Back to Journals » OncoTargets and Therapy » Volume 11
Long-term survival with transarterial chemoembolization and radioembolization in a patient with cancers of unknown primary
Authors Aktas G , Kus T, Metin T, Kervancioglu S, Elboga U
Received 3 October 2017
Accepted for publication 6 February 2018
Published 4 April 2018 Volume 2018:11 Pages 1885—1889
DOI https://doi.org/10.2147/OTT.S153122
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ingrid Espinoza
Gokmen Aktas,1 Tulay Kus,2 Taylan Metin,3 Selim Kervancioglu,4 Umut Elboga5
1Department of Internal Medicine, Division of Medical Oncology, School of Medicine, University of Kahramanmaras Sutcu Imam, Kahramanmaraş, Turkey; 2Division of Medical Oncology, Adiyaman Training and Research Hospital, Adiyaman, Turkey; 3Department of Internal Medicine, School of Medicine, Gaziantep Oncology Hospital, University of Gaziantep, Gaziantep, Turkey; 4Department of Radiology, Faculty of Medicine, University of Gaziantep, Gaziantep, Turkey; 5Department of Nuclear Medicine, Faculty of Medicine, University of Gaziantep, Gaziantep, Turkey
Abstract: Cancers of unknown primary (CUP) are histologically proven metastatic malignant tumors without an identified primary site before treatment. The common characteristics are early dissemination, lower response to chemotherapy and poor prognosis with short life expectancy. Treatment was directed according to the presence of localized or disseminated disease. The most frequent site of metastasis is the liver, which is a suitable target organ for arterial-directed therapies. We report a case of 53-year-old woman who was diagnosed with CUP and suspected with intracellular cholangiocellular carcinoma (ICC), presented with a very large, unresectable, chemotherapy-refractory hepatic mass and treated with transarterial chemoembolization and transarterial radioembolization and surprisingly followed for 48 months with minimally progressive and stable disease. Arterial-directed therapies, an important therapeutic option in unresectable liver tumors, can provide survival benefit even for ICC and CUP which are very large in size.
Keywords: cancers of unknown primary, TACE, TARE
Introduction
Cancers of unknown primary (CUP) are histologically proven metastatic malignant tumors without an identified primary site before treatment. The common characteristics are early dissemination, lower response to chemotherapy and poor prognosis with short life expectancy such as a median overall survival (OS) of 6–9 months for patients with only lymph node metastasis and 2–4 months for patients with extranodal disease.1,2 Young age, good performance status (PS), patients with <3 organ sites involved, treated patients, the absence of liver metastases, a lower serum lactate dehydrogenase (LDH) and higher serum albumin levels were defined as favorable prognostic factors for CUP.1,3,4
Because CUP are mostly refractory to systemic therapy, treatment was directed according to the presence of localized or disseminated disease. The most frequent site of metastasis is the liver which is a suitable target organ for arterial-directed therapies. In patients with unresectable liver lesions, locoregional treatment options including transarterial chemoembolization (TACE), hepatic arterial chemotherapy and transarterial radioembolization (TARE) have been used to increase OS. Both TACE and TARE can be used together with chemotherapy to achieve more longer survival or as a monotherapy for patients with chemotherapy refractory disease as in our case.5,6 We report a case of 53-year-old woman who was diagnosed with CUP, presented with a very large, unresectable, chemotherapy-refractory hepatic mass and treated with TACE and TARE and surprisingly followed for 48 months with minimally progressive and stable disease.
Case report
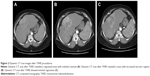
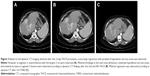
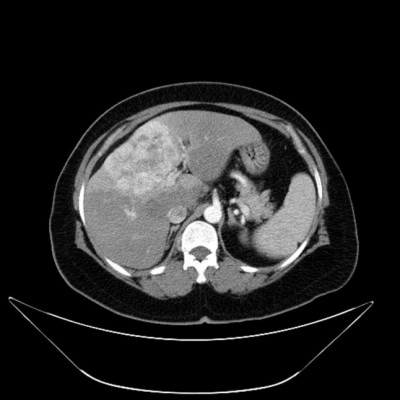
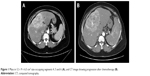
A 53-year-old female patient presented with blunt abdominal pain in the right upper quadrant. Liver ultrasonography showed a hypoechoic heterogeneous mass lesion with irregular lobule contours in the dimensions of 12 × 10 cm2 extending from segment 4 to segment 6, which was compatible with hepatocellular carcinoma. The present lesion was interpreted as hemangioma in upper abdominal magnetic resonance imaging (MRI). Blood counts and liver function tests were within normal limits in laboratory findings. Carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), carbohydrate antigen 15-3 (CA15-3), carbohydrate antigen 125 (CA 125) and alpha-fetoprotein (AFP) were within normal limits. In the dynamic liver computed tomography (CT) scan, 12 × 9 × 6.5 cm3 irregular lobule contoured lesions extending to liver segments 4, 5 and 6 were clearly visible in the early arterial phase, with delayed phase contrast (wash-out phenomenon), causing pressure on the left portal vein and portal hilus, and it was initially evaluated favorable for hepatocellular carcinoma (HCC) (Figure 1A). There was no other mass on systemic CT scans. Fine needle aspiration biopsy of the liver was reported as adenocarcinoma. Cytokeratin 7+, cytokeratin 20+, CEA focal+, estrogen-, WT-1-, TTF-1- and CDX2- reacted with established immunohistochemical stains (mason tricrom and reticulin staining were also applied). As a result, pathological report reported that differential diagnosis of cholangiocellular carcinoma and metastatic adenocarcinoma could not be performed. Upper and lower endoscopes, breast scans, gynecological examinations and ultimately positron emission tomography (PET) scan were performed to investigate the primary site, but no primary site was found. The patient was in the intermediate-risk group according to Ioannina Score for CUP Outpatient Oncologic Prognostication (I-SCOOP) with Eastern Cooperative Oncology Group (ECOG) PS of 1. Considering the diagnosis of CUP and cholangiocarcinoma as a possible diagnosis, carboplatin with gemcitabine, which is an effective systemic chemotherapy for both types of cancers, was initiated. However, after 3 cycles, >25% increase in the size of lesion (15 × 10 × 8 cm3) was detected in the CT, and FOLFOX-6 (oxaliplatin with 5-fluorouracil) chemotherapy was initiated as 2nd line chemotherapy. The patient was stable radiologically according to next 3-month evaluation. Chemotherapy could not be continued due to resistant thrombocytopenia that developed after 6 months of treatment and newly developed lymph nodes (Figure 1B). The patient was evaluated for arterial-directed treatments. The patient underwent TARE (Figure 2) for 3 times without stasis in total and irinotecan–lipidol (100 mg) TACE for 4 times based on intermittent CT images for 40 months (Figures 2 and 3). The resinous radiomicrospheres were used in intra-arterial Y-90 microsphere treatment. A council consisting of a gastroenterologist, a surgeon, a medical oncologist, an interventional radiologist and a nuclear medicine doctor was established for every treatment decision of the patient. The patient is still being followed with a stable disease. The patient had no complaints other than intermittent abdominal pain during the treatment. There were no side effects other than minimal liver enzyme elevation.
  | Figure 1 Mass in 12 × 9 × 6.5 cm3 size occupying segments 4, 5 and 6 (A), and CT image showing progression after chemotherapy (B). |
Written informed consent was obtained from the patient to have the case details and any accompanying images published.
Discussion
CUP consist of heterogeneous diseases that have variable clinical outcomes. Petrakis et al7 investigated estimation of death hazard of patients with CUP by clinical, pathological and laboratory parameters. Multivariate analysis showed that leukocytosis (hazard ratio [HR] 0.37, p = 0.001, cutoff <10,000/mm3 leukocytes), clinicopathological CUP subgroup (visceral vs nodal–neuroendocrine–mucinous peritoneal vs nodal–axillary–serous peritoneal–squamous head neck, HR 2.44, p = 0.001 for the visceral subgroup) and PS (0 vs 1, HR 0.58, p = 0.002 for PS 0–1) were independent prognostic parameters for survival. These 3 parameters made up I-SCOOP, which classify patients in low-, intermediate- and high-risk groups with median survival times of 36, 11–14 and 5–8 months, respectively.7 Although the presented case is classified as the intermediate-risk group with visceral involvement according to I-SCOOP, the patient survived for ~48 months with stable disease by using locoregional treatments. Moreover, our patient did not respond to 2nd line conventional chemotherapy, which is also a non-favorable prognostic factor. In this regard, this case presented an unexpected clinical course for CUP, suggesting that arterial-directed therapies are beneficial even for a very large hepatic mass of 15 cm.
Liver metastasis was defined as one of the poor prognostic factors for CUP, and patients with liver metastases are resistant to conventional chemotherapy as in our case.8,9 Thus, importance of arterial-directed therapies is increasing. TACE provides a dual therapeutic approach to tumors combining chemotherapeutic agents with hepatic artery embolization. This procedure increases the local chemotherapy concentration and reduces drug clearance from the liver. TARE using yttrium-90-resin microspheres ((90)Y-RE) delivers targeted radiation therapy to tumor and reduces oxygen and nutrient delivery to the tumor like TACE. These are applied principally for the treatment of unresectable hepatic metastatic colorectal carcinoma (CRC) and HCC. In the patients with HCC, Y90 microsphere treatment has been used either to convert non-resectable disease to a disease that is appropriate for resection/transplantation/local ablative therapies or to achieve better survival. In patients with CRC who progressed with systemic chemotherapy, progression-free survival for ~2.6–4.9 months and OS of 7.0–18.3 months had been obtained with TARE. Patients responding to radioembolization and patients with only liver disease have been shown to have a greater survival advantage for CRC. Although tumor size <5 cm and earlier stage are independent predictive and prognostic factors for arterial directed therapies in multivariate analysis, benefit can be provided even in very large hepatic masses and patients who presented stable disease after local therapies as in our case.5,10,11
There are a few studies including a small number of patients with CUP presenting the experiences of arterial-directed therapy.12,13 Kiefer et al14 applied chemoembolization with cisplatinum, doxorubicin, mitomycin C, ethiodol and polyvinyl alcohol primarily on patients who have intracellular cholangiocellular carcinoma (ICC) and adenocarcinoma of unknown primary. They obtained improved survival rates: median survival from time of first chemoembolization was 15 months, with 1- and 2-year survival of 61% and 27%, respectively. There was no statistically significant difference in survival of patients with ICC and adenocarcinoma of unknown primary. Improved OS was also seen in patients who received systemic chemotherapy (median OS: 28 months).14 ICC and CUP have poor prognosis with 1-year survival of 25% and 15%, respectively, and low response to systemic chemotherapies. Studies on biliary cancer treatment mostly included extrahepatic tumors, ampulla of Vater carcinoma and gallbladder carcinoma, which are not suitable for chemoembolization procedures.15 In this regard, ICC patients should be evaluated separately from other biliary cancers, as they may be appropriate for arterial-directed treatments. In the presence of unresectable hepatic metastases or ICC that may be available for arterial-directed treatments, local treatment and improved survival outcomes can be achieved even for patients with CUP, which is known to have short survival. It was not possible to distinguish ICC or CUP in our case, but a very good OS of 48 months was obtained by adding TACE and TARE treatments for these 2 chemotherapy-resistant and short-term survival cancers, as compared to the case series in the literature.
Conclusion
Arterial-directed therapies, an important therapeutic option in unresectable liver tumors, can provide survival benefit even for ICC and CUP which are very large in size.
Disclosure
The authors report no conflicts of interest in this work.
References
Hemminki K, Bevier M, Hemminki A, Sundquist J. Survival in cancer of unknown primary site: population-based analysis by site and histology. Ann Oncol. 2012;23(7):1854–1863. | ||
Hemminki K, Riihimäki M, Sundquist K, Hemminki A. Site-specific survival rates for cancer of unknown primary according to location of metastases. Int J Cancer. 2013;133(1):182–189. | ||
Chen KW, Liu CJ, Lu HJ, et al. Evaluation of prognostic factors and the role of chemotherapy in unfavorable carcinoma of unknown primary site: a 10-year cohort study. BMC Res Notes. 2012;5:70. | ||
Cosimelli M, Golfieri R, Cagol PP, et al; Italian Society of Locoregional Therapies in Oncology (SITILO). Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103(3):324–331. | ||
Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12(12):1711–1720. | ||
Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379(9824):1428–1435. | ||
Petrakis D, Pentheroudakis G, Voulgaris E, Pavlidis N. Prognostication in cancer of unknown primary (CUP): development of a prognostic algorithm in 311 cases and review of the literature. Cancer Treat Rev. 2013;39(7):701–708. | ||
Fehri R, Rifi H, Alboueiri A, et al. Carcinoma of unknown primary: retrospective study of 437 patients treated at Salah Azaiez Institute. Tunis Med. 2013;91(3):205–208. | ||
Lazaridis G, Pentheroudakis G, Fountzilas G, Pavlidis N. Liver metastases from cancer of unknown primary (CUPL): a retrospective analysis of presentation, management and prognosis in 49 patients and systematic review of the literature. Cancer Treat Rev. 2008;34(8):693–700. | ||
Golfieri R, Mosconi C, Giampalma E, et al. Selective transarterial radioembolisation of unresectable liver-dominant colorectal cancer refractory to chemotherapy. Radiol Med. 2015;120(8):767–776. | ||
Benson AB 3rd, Geschwind JF, Mulcahy MF, et al. Radioembolisation for liver metastases: results from a prospective 151 patient multi-institutional phase II study. Eur J Cancer. 2013;49(15):3122–3130. | ||
Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres – safety, efficacy, and survival. Radiology. 2008;247(2):507–515. | ||
Gulec SA, Mesoloras G, Dezarn WA, McNeillie P, Kennedy AS. Safety and efficacy of Y-90 microsphere treatment in patients with primary and metastatic liver cancer: the tumor selectivity of the treatment as a function of tumor to liver flow ratio. J Transl Med. 2007;5:15. | ||
Kiefer MV, Albert M, McNally M, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer. 2011;117(7):1498–1505. | ||
Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990–2009. World J Gastroenterol. 2009;15(34):4240–4262. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.