaling plays a crucial role in the survival and maintenance of CSCs, we examined the effect of longdaysin on the stemness of breast cancer cells using a sphere formation assay. The breast cancer Hs578T cells were incubated with longdaysin at different concentrations for 1 week. The images of tumor spheres of Hs578T cells showed markedly decreased number and size of tumor spheres in cells treated with longdaysin (Figure 6A and B). Furthermore, longdaysin treatment downregulated the expression of stemness marker genes, CD44, Slug, and Snail, in Hs578T cells (Figure 6C and D). As expected, CK1δ/ε knockdown attenuated tumor sphere formation and inhibited expressions of stemness genes CD44, Slug, and Snail in mRNA and their protein levels in Hs578T cells (Figure 6C and D). Longdaysin had little effects on the sphere formation, and expression of stemness marker genes in CK1δ/ε-silenced cells (Figure 6A–C). It has been well established that CD44, Slug, and Snail are the target genes of Wnt/β-catenin signaling.26,27 Thus, it is fairly reasonable to assume longdaysin-induced inhibition of stemness may be associated with its antagonistic effects on Wnt/β-catenin signaling through targeting CK1δ/ε.
Suppression of tumor growth in vivo and inhibition of Wnt/β-catenin signaling in MDA-MB-231 xenografts by longdaysin
To determine the inhibitory effect of longdaysin on the Wnt/β-catenin pathway in breast cancer cells in vivo, MDA-MB-231 cells were injected s.c. into nude mice. The tumors were measured every 3 days. Once the volumes of the tumors reached approximately 50 mm3, mice were randomly divided into two groups, and i.p. injected with vehicle or longdaysin at 5 mg/kg every 3 days, respectively. After 3-week treatment, the mice were sacrificed and tumors were removed and weighed. The treatment regimen with longdaysin obviously inhibited tumor growth (Figure 7A–C), and decreased tumor cell density (Figure 7D) and proliferation as indicated by Ki-67 staining (Figure 7E). However, longdaysin did not influence body weight, suggesting minimal toxicity.
To investigate the effect of longdaysin on Wnt/β-catenin signaling in MDA-MB-231 xenografts, immunohistochemical staining, immunoblot analyses, and real-time PCR were performed. As indicated by immunohistochemical staining, administration of longdaysin markedly reduced the expression of active β-catenin and total β-catenin (Figure 7F and G). Moreover, Western blot demonstrated that longdaysin treatment decreased the expression of phosphorylated and total LRP6, phosphorylated DVL2, and active and total β-catenin (Figure 7H). The Wnt-inhibitory effect of longdaysin was further confirmed by real-time PCR, which showed reduced mRNA expression of the Wnt target genes including Axin2, DKK1, LEF1, and Survivin in longdaysin-treated group compared with control group (Figure 7I).
We further explored the effect of longdaysin on the expression of stemness-related Wnt target genes CD44, Slug, and Snail. Longdaysin treatment inhibited both mRNA and protein levels of CD44, Slug, and Snail (Figure 7H and I).
Discussion
Longdaysin is a novel chemical regulator of circadian clock. This compound markedly slows the circadian clock both in a variety of mammalian cells and in zebrafish in vivo through inhibition of the protein kinases CK1δ, CK1α, and ERK2. Longdaysin may form the basis for therapeutic strategies directed toward circadian disorders, such as shift-work fatigue and jet lag.23 However, little is known about the effect of longdaysin on the Wnt/β-catenin pathway. In this study, longdaysin was identified as a potent inhibitor of Wnt/β-catenin signaling through targeting CK1δ and CK1ε. In MDA-MB-231 breast cancer xenografts, longdaysin suppressed in vivo tumor growth, concomitant with reduced expression of phosphorylated and total LRP6, phosphorylated DVL2, active and total β-catenin, and Wnt target genes. Our results indicate that longdaysin may have therapeutic potential for the treatment of breast cancer.
Aberrant regulation of CK1δ and CK1ε is involved in the pathogenesis of several human cancers including breast cancer.2 CK1ε has been found to be an essential regulator of β-catenin activity and proliferation in breast cancer cells. The expression of CK1ε could promote oncogenic transformation of mammary epithelial cells in a β-catenin-dependent manner.28 Hirner et al reported that inactivating mutations in CK1δ could attenuate SV40-mediated cellular transformation in vitro and SV40-induced mouse mammary carcinogenesis in vivo.29 Rosenberg et al demonstrated that CK1δ is frequently amplified and overexpressed in a subset of human breast cancers. Knockdown or inhibition of CK1δ induced breast tumor regression in patient-derived and cell line orthotopic xenograft models of TNBC and HER2+ breast cancer.30 Recently, Janovska et al identified CK1δ/ε as a novel therapeutic target in chronic lymphocytic leukemia.31 These studies indicate that CK1δ and CK1ε may be therapeutic targets for cancer drug development. Our results demonstrated that longdaysin could inhibit the Wnt/β-catenin signaling through inhibition of CK1δ/ε (Figure 8). LRP6 and DVL2 are well known as specific substrates for CK1. Longdaysin markedly decreased phosphorylation of LRP6 and DVL2, and reduced the levels of active β-catenin and total β-catenin protein, finally leading to the transcriptional downregulation of Wnt target genes. Consistently, knockdown of CK1δ/ε also decreased protein levels of phosphorylated and total LRP6, phosphorylated DVL2, active β-catenin, and total β-catenin in breast cancer cells, resulting in downregulation of the mRNA expression of Wnt target genes. Moreover, the effects of longdaysin on Wnt/β-catenin occurred at concentrations comparable to those required for inhibiting colony formation, migration, and invasion in breast cancer cells. These results suggest that the antitumor activity of longdaysin is associated with its inhibitory effects on the Wnt/β-catenin signaling pathway.
Our results showed that longdaysin treatment dose-dependently inhibited colony formation, migration, invasion, and formation of tumor spheres in breast cancer cells, while knockdown of CK1δ/ε abrogated its effects. Additionally, we did not observe any synergistic or additive effect after combined CK1δ/ε knockdown and longdaysin treatment. These results strongly suggest that longdaysin exhibits its antitumor activity against breast cancer via targeting CK1δ/ε.
Increasing evidence has demonstrated that the Wnt/β-catenin pathway regulates self-renewal and stemness properties of CSCs.32 Several Wnt target genes have been proposed as CSC markers including CD44, Slug, and Snail in breast cancer.33,34 Thus, targeting the Wnt/β-catenin pathway can potentially eliminate CSC populations in breast cancer. Our results showed that longdaysin significantly inhibited sphere formation of breast cancer cells and decreased the expression of stemness marker genes CD44, Slug, and Snail. In the MDA-MB-231 xenografts, longdaysin suppressed tumor growth in vivo and reduced both mRNA and protein levels of CD44, Slug, and Snail. These results suggest that longdaysin may be an efficient inhibitor of breast CSCs. Further investigation is needed to characterize the inhibitory action of longdaysin on breast CSCs.
Conclusion
Our results showed that longdaysin is able to inhibit the Wnt/β-catenin pathway by targeting CK1δ/ε. This compound markedly decreased phosphorylation of LRP6 and DVL2, and reduced the levels of active β-catenin and total β-catenin protein, finally leading to the transcriptional downregulation of Wnt target genes. We further demonstrated that longdaysin could repress breast cancer cell colony formation, migration, and invasion in a CK1δ/ε-dependent manner. In breast cancer xenografts, longdaysin suppressed in vivo tumor growth with concurrent inhibition of Wnt/β-catenin signaling. To our knowledge, this is the first study providing evidence that longdaysin is a potent antitumor agent. It exhibited antitumor activity against breast cancer via inhibition of CK1δ/ε-dependent Wnt signaling.
Abbreviations
CSCs, cancer stem cells; DMSO, dimethyl sulfoxide; i.p., intraperitoneally; s.c., subcutaneously.
Data sharing statement
All data underlying the findings described in this manuscript are fully available without restrictions.
Acknowledgments
The authors would like to thank the Cancer Research Center, Department of Pharmacology, Shenzhen University Health Science Center, for providing the facilities to carry out this study. This work was supported by the National Natural Science Foundation of China (Grants 31870754, 81802662 and 81372342), the Natural Science Foundation of Guangdong Province (Grant 2017A030310329), the Shenzhen Peacock Innovation Team Project (Grant KQTD20140630100658078), the Key Laboratory Project of Shenzhen (Grant ZDSY20130329101130496), the Shenzhen Peacock Plan (Grants 827000183 and 827000186), and the Shenzhen Basic Research Program (Grants JCYJ20150525092941006, JCYJ20170302143447936 and JCYJ20170817094611664).
Disclosure
The authors report no conflicts of interest in this work.
References
Knippschild U, Gocht A, Wolff S, Huber N, Löhler J, Stöter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17(6):675–689. | ||
Knippschild U, Wolff S, Giamas G, et al. The role of the casein kinase 1 (CK1) family in different signaling pathways linked to cancer development. Onkologie. 2005;28(10):508–514. | ||
Cheong JK, Virshup DM. Casein kinase 1: complexity in the family. Int J Biochem Cell Biol. 2011;43(4):465–469. | ||
Price MA. CKI, there’s more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 2006;20(4):399–410. | ||
Schittek B, Sinnberg T. Biological functions of casein kinase 1 isoforms and putative roles in tumorigenesis. Mol Cancer. 2014;13(1):231. | ||
Knippschild U, Kruger M, Richter J, et al. The CK1 family: contribution to cellular stress response and its role in carcinogenesis. Front Oncol. 2014;4(1):96. | ||
Richter J, Ullah K, Xu P, et al. Effects of altered expression and activity levels of CK1delta and varepsilon on tumor growth and survival of colorectal cancer patients. Int J Cancer. 2015;136(12):2799–2810. | ||
Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. | ||
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. | ||
Macdonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. | ||
Cruciat CM. Casein kinase 1 and Wnt/β-catenin signaling. Curr Opin Cell Biol. 2014;31:46–55. | ||
Del Valle-Pérez B, Arqués O, Vinyoles M, de Herreros AG, Duñach M. Coordinated action of CK1 isoforms in canonical Wnt signaling. Mol Cell Biol. 2011;31(14):2877–2888. | ||
Liu C, Li Y, Semenov M, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108(6):837–847. | ||
Klimowski LK, Garcia BA, Shabanowitz J, Hunt DF, Virshup DM. Site-specific casein kinase 1epsilon-dependent phosphorylation of dishevelled modulates beta-catenin signaling. FEBS J. 2006;273(20):4594–4602. | ||
Swiatek W, Tsai IC, Klimowski L, et al. Regulation of casein kinase I epsilon activity by Wnt signaling. J Biol Chem. 2004;279(13):13011–13017. | ||
Cong F, Schweizer L, Varmus H. Casein kinase Iepsilon modulates the signaling specificities of dishevelled. Mol Cell Biol. 2004;24(5):2000–2011. | ||
Niehrs C, Shen J. Regulation of LRP6 phosphorylation. Cell Mol Life Sci. 2010;67(15):2551–2562. | ||
Etchegaray JP, Machida KK, Noton E, et al. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol Cell Biol. 2009;29(14):3853–3866. | ||
Brennan KC, Bates EA, Shapiro RE, et al. Casein kinase iδ mutations in familial migraine and advanced sleep phase. Sci Transl Med. 2013;5(183):183ra56, 1–11. | ||
Meng QJ, Maywood ES, Bechtold DA, et al. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc Natl Acad Sci U S A. 2010;107(34):15240–15245. | ||
Meng QJ, Logunova L, Maywood ES, et al. Setting clock speed in mammals: the CK1ε tau mutation in mice accelerates circadian pacemakers by selectively destabilizing period proteins. Neuron. 2008;58(1):78–88. | ||
Maywood ES, Chesham JE, Smyllie NJ, Hastings MH. The Tau mutation of casein kinase 1epsilon sets the period of the mammalian pacemaker via regulation of Period1 or Period2 clock proteins. J Biol Rhythms. 2014;29(2):110–118. | ||
Hirota T, Lee JW, Lewis WG, et al. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIα as a clock regulatory kinase. PLoS Biol. 2010;8(12):e1000559. | ||
Wang Z, Li B, Zhou L, et al. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc Natl Acad Sci U S A. 2016;113(46):13150–13155. | ||
Jiang J. CK1 in developmental signaling: hedgehog and Wnt. Curr Top Dev Biol. 2017;123:303–329. | ||
Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int J Oncol. 2017;51(5):1357–1369. | ||
Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2(2):a002915. | ||
Kim SY, Dunn IF, Firestein R, et al. CK1ε is required for breast cancers dependent on β-catenin activity. PLoS One. 2010;5(2):e8979. | ||
Hirner H, Günes C, Bischof J, et al. Impaired CK1 delta activity attenuates SV40-induced cellular transformation in vitro and mouse mammary carcinogenesis in vivo. PLoS One. 2012;7(1):e29709. | ||
Rosenberg LH, Lafitte M, Quereda V, et al. Therapeutic targeting of casein kinase 1δ in breast cancer. Sci Transl Med. 2015;7318(318):318ra202. | ||
Janovska P, Verner J, Kohoutek J, et al. Casein kinase 1 is a therapeutic target in chronic lymphocytic leukemia. Blood. 2018;131(11):1206–1218. | ||
De Francesco EM, Sotgia F, Lisanti MP. Cancer stem cells (CSCs): metabolic strategies for their identification and eradication. Biochem J. 2018;475(9):1611–1634. | ||
Lee SY, Jeong EK, Ju MK, et al. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16(1):10. | ||
Wu Y, Sarkissyan M, Vadgama JV. Epithelial-mesenchymal transition and breast cancer. J Clin Med. 2016;5(2):E13. |
Supplementary material
  | Figure S1 The basal expression of CK1α, CK1δ, and CK1ε in HEK293T cells detected by immunoblotting. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.