Back to Journals » Cancer Management and Research » Volume 12
Long-Term Survival After Nasopharyngeal Carcinoma Treatment in a Local Prefecture-Level Hospital in Southern China
Authors Du Y , Zhang W, Lei F, Yu X, Li Z, Liu X, Ni Y , Deng L, Ji M
Received 8 November 2019
Accepted for publication 31 January 2020
Published 24 February 2020 Volume 2020:12 Pages 1329—1338
DOI https://doi.org/10.2147/CMAR.S237278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Yun Du,1,2 Wentong Zhang,3 Feng Lei,4 Xia Yu,1 Zhuming Li,1 Xiaodong Liu,1 Yanan Ni,1 Li Deng,4 Mingfang Ji1
1Cancer Research Institute of Zhongshan City, Zhongshan City People’s Hospital, Zhongshan 528400, People’s Republic of China; 2Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Solna, Stockholm, Sweden; 3Department of Chinese Medicine, Southern Medical University, Guangzhou, People’s Republic of China; 4Department of Radiotherapy, Zhongshan City People’s Hospital, Zhongshan, People’s Republic of China
Correspondence: Mingfang Ji
Cancer Research Institute of Zhongshan City, Zhongshan City People’s Hospital, No. 2. Sunwen Road East, Zhongshan 528400, Guangdong, People’s Republic of China
Tel +86 135 3206 2222
Fax +86 076488822698
Email [email protected]
Purpose: NPC is a malignant and invasive tumor with the incidence rate of 19/100,000 per year in Zhongshan City, a prefecture city in southern China. Long-term survival analysis on intensity-modulated radiotherapy (IMRT)-based treatment in local prefecture-level hospitals have not been investigated. We aimed to evaluate the 5-year clinical outcomes and prognostic factors of NPC treated with IMRT in Zhongshan City People’s Hospital (ZSPH), a prefecture-level hospital in South China.
Patients and Methods: The number of 149 newly diagnosed non-metastatic NPC cases treated with IMRT were included from Zhongshan City People’s Hospital between January 2010 and December 2011. The survival outcomes, treatment toxicities and prognostic factors were analyzed by Kaplan-Meier method and Cox proportional hazards model.
Results: With a median follow-up period of 65 months for the cohort, the 5-year local recurrence-free survival (LRFS), regional recurrence-free survival (RRFS) and distant metastasis-free survival (DMFS) and overall survival (OS) were 86.80%, 94.80%, 86.10% and 80.50%, respectively. The 5-year OS rates were 100%, 95.2%, 87% and 67.2% for stage I, II, II and IVa-b, respectively (P=0.004). The 5-year LRFS rates were 97.2%, 96.0%, 90.4% and 72.0% for T1, T2, T3 and T4, respectively (P=0.001); the 5-year DMFS rates were 100% for T1, 96.8% for T2, 81.9% for T3 and 74.6% for T4 (P=0.022). A multivariate analysis revealed tumor stage as an independent prognostic factor for LRFS, DMFS and OS. No patients died from acute toxicities. Late toxicities were observed for 130 (87.2%) patients, and most late toxicities were graded I/II.
Conclusion: NPC treatment effect in a prefecture-level hospital in South China was comparable to international results and toxicities were tolerable. Tumour stage was an independent prognostic factor for survival outcome. More NPC survival data from local and remote places are needed.
Keywords: nasopharyngeal carcinoma, intensity-modulated, radiotherapy, prognosis, long-term survival results
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor derived from the nasopharyngeal epithelium. It has a unique, unbalanced and endemic distribution. Data1 show that 86,691 cases occurred in the world in 2012, and 81% of them occurred in Asia, with a rather high density in the cities of Zhongshan and Sihui of Guangdong, China.2 Given the deep and small anatomy and the highly radiosensitive feature of NPC, surgery is significantly challenging, and radiotherapy is the core curative treatment for NPC. In the era of two-dimensional conventional therapy (2D-CRT), a retrospective study of many cases, conducted by Lee et al3 reported that the 5-year LRFS (Local Recurrence-Free Survival), RRFS (Regional Recurrence-Free Survival), DMFS (Distant Metastasis-Free Survival) and OS (Overall Survival) rates were 66%, 67% and 62% and 52%, respectively. Later, Lee et al4 assessed the therapeutic gains between conventional radiotherapy and IMRT (Intensity-Modulated Radiation Therapy) and showed that IMRT was superior in terms of dose distribution and protecting organs at risk (OARs). Encouraging results from IMRT studies have been reported consistently. Sun et al5 reported the results of IMRT treatments on 868 NPC cases from the Sun Yat-Sen University Cancer Centre (SYSUCC) with 5-year LRFS rate of 91.8%, a RRFS rate of 96.4% and a DMFS rate of 84.6%. Wang et al6 reported the results of IMRT treatment on 695 cases of NPC from the Sichuan Cancer Hospital (SIH): the 5-year LRFS, RRFS, DMFS and OS were 89.8%, 95.2%, 74.1% and 77.1%, respectively. The treatment outcomes of NPC from the Guangxi Medical University Assistant Tumour Hospital (GXMUTH),7 Pamela Youde Nethersole Eastern Hospital (PYNE)8 and many other provincial or large college-affiliated hospitals were analyzed. It seems that in the past two decades, China has made great progress in NPC treatment.9 However, almost all studies were conducted in large provincial hospitals in China. In 2019 Chinese conference on oncology, Chinese National Cancer Center announced that 5-year OS rate was 47.3% (95% CI: 46.7–47.9%) according to population-based National Cancer Registry Database. It revealed the survival outcomes might not be satisfactory since most published data were released by large institutes where the technique and economic is advanced. In fact, most patients were treated in local prefecture-level hospitals due to financial problems, convenience near home and so forth. Despite, the analysis of patients from less-known prefecture-level hospitals are rarely seen. Few systematic studies have analyzed clinical outcomes of NPC cases from Zhongshan City People’s Hospital (ZSPH), which is located in a city with one of the highest cumulative incidence rates of NPC.2 Therefore, we performed a retrospective analysis of the long-term therapeutic effects and the prognostic factors for 149 nasopharyngeal carcinoma patients who received full-course IMRT between January 2010 and December 2011 at Zhongshan City People’s Hospital.
Materials and Methods
Study Patients
A total of 528 NPC patients were primarily diagnosed and pathologically confirmed between January 2010 and December 2011 at Zhongshan City People’s Hospital. 325 patients receiving 2D-CRT and 3-demensional conformal radiation therapy (3D-CRT) were excluded. Meanwhile, patients with distant metastasis, radiotherapy history before treatment were also excluded. Finally, 149 patients who had full-course IMRT and complete baseline clinical information were entered into the analysis. Written informed consent was obtained from all the patients before treatment.
Pre-Treatment Workup
Auxiliary examinations consisted of blood testing, fiberoptic nasopharyngoscopy, magnetic resonance imaging (MRI) of the nasopharynx and neck (computed tomography was used instead if the patient had an MRI test contraindication), chest radiography or computed tomography (CT), abdominal sonography or CT. Patients staged as N2-3 according to the 7th Union for International Cancer Control and American Joint Cancer Committee (UICC/AJCC) underwent 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET-CT).
Radiotherapy
All of the patients received IMRT that was performed according to guidelines. Each patient underwent a CT scan with serial 3 mm slices, from which two sets of images with and without contrast were obtained from the vertex through the clavicles. The target volume and OARs were defined according to the guidelines of the International Commission on Radiation Units and Measurements reports 50 and 62 (ICRU50 and 62). The primary nasopharyngeal gross tumour volume (GTVnx) and the involved cervical lymph nodes (GTVnd-L/R) were assessed by CT/MRI/PET-CT, clinical and endoscopic examinations. High-risk clinical target volume (CTV1) included GTVnx plus a 5- to 10-mm margin (a smaller margin for the primary tumour when it is adjacent to the brainstem or spinal cord). Low-risk clinical target volume (CTV2) included CTV1 plus a 5- to 10-mm margin, parapharyngeal spaces, posterior third of the nasal cavities and maxillary sinus, skull base, retropharyngeal nodal regions and the selective neck area (II, III, IVa and IVb). Planning target volume (PTV) was delineated as CTV plus a 3-mm margin. The total prescribed does were as follows: PTVnx 68.0 to 78.0 Gy, PTVnd 60.0 to 74.0 Gy, PTV1 60.0 to 68 Gy and PTV2 54.0 to 60.0 Gy in 30 to 37 fractions. The per-fraction dose was 1.8 to 2.2 Gy with five daily fractions per week for 6 to 7 weeks.
Chemotherapy
Of the 119 patients with stage III/IVa-b, chemotherapy was administered to 117 (98.3%) patients. Radiotherapy alone was administered to the remaining 2 patients. Concurrent chemotherapy (CCRT) was delivered to 4 patients, induction chemotherapy (ICT) + CCRT to 48 patients, CCRT + adjuvant chemotherapy (ACT) to 1 patient, ICT+CCRT+ACT to 47 patients, ICT to 13 patients, and ICT+ACT to 4 patients. Of the patients with stage I/II NPC, CCRT was delivered to 5 patients, ICT + CCRT to 6 patients, ICT to 3 patients and CCRT + AC to 1 patient. The ICT regimens included TPF (docetaxel 60 mg/m2 on day 1, cisplatin 60 mg/m2 on day 1 and fluorouracil 600 mg/m2/d continuously on days 1 through 5), PF (cisplatin 100 mg/m2 on day 1 and 5, Fu 1000 mg/m2/d continuously on day 1 through 5) and GP (gemcitabine 1 g/m2 on days 1 and 8 and cisplatin 80 mg/m2 on day 1) for 2 to 3 cycles. The CCRT regimens included cisplatin/nedaplatin 40 mg/m2 per week. For patients who received the ACT, PF, GP, TPF regimens (the same as ICT), treatment was repeated every 3 weeks for 1 to 3 cycles.
Endpoints and Follow-Up
The primary endpoint in the study was OS, and the secondary endpoints were LRFS, RRFS and DMFS. OS, LRFS, RRFS and DMFS referred to the duration from the date of pathological diagnosis of NPC to the date of any cause of death, local recurrence, regional lymph node recurrence or distant metastasis. During the treatment period, physical examinations and blood tests were performed weekly. Acute radiation toxicity was evaluated on a weekly basis in accordance with the Radiation Therapy Oncology Group (RTOG) criteria. After treatment, patients were recommended to be assessed regularly for treatment response and toxicity (every 3 months during the first 2 years and then every 6 to 12 months during years 3 to 5). Each follow-up examination included a blood test, physical examination, ultrasonography of the abdomen and chest X-ray, and a CT/MR of the head and neck region. Late toxicities were defined according to the RTOG scoring criteria and the Common Terminology Criteria for Adverse Events (Version 3.0). Late reactions referred to those that occurred 3 months after radiotherapy or those that lasted for longer than 3 months. Patients with regular follow-up examinations were entered into the analysis of late toxicities. Patients with signs of local-regional recurrence, distant metastasis or severe complications received additional tests.
Statistical Analysis
The statistical analysis was performed using the SPSS 20.0 software, and the survival proportion was calculated by the Kaplan-Meier method and tested by Log rank test. The multivariate Cox proportional hazards model was conducted to estimate hazard ratios (HRs) and 95% confidence intervals (CIs); age, gender, Karnofsky performance score (KPS), radiation dose, T category, N category, overall stage and chemotherapy were included as covariates. All statistical tests were two-sided, and P < 0.05 was defined as statistically significant.
Results
Baseline Characteristics
The baseline demographic and clinical characteristics of the patients are detailed in Table 1. For the enrolled cohort, the male (n =119)-to-female (n =30) ratio was 4:1, and the median age was 45 (range, 12 to 74 years) years old. The proportions of patients classified as stage I, II, III and IVa-b were 6%, 14.1%, 36.2% and 43.6%, respectively. Overall, 135 (90.6%) patients received chemotherapy. The median radiation GTVnx dose given to the subjects was 74 Gy (range, 56 to 80 Gy). The median follow-up duration was 65 months (range, 6 to 83 months).
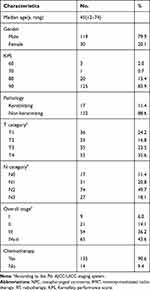 |
Table 1 Baseline Characteristics of the 149 Patients with NPC Receiving IMRT |
Overall Survival and Failure Pattern
Altogether, 29 (19.5%) subjects died. The 3- and 5-year OS rates for the whole series were 89.9% and 80.5%, respectively. As is shown in Figure 1D and Table 2, the 5-year OS rates were 100%, 95.2%, 87% and 67.2% for stage I, II, II and IVa-b, respectively (P=0.004). Of the 149 patients, 45 subjects developed failure after treatment. The failed cases included 20 cases of local failure, 7 cases of regional failure, and 21 cases of distant metastasis. The cumulative survival incidence is shown in Table 3,5,6,8,10–15 together with the reported data from previous literature.
 |
Table 2 Univariate Analysis on Clinical Stage of NPC Patients Receiving IMRT |
 |
Table 3 Comparison NPC Treatment Outcomes from Different Institutes in the IMRT Era |
 |
Table 4 Multivariate Analysis of Prognostic Factors for NPC Patients Receiving IMRT |
 |
Table 5 Acute Radiation Reaction of IMRT on Patients with Primary NPCa |
 |
Table 6 Incidence of Major Late Toxicities for the 149 Patients Receiving IMRT |
Local Control
With a median interval of 19 months (range, 6 to 53 months), 20 (13.4%) patients developed local recurrence, including 17 in the nasopharynx, 2 in the skull base and 1 in the sphenoid sinus. The corresponding 3- and 5-year LRFS rates for the series were 91.1% and 86.8% (Table 2). The 5-year LRFS rates were 97.2%, 96.0%, 90.4% and 72.0% for T1, T2, T3 and T4, respectively, and T stage significantly affected 5-year LRFS (P=0.001) (Figure 1A).
Regional Control
With a median interval of 30 months (range, 13 to 57 months), 7 (4.7%) patients developed regional lymph node recurrence, of which 5 cases were combined with local recurrence. The corresponding 3-and 5-year RRFS were 97.2% and 94.8%, respectively. RRFS and T, N and overall stages were not statistically significant (P>0.05) (Figure 1B and Table 2).
Distant Control
By the last follow-up day, distant metastasis was found in 21 (14.1%) patients, including 10 in bone, 11 in lung, 7 in liver and 1 in the parotid. Of the 21 patients with distant metastasis, 18 (85.7%) cases of distant metastasis occurred in the first 3 years. The actuarial 3-and 5-year DMFS were 87.6% and 86.1%, respectively. The cumulative incidence of DMFS was associated strongly with N-stage (P=0.022): DMFS was 100% for N0, 96.8% for N1, 81.9% for N2 and 74.6% for N3 cases (Figure 1C and Table 2).
Prognostic Factors
A multivariate analysis was performed to screen for independent prognostic factors, and the results are presented in Table 4. Tumour stage was the key prognostic factor of LRFS, DMFS and OS (P< 0.05 for all rates, shown on Table 4). Intriguingly, the DMFS for females seemed to be better than for males; however, the difference was only marginally significant (HR, 6.45; 95% CI, 0.86–48.15; P = 0.069). Additionally, age was an independent factor for OS (HR, 3.5, 95% CI: 1.06–11.62, P=0.041).
Irradiation Complications
Acute Toxicities
Therapy-related adverse events were evaluated during the entire treatment. None of the patients cancelled radiotherapy, but 10 patients with stage IVa-b disease gave up concurrent chemotherapy due to the serious induction of chemotherapy toxicities. The incidences not less severe than grade 3, including acute mucositis, dermatitis, haematological toxicity and acute xerostomia occurred at a rate of 20.8%, 22.1%, 9.40% and 0%, respectively (Table 5). Five patients (3.4%) with large cervical lymph nodes were classified as having a grade 4 acute dermatitis, and 3 cases (2%) were defined as grade 4 haematological toxicity.
Late Toxicities
Data on the late toxicities of 130 cases (87.2%) is detailed in Table 6. The major symptoms were mild or moderate late injuries, in which hearing impairment (25/130, 19.2%), tinnitus (12/130, 9.2%), xerostomia (107/130, 82.3%) and temporal lobe injury (17/130, 13.1%) were the most common reactions affecting the quality of life of the patients. One patient with local-recurrence suffered nasopharyngeal necrosis after re-radiotherapy and died of nasopharyngeal massive haemorrhage; one patient experienced grade 4 dysphagia and was subsequently assisted with gastric fistula and parenteral nutrition support; and one patient developed double stress mixed deafness.
Discussion
The results of our study show that IMRT achieved satisfactory survival outcomes in treating NPC with tolerable toxicities, compared to 2D-CRT and 3D-CRT, even in a prefecture-level and non-affiliated hospital in China. Clinical stage was an obvious prognostic factor associated with LRFS, DMFS and OS, but not RRFS.
We reported that the 5-year OS rates were 100%, 95.2%, 87% and 67.2% for stage I, II, II and IVa-b, respectively, after IMRT treatment in ZSPH. These results demonstrate that the efficacy of IMRT is high, compared to traditional radiotherapy. Lee et al3 reported that LRFS, RRFS, DMFS and OS were 66%, 67%, 62% and 52%, respectively, by 2D-CRT, while in the present study, the treatment outcomes were increased by 20.8%, 27.8%, 24.1% and 28.5% respectively. We proposed several possible reasons for this progress. First, the accuracy of IMRT can effectively protect the OARs while also improving the dose in gross,16 thus substantially improving the local-regional control of NPC.17 Second, the median dose in the study by Lee et al was 65 Gy; the dose of radiotherapy was an independent prognostic factor for local control, and a dose of 70 Gy was recommended.18 Third, the application of chemotherapy is more widely used in the current IMRT age, with the benefit that chemotherapy can increase the sensitivity of radiotherapy,19 and reduce the risk of distant metastasis.20 Fourth, imaging examination in the IMRT era is superior to that of the past, an improvement which facilitates better treatment. We also observed that T and N stage and age were independent predictors for OS in the multivariate Cox proportional hazards model analysis, which was consistent with previous studies21–23 and which demonstrated the rationality of the staging system based on imaging guidance in the IMRT age.
The present study showed that LRFS was correlated with T stage, and the LRFS of T1-2 cases was significantly higher than that of T3-4 cases in the multivariate analysis (HR=4.51, 95% CI: 1.33–15.1, P=0.017). The multivariate analysis indicated that T stage was a prognostic factor for LRFS. Several articles5,14,24 also support this conclusion, since IMRT technology enables coverage of irregularly shaped tumours while limiting the dose to critical organs. The 5-year LRFS for stages T1, T2, T3, and T4 were 97.2%, 96%, 90.4%, and 72%, respectively, and the overall 3-year and 5-year LFRS was 91.1% and 86.8%. The LRFS rates of patients with T1-3 stage disease were comparable to those observed at other provisional-level or international institutes,5,13 but the LRFS rate for stage T4 was lower. The 5-year LRFS rate was slightly lower in the present study than that observed at other large institutes. We propose possible reasons for this result: 10 cases with stage T4 disease received less than three cycles of concurrent chemotherapy because of patient refusal and treatment toxicities. Previous studies25,26 have shown that the total dose of concurrent chemotherapy has a potential effect on loco-regional control. Interestingly, we found in the stratified analysis that LRFS rate was significantly associated with N stage (P=0.023) (Table 2). However, in the multivariate analysis, no positive conclusions were obtained. Referring to previous literature,13,24 significant correlations have not been found between LRFS and N stage. Consequently, we considered that the correlation found in this study might have been caused by an error of the single factor analysis in Log-rank, and more research is warranted.
This study showed that the 5-year RRFS rate was 94.4%, which is comparable to other studies. Results also showed that N stage was not a prognostic factor for RRFS and that finding was consistent with previous studies.5,7,13 However, the relationship between RRFS and N stage was controversial. Previous studies4,24 have shown that RRFS was correlated with N stage, and as the T stage elevated, LRFS gradually decreased. Therefore, the prognostic factors of RRFS warrant further exploration.
In our series, 21 patients developed distant metastasis, with a 5-year DMFS rate of 86.1%. Moreover, distant metastasis accounted for 44.7% of the NPC failure patterns, which was approximately half of the rate (73.6%) reported by Wang et al.6 Apparently, in comparison to other results in Table 6, we achieved a satisfactory DMFS. An explanation to that is that in this study, 112 (94.1%) patients with stage III and IV received ICT, and in the IMRT era, ICT applied in loco-regionally advanced nasopharyngeal carcinoma can significantly reduce the risk of distant metastasis.20,27–29 In addition, N stage was an independent predictor for DMFS in the multivariate Cox proportional hazards model analysis due to lymphatic drainage into the blood. That finding was consistent with the latest research.21,22,30
In general, acute and chronic toxicities were tolerable. Acute injuries grade 3 and above accounted for less than 30% of all toxicities, and those injuries improved after symptomatic treatment. The proportion of severe radiation injury was much lower than what was typically seen in the 2D-CRT age.3 Chronic radiation injuries, including hearing system damage, chronic xerostomia, and brain damage, were the most troublesome injuries observed in this study. Fortunately, most patients with severe hearing damage can be treated with ear canal surgery. However, temporal lobe injury remained at a high level (17/130, 12.1%) since most cases were advanced at diagnose.
However, this research has several limitations. First, patients with stage I disease only accounted for 6.0% of the cohort, due to atypical symptoms. Second, the regimens are slightly different from the National Comprehensive Cancer Network (NCCN) guidelines. However, it is inevitable since patients’ refusal and financial conditions have to be considered in Prefecture-level Hospitals in China. Third, the follow-up period was not long enough. Forth, patients with distant metastasis, radiotherapy history before treatment were excluded. Thus, the true survival outcome in the hospital might be a little less satisfactory than analysis above. In future research, we will enlarge the sample size, include patients with distant metastasis and incomplete radiotherapy and continue to monitor the long-term quality of life of the patients.
In conclusion, our long-term survival results further demonstrate that IMRT plays an essential role in the treatment of NPC. Moreover, this study provides NPC treatment data in a prefecture-level hospital located in one of the highest epidemic city in South China. The data showed satisfactory survival outcome and provide evidence for NPC patients in local high-risk areas. Since patients could choose to accept treatment in local hospitals, they might have better family care and nutrition which is essential for prognosis.31 It’s good for the sanitary system. But still, more NPC survival data from local and remote places are needed.
Ethics Statement
This study was approved by research ethics committee of Zhongshan City People’s Hospital regarding the consents of patient or a parent for patients under 18 years old for radiotherapy and chemotherapy. This study was conducted in accordance with the Declaration of Helsinki. We also confirm that the privacy of the participants was anonymized.
Acknowledgments
We acknowledge support from the Natural Science Foundation of China, Grant No. 81572062 and language editing from American Journal Experts.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4(9):e609–e616. doi:10.1016/S2214-109X(16)30143-7
2. Wei KR, Yu YL, Yang YY, et al. Epidemiological trends of nasopharyngeal carcinoma in China. Asian Pac J Cancer Prev. 2010;11(1):29–32.
3. Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23(2):261–270. doi:10.1016/0360-3016(92)90740-9
4. Lee AW, Ng WT, Chan LL, et al. Evolution of treatment for nasopharyngeal cancer–success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110(3):377–384. doi:10.1016/j.radonc.2014.02.003
5. Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403. doi:10.1016/j.radonc.2013.10.020
6. Wang W, Feng M, Fan Z, Li J, Lang J. Clinical outcomes and prognostic factors of 695 nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Biomed Res Int. 2014;2014:814948.
7. Chen X, Zhu X, Liang Z, et al. Long-term outcomes of neoadjuvant chemotherapy followed by concurrent chemoradiotherapy (CCRT) vs CCRT alone for nasopharyngeal carcinoma in the era of intensity-modulated radiation therapy using propensity score matching method. Onco Targets Ther. 2017;10:2909–2921. doi:10.2147/OTT.S135590
8. Ng WT, Lee MC, Hung WM, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011;79(2):420–428. doi:10.1016/j.ijrobp.2009.11.024
9. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi:10.1016/S0140-6736(19)30956-0
10. Cao CN, Luo JW, Gao L, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for T4 nasopharyngeal carcinoma. Oral Oncol. 2013;49(2):175–181. doi:10.1016/j.oraloncology.2012.08.013
11. Wang L, Guo Y, Xu J, et al. Clinical analysis of recurrence patterns in patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Ann Otol Rhinol Laryngol. 2017;126(12):789–797. doi:10.1177/0003489417734229
12. Jiang F, Jin T, Feng XL, Jin QF, Chen XZ. Long-term outcomes and failure patterns of patients with nasopharyngeal carcinoma staged by magnetic resonance imaging in intensity-modulated radiotherapy era: the Zhejiang Cancer Hospital’s experience. J Cancer Res Ther. 2015;11(Suppl 2):C179–C184. doi:10.4103/0973-1482.168181
13. Zhao W, Lei H, Zhu X, Li L, Qu S, Liang X. Investigation of long-term survival outcomes and failure patterns of patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: a retrospective analysis. Oncotarget. 2016;7(52):86914–86925. doi:10.18632/oncotarget.v7i52
14. Wolden SL, Chen WC, Pfister DG, Kraus DH, Berry SL, Zelefsky MJ. Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer: update of the Memorial Sloan-Kettering experience. Int J Radiat Oncol Biol Phys. 2006;64(1):57–62. doi:10.1016/j.ijrobp.2005.03.057
15. Tham IW, Hee SW, Yeo RM, et al. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy-the national cancer centre singapore experience. Int J Radiat Oncol Biol Phys. 2009;75(5):1481–1486. doi:10.1016/j.ijrobp.2009.01.018
16. Tuan JK, Ha TC, Ong WS, et al. Late toxicities after conventional radiation therapy alone for nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):305–311. doi:10.1016/j.radonc.2011.12.028
17. Zhang MX, Li J, Shen GP, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer. 2015;51(17):2587–2595. doi:10.1016/j.ejca.2015.08.006
18. Kam MK, Teo PM, Chau RM, et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60(5):1440–1450. doi:10.1016/j.ijrobp.2004.05.022
19. Chen YP, Wang ZX, Chen L, et al. A Bayesian network meta-analysis comparing concurrent chemoradiotherapy followed by adjuvant chemotherapy, concurrent chemoradiotherapy alone and radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2015;26(1):205–211. doi:10.1093/annonc/mdu507
20. Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a Phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–1520. doi:10.1016/S1470-2045(16)30410-7
21. Ren Y, Qiu H, Yuan Y, et al. Evaluation of 7th edition of AJCC staging system for nasopharyngeal carcinoma. J Cancer. 2017;8(9):1665–1672. doi:10.7150/jca.19197
22. Liang ZG, Chen XQ, Niu ZJ, et al. Recommendations for updating T and N staging systems for nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy. PLoS One. 2016;11(12):e0168470. doi:10.1371/journal.pone.0168470
23. OuYang PY, Zhang LN, Tang J, et al. Evaluation of body mass index and survival of nasopharyngeal carcinoma by propensity-matched analysis: an observational case-control study. Medicine. 2016;95(2):e2380. doi:10.1097/MD.0000000000002380
24. Wu LR, Liu YT, Jiang N, et al. Ten-year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: an analysis of 614 patients from a single center. Oral Oncol. 2017;69:26–32. doi:10.1016/j.oraloncology.2017.03.015
25. Lee AW, Tung SY, Ngan RK, et al. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 trials. Eur J Cancer. 2011;47(5):656–666. doi:10.1016/j.ejca.2010.10.026
26. Loong HH, Ma BB, Leung SF, et al. Prognostic significance of the total dose of cisplatin administered during concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):300–304. doi:10.1016/j.radonc.2011.12.022
27. Hui EP, Ma BB, Leung SF, et al. Randomized Phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Onco. 2009;27(2):242–249. doi:10.1200/JCO.2008.18.1545
28. OuYang PY, Zhang LN, Xiao Y, et al. Validation of published nomograms and accordingly individualized induction chemotherapy in nasopharyngeal carcinoma. Oral Oncol. 2017;67:37–45. doi:10.1016/j.oraloncology.2017.01.009
29. Zhang LL, Zhou GQ, Li YC, et al. Induction chemotherapy has no prognostic value in patients with locoregionally advanced nasopharyngeal carcinoma and chronic hepatitis B infection in the IMRT era. Transl Oncol. 2017;10(5):800–805. doi:10.1016/j.tranon.2017.07.001
30. Ai QY, King AD, Mo FKF, et al. Prediction of distant metastases from nasopharyngeal carcinoma: improved diagnostic performance of MRI using nodal volume in N1 and N2 stage disease. Oral Oncol. 2017;69:74–79. doi:10.1016/j.oraloncology.2017.04.008
31. Huang JF, Sun RJ, Jiang WJ, et al. Systematic nutrition management for locally advanced nasopharyngeal carcinoma patients undergoing radiotherapy. Onco Targets Ther. 2019;12:8379–8386. doi:10.2147/OTT.S213789
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

