Back to Journals » OncoTargets and Therapy » Volume 15
Long-Term Survival of FOLFIRINOX +toripalimab in a Patient with Metastatic Pancreatic Ductal Adenocarcinoma: A Case Report
Authors Jiang T , Gao C, Luo Y , Ye Z, Wang B
Received 7 April 2022
Accepted for publication 3 August 2022
Published 25 August 2022 Volume 2022:15 Pages 883—890
DOI https://doi.org/10.2147/OTT.S369772
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Ting Jiang,1,2 Chen Gao,3 Yiyang Luo,4 Zixiang Ye,5 Binbin Wang1
1Department of Oncology, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 2Department of Oncology, The First Affiliated Rehabilitation Hospital of Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 3Department of Radiology, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 4First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China; 5Peking University China-Japan Friendship School of Clinical Medicine, Beijing, People’s Republic of China
Correspondence: Binbin Wang, Department of Oncology, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China, 310006, Tel +86-13588887210, Email [email protected]
Background: Pancreatic ductal adenocarcinoma (PDAC) remains one of the most fatal diseases, with its morbidity and mortality showing an upward trend. The application of monotonous immune checkpoint inhibitor (ICI) in PDAC comes to a disappointing endpoint, despite of its great advancements achieved in cancer treatment. However, a promising efficacy can be obtained on condition that ICIs are used in combination with chemotherapy.
Case: We reported a patient suffering from metastatic PDAC with proficient mismatch repair (pMMR) and low expression of programmed cell death ligand 1 (PD-L1). The patient survived for a remarkably long time and showed favorable tolerance to the combination of FOLFIRINOX+Toripalimab (a novel PD-1 inhibitor) administrated after chemoradiotherapy and targeted therapy. Today, the survival benefits gained from this therapy will continue to have a positive impact on him.
Conclusion: FOLFIRINOX+Toripalimab potentially serves as a novel therapeutic strategy for PDAC in late stage, with durable benefits and manageable toxicity in patients, which is still required to be validated in further research.
Keywords: pancreatic ductal adenocarcinoma, programmed cell death protein 1, chemotherapy, long-term survival, favorable tolerance
Plain Language Summary
Pancreatic ductal adenocarcinoma (PDAC) still remains one of the most fatal diseases with increasing morbidity and mortality. Due to the lack of effective treatment, the 5-year overall survival remains less than 5%, resulting in poor prognosis.
Recent years have witnessed the great progress of monotonous immune checkpoint inhibitor (ICI) achieved in cancer treatment. In contrast, its application in PDAC comes to a disappointing endpoint. Increasing evidences have proved the promising efficacy of ICIs combined with chemotherapy.
Toripalimab, a novel programmed cell death protein-1 (PD-1) antibody, has elicited robust efficacy in a slew of cancer types, but remains an unexplored efficacy in PDAC.
We reported a patient suffering from metastatic PDAC with programmed cell death ligand 1 (PD-L1) low expression and proficient mismatch repair (pMMR), who experienced a strinkingly long-term progression-free survival(over 30 months) and favorable tolerance to the combinative therapy of FOLFIRINOX+Toripalimab, followed by Toripalimab maintenance as the later-line treatment.
Therefore, FOLFIRINOX+Toripalimab could be a novel therapy as the later-line treatment for PDAC to prolong its survival.
Introduction
Of cancer-related death in human malignancies, pancreatic ductal adenocarcinoma (PDAC) ranks the fourth, with increasing morbidity and mortality.1 The difficulty in the early detection of PDAC attributes to its shady position, resulting in the diagnosis at the advanced stage. Over the past decade, a series of crucial Phase III clinical trials have introduced FOLFIRINOX (a combination of 5-fluorouracil, Leucovorin, Irinotecan, and Oxaliplatin) and AG (Nab-paclitaxel plus Gemcitabine) as the standard first-line treatment for PDAC patients.2,3 The POLO research has proposed Olaparib as the key therapy for germline BRCA1/2-mutated PDAC,4 however, the results showed that the 5-year overall survival (OS) remained less than 5%.5
Monotonous Pembrolizumab has been approved for pre-treatment in patients with deficient mismatch repair (dMMR)/microsatellite instability-high (MSI-H), or in patients with a high tumor mutation burden (TMB)≥10mut/Mb, regardless of cancer type.6,7 Unfortunately, this therapy showed disappointing results in PDAC as well, which probably due to its complicated immune-suppressive tumor environment, including dense desmoplastic stroma, low number of tumor-infiltrating lymphocytes (TILs), and high level of myeloid-derived suppressor cells (MDSCs). Worse still, PDAC is characterised by low possibility of dMMR/MSI-H and low TMB.8,9 In order to obtain an improved synergistic outcome, chemo-immunotherapy has now become a common practice. In untreated metastatic PDAC, the objective response rate (ORR) of CPI-613 (targeted on mitochondria) + FOLFIRINOX was 61%, resulting in a progression-free survival (PFS) of 9 months and an OS of 19 months.10 A recent study has found that using AG+Pembrolizumab as the first-line treatment in PDAC could achieve an improved PFS and OS of 8.1 months and 15 months, respectively.11 APX005M, a CD40 agonist that up-regulates the immune system, in combination with Nivolumab and AG, has achieved a disease control rate (DCR) of 92% in untreated PDAC.12 Toripalimab, a novel programmed cell death protein-1(PD-1) antibody developed by a Chinese company, has elicited robust efficacy in a slew of cancer types. However, little literature has been published on Toripalimab with a focus on PDAC. In this article, we shared a case of a metastatic PDAC patient who maintained a significant and persistent positive response to FOLFIRINOX+ Toripalimab, hoping to introduce a novel therapy for PDAC and contribute to the research in this field, so as to prolong the survival.
Case Report
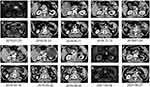
On January 03, 2018, the abdominal computerised tomography (CT) revealed a solid tumor (34*29mm) at pancreatic body without retroperitoneal lymph node metastasis of a 57-year-old male who suffered from nausea and vomiting for 2 months (Figure 1A and K). The serum level of carcinoembryonic antigen (CEA) was 10.4ng/mL(normal<5ng/mL) and that of cancer antigen 19–9(CA19-9) was 73.7U/mL (normal<37U/mL) (Figure 2). Subsequently, chest CT examination was performed and no lung metastasis was found. The patient’s medical history included type II diabetes, which was under control with regular medication. On January 08, 2018, the patient underwent a resection of the pancreatic body, tail and spleen. Postoperative pathology confirmed the poorly differentiated PDAC (50*40*40mm), along with 4 peripancreatic lymph node metastasis, among a total of 24 sites. Immunohistochemical findings showed the presence of CK(7) and Villin, and 22C3 antibody showed PD-L1(20%+), MLH1(+), MSH2(+), MSH6(+), PMS2(+), indicating proficient mismatch repair (pMMR) (Figure 3). On February 23, 2018, CT scan revealed that the patients had retroperitoneal lymph nodes metastasis (Figure 1L). Based on the above information, the patient was classified as T3N2M1 (stage IV) by the American Joint Committee on Cancer (AJCC) stage 8th edition. From February 25, 2018 to June 12, 2018, the patient received GS for 6 cycles every 3 weeks(Gemcitabine 1000mg/m2, day 1, day 8 +S-1 60mg orally twice per day, day 1–14). From April 26, 2018 to June 04, 2018, the patient also underwent radiotherapy for tumor and lymph nodes drainage area, with a dosage of 50.4Gy/28F, five times a week. The follow-up examinations indicated no recurrence in pancreas with shrunken retroperitoneal lymph nodes (Figure 1M) and normal boundaries of tumor markers (Figure 2).
 |
Figure 2 The serum monitoring of tumor markers. Abbreviations: CEA, carcinoembryonic antigen (ng/mL); CA19-9, cancer antigen 19–9 (U/mL). |
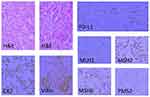 |
Figure 3 The histopathology and IHC of the PDAC tissues of this patient (200×). Abbreviations: IHC, immunohistochemistry; PDAC, Pancreatic ductal adenocarcinoma. |
On November 13, 2018, the PFS of the patients was 9 months. CT scan showed that the patient had PDAC-originated lung metastases on the upper lobe of the left lung (12*11mm) and the middle lobe of the right lung (15*13mm) (Figure 4A and B), as well as the enlarged retroperitoneal lymph nodes metastasis (Figure 1N). In addition, CEA was abnormally elevated to 12.6ng/mL (Figure 2). From November 24, 2018, the patient was administrated with 12mg of Anlotinib daily every 3 weeks for 14 days. Following 2 courses of Anlotinib, both lung lesions showed a partial regression with decreased tumor markers but retroperitoneal lymph nodes were enlarged (Figures 4C, D, 2 and 1O). Considering the regression of the lung lesions, we prescribed another 3 courses of Anlotinib, whereby the left lung lesion and retroperitoneal lymph nodes increased in size (Figures 4E, F and 1P). At this point, genetic testing for BRCA1/2 mutation (blood sample) was conducted, but it was found to be a wild-type. Since March 28, 2019, we prescribed the combined therapy of FOLFIRINOX (Oxaliplatin 85mg/m2, Leucovorin 400mg, Irinotecan 180mg/m2 all on day 1, followed by 5-fluorouracil 2400 mg/m2 delivered by continuous infusion for 46 hours) every 2 weeks, and Toripalimab (a fixed dose of 240mg, day 1) every 3 weeks. After 4 cycles of FOLFIRINOX and 3 cycles of Toripalimab, effective responses were observed, indicating the regressed bilateral pulmonary lesions and retroperitoneal lymph nodes and normal tumor markers (Figures 4G, H, 1Q and 2). Following another 4 cycles of FOLFIRINOX and 5 cycles of Toripalimab, the regressed bilateral pulmonary lesions remained stable and retroperitoneal lymph nodes further shrunk (Figures 4I, J and 1R). Since September 12, 2019, the patient was administrated with the monotonous Toripalimab maintenance treatment. By the time we reported this case, he had successfully received 8 cycles of FOLFIRINOX+Toripalimab and 27 cycles of Toripalimab maintenance therapy. The last time for Toripalimab maintenance was March 30, 2021. The patient had experienced PFS for more than 30 months with an ongoing effective response. During the regular follow-up of maintenance therapy, no pancreatic recurrence was observed, and the retroperitoneal lymph nodes were eliminated (Figure 1B–J, S and T). Both lung lesions and tumor makers remained stable within the normal range (Figures 4K–N and 2). The patient’s entire treatment timeline was displayed in Figure 5.
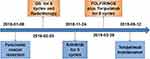 |
Figure 5 The entire treatment timeline of this patient. |
According to the Common Terminology Criteria for Adverse Events version 4.03, during the clinical process of FOLFIRINOX+Toripalimab, the patient developed grade II myelosuppression (white blood cell 2.7*10^9/L) and grade I transaminase elevation (alanine aminotransferase 104U/L, aspartate transaminase 63U/L), and grade II Peripheral neurotoxicity, all of which recovered after corresponding interventions. It is of note that on May 18, 2020, grade II immune-related hypothyroidism occurred (14 months after the initiation of Toripalimab, the 21st cycle). The testing of the thyroid function showed that thyroid-stimulating hormone (TSH) was 87.2mIU/L, total thyroxine 4(TT4) was 11.71nmol/L, free thyroxine 3(FT3) was 1.54pmol/L, and free thyroxine 4(FT4) was 5.15pmol/L. Ultrasonic meanwhile, showed diffuse thyroid disease. Following a daily administration of 150ug dose of levothyroxine for 6 months, the thyroid hormone returned to normal. No grade III or higher toxicities appeared and the patient showed favourable tolerance to this combinative therapy with a favorable quality of life correspondingly.
Discussion
To the best of our knowledge, this is the first time that a patient with metastatic PDAC has achieved remarkably long-term survival as a result of FOLFIRINOX+Toripalimab, followed by Toripalimab maintenance therapy. Several published results also supported the survival benefit of Toripalimab+chemotherapy for pancreatic cancer.13,14 According to the National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology (CSCO) guidelines, the patient with pMMR and low expression of PD-L1 (≥50% was regarded as high expression) was not sensitive to monotonous immunotherapy. However, it tends to shape PD-L1ʹs predicting role in pan-cancer when prescribing chemo-immunotherapy.15,16 For example, Food and drug administration (FDA) has approved Pembrolizumab plus chemotherapy in non-small cell lung cancer in 2017 with unrestricted PD-L1 expression based on the clinical trial Keynote-021.17 The patient has been previously treated with chemoradiotherapy and molecular targeted therapy, but the PFS was unsatisfactorily short. He and his families strongly desired for a more effective therapy to prolong the survival, but the second-line options in NCCN/CSCO guidelines for PDAC progressing from previous Gemcitabine or 5-fluorouracil-based regimens were lacking with quite short survival. The longest reported median OS of Nano-liposomal irinotecan (nal-IRI)+ 5-fluorouracil/leucovorin (5-FU/LV) was only 6.1 months.18 Considering that the patient had a good performance status (PS=0) with PD-L1(20%+) and was willing to fight for a prolonged survival, we initiated him the FOLFIRINOX plus Toripalimab. Through this, he has obtained a durable survival.
Immunotherapy has developed greatly in lung cancer and melanoma, however, current evidence from numerous trials has yielded pessimistic outcome in PDAC. A basket trial, KEYNOTE-028, aiming to evaluate the efficacy of Pembrolizumab across 20 types of PD-L1-positive cancer, enrolled 24 PDAC patients. However, the final result was negative without objective response, and PFS was 1.7 months shorter than that of the whole group(2.2 months).19 Another basket study revealed none of its 14 patients with advanced pancreatic cancer obtained an objective response to PD-L1 antibody BMS-936559.20 Therefore, monotonous immunotherapy may not act as well in PDAC as in other cancer types. This probably attributes to its immunologically “cold” environment – featured with dense desmoplastic stroma, low TILs levels, increased Tregs and MDSCs. Additionally, MSI-H/dMMR rarely occurs in PDAC, with an approximate incidence of less than 1%.8 Compared with high TMB(10mut/Mb) in non-small cell lung cancer, the average level of TMB in PDAC was only 1mut/Mb.9
Such good curative effect on Folfirinox+Toripalimab may possess underlying uniqueness, providing insights that deserve further research and exploration. The chemo-immunotherapy that the patient received was reported to have synergistic reactions. The mechanism of internal interaction involved that chemotherapy altering the immune-suppressive environment by increasing tumor immunogenicity, and depleting Tregs and MDSCs to facilitate the T cells killing of tumor cells. Studies have found that 5-fluorouracil and oxaliplatin are capable of inducing immunogenic apoptosis of cancer cells, as well as activating immune responses.21,22 5-fluorouracil accelerated MDSCs apoptosis and stimulated IFN-γ production by CD8+ T cell, thereby improving T cell-dependent anti-tumor reactions.23 Oxaliplatin can also up-regulate CD8+/Treg ratio and down-regulate MDSCs.24 This may be partly responsible for the long-term survival of the patient. Toripalimab is the first recombinant humanized anti-PD-1 inhibitor developed by China has shown potent antitumor activity in a variety of cancer types. Unlike other PD-1 inhibitors, it binds to the FG loop of the PD-1 receptor identified via unconventional three-dimensional structure analysis.25 Increasing studies have shown that Toripalimab alone or combined with chemotherapy exhibits promising effects and favourable tolerability in various malignancies and the adverse reactions to Toripalimab are acceptable and generally manageable.26,27
In recent years, an evergrowing number of efforts have been made to explore chemo-immunotherapy, with encouraging results in its ability to overcome primary resistance to PDAC. A clinical study has found that AG+Pembrolizumab as the first-line treatment of PDAC was superior to AG in the MPACT study in terms of PFS (8.1 versus 5.5 months) and OS (15 versus 8.5 months).3,11 CD40 also had a critical function in motivating the immune system. In a study on the efficacy of CD40 agonist (APX005M) combined with Nivolumab and AG in untreated PDAC, 58% (14/24) ORR was observed and 33% (8/24) achieved a stable response.12 Based on this, FDA has approved it as “orphan medicine” for the treatment of pancreatic cancer. Further study should be conducted despite of the promising efficacy of chemo-immunotherapy on pancreatic cancer.
As the patient was in the metastatic stage and had already received Toripalimab therapy (35 cycles) for 2 years, the follow-up treatment regimen remained controversial – whether to continue the immunotherapy maintenance or stop after 2 years. A list of phase III trials targeting multi-cancer types supported continuous immunotherapy for 2 years (35 cycles).28–30 In addition, this patient has experienced persistent benefits, but did experience immune-related hypothyroidism. Therefore, after 35 cycles of treatment with Toripalimab, we recommended him to follow up with close monitoring. However, due to the serious environment of novel Coronavirus Disease-2019 (COVID-19), the patient did not continue to follow up.
Nevertheless, several limitations do exist in this case report. We did not analyze and monitor the dynamic changes in the number ratio and functional status of immune cell subsets to tumor tissues during chemo-immunotherapy, such as the expression of CD4+T and CD8+T. The reasons are as follows. Due to the difficulty in obtaining the biopsy of metastatic retroperitoneal lymph nodes, we had to consider whether to test the tumor immune infiltrating cells via lung metastasis biopsy, but the patient strongly refused for personal reasons - he did not want to endure the repeated puncture biopsy and testing expense. This case report suggested the combinative therapy as a potentially novel therapy to improve the survival of PDAC. However, further validation is required due to the lack of solid evidence.
Conclusion
To sum up, we reported for the first time that the combinative therapy of FOLFIRINOX+Toripalimab has impressive efficacy and favourable tolerance in patients suffering from metastatic PDAC with PD-L1 positive and pMMR after chemoradiotherapy and targeted therapy. This novel strategy could be a potential alternative for the optimal performance of PDAC patients.
Ethics Statement
This case report was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang Chinese Medical University.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Funding
This work was supported by 2022 National Traditional Chinese Medicine administration Project for the Traditional Chinese Medicine Rehabilitation Service Capability Improvement (No. 2021-242).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi:10.1056/NEJMoa1011923
3. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi:10.1056/NEJMoa1304369
4. Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–327. doi:10.1056/NEJMoa1903387
5. Deplanque G, Demartines N. Pancreatic cancer: are more chemotherapy and surgery needed? Lancet. 2017;389(10073):985–986. doi:10.1016/S0140-6736(17)30126-5
6. Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med. 2017;377(15):1409–1412. doi:10.1056/NEJMp1709968
7. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, Phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi:10.1016/S1470-2045(20)30445-9
8. Hu ZI, Shia J, Stadler ZK, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res. 2018;24(6):1326–1336. doi:10.1158/1078-0432.CCR-17-3099
9. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi:10.1038/nature12477
10. Alistar A, Morris BB, Desnoyer R, et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, Phase 1 trial. Lancet Oncol. 2017;18(6):770–778. doi:10.1016/S1470-2045(17)30314-5
11. Weiss GJ, Blaydorn L, Beck J, et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs. 2018;36(1):96–102. doi:10.1007/s10637-017-0525-1
12. O’hara MH, O’reilly EM, Rosemarie M, et al. A Phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (Gem) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PDAC) patients. Cancer Res. 2019;79:13.
13. Shui L, Cheng K, Li X, et al. Durable Response and Good Tolerance to the Triple Combination of Toripalimab, Gemcitabine, and Nab-Paclitaxel in a Patient With Metastatic Pancreatic Ductal Adenocarcinoma. Front Immunol. 2020;11:1127. doi:10.3389/fimmu.2020.01127
14. Xu H, Wang X, Zhou S, Hu Q, Cao D. Efficacy of chemotherapy combined with toripalimab in PD-L1-positive and high tumor mutation burden pancreatic acinar cell carcinoma: case report. Tumori. 2021;107(6):NP24-NP27. doi:10.1177/0300891620980792
15. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi:10.1056/NEJMoa1810865
16. Kato K, Sun JM, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: the Phase 3 KEYNOTE-590 study. Ann Oncol. 2020;31:S1192–S1193. doi:10.1016/j.annonc.2020.08.2298
17. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi:10.1016/S1470-2045(16)30498-3
18. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545–557. doi:10.1016/S0140-6736(15)00986-1
19. Ott PA, Bang YJ, Piha-Paul SA, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37(4):318–327. doi:10.1200/JCO.2018.78.2276
20. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi:10.1056/NEJMoa1200694
21. Correale P, Aquino A, Giuliani A, et al. Treatment of colon and breast carcinoma cells with 5-fluorouracil enhances expression of carcinoembryonic antigen and susceptibility to HLA-A(*)02.01 restricted, CEA-peptide-specific cytotoxic T cells in vitro. Int J Cancer. 2003;104(4):437–445. doi:10.1002/ijc.10969
22. Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi:10.1038/onc.2009.356
23. Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–3061. doi:10.1158/0008-5472.CAN-09-3690
24. Gonzalez-Aparicio M, Alzuguren P, Mauleon I, et al. Oxaliplatin in combination with liver-specific expression of interleukin 12 reduces the immunosuppressive microenvironment of tumours and eradicates metastatic colorectal cancer in mice. Gut. 2011;60(3):341–349. doi:10.1136/gut.2010.211722
25. Zhang L, Hao B, Geng Z, et al. Toripalimab: the first domestic anti-tumor PD-1 antibody in China. Front Immunol. 2021;12:730666. doi:10.3389/fimmu.2021.730666
26. Mai HQ, Chen QY, Chen D, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021;27(9):1536–1543. doi:10.1038/s41591-021-01444-0
27. Wang ZX, Cui C, Yao J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (Jupiter-06): a multi-center phase 3 trial. Cancer Cell. 2022;40(3):277–288.e3. doi:10.1016/j.ccell.2022.02.007
28. Paz-Ares L, Vicente D, Tafreshi A, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15(10):1657–1669. doi:10.1016/j.jtho.2020.06.015
29. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi:10.1016/S0140-6736(21)00797-2
30. Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi:10.1016/S0140-6736(21)01234-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.