Back to Journals » Journal of Pain Research » Volume 10
Long-term safety and analgesic efficacy of buprenorphine buccal film in patients with moderate-to-severe chronic pain requiring around-the-clock opioids
Authors Hale M, Urdaneta V, Kirby MT, Xiang Q, Rauck R
Received 19 August 2016
Accepted for publication 14 October 2016
Published 25 January 2017 Volume 2017:10 Pages 233—240
DOI https://doi.org/10.2147/JPR.S120170
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Martin Hale,1 Veronica Urdaneta,2 M Todd Kirby,3 Qinfang Xiang,4 Richard Rauck5
1Gold Coast Research, LLC, Plantation, FL, USA; 2Pharmacovigilance and Risk Management, 3Clinical Development, 4Biometrics, Endo Pharmaceuticals Inc., Malvern, PA, USA; 5Carolinas Pain Institute, Wake Forest Baptist Health, Winston Salem, NC, USA
Background: This open-label, single-arm study was conducted to evaluate the long-term safety and efficacy of a novel buprenorphine formulation, buprenorphine buccal film, in the treatment of moderate-to-severe chronic pain requiring around-the-clock opioids.
Methods: The primary purpose of this study was to evaluate the long-term safety and tolerability of buprenorphine buccal film. Five hundred and six patients who completed previous studies with buprenorphine buccal film (n=445; rollover patients) or were recruited de novo for this study (n=61) were enrolled in this study. All patients underwent a dose titration period of ≤6 weeks, during which doses of buprenorphine buccal film were adjusted to a maximum 900 µg every 12 hours, depending on tolerability and the need for rescue medication. An optimal dose was defined as the dose that the patient found satisfactory for both pain relief and tolerability, without the need for rescue medication or with ≤2 tablets of rescue medication per day. Once the optimal dose was reached, treatment was continued for ≤48 weeks. Pain intensity was measured throughout the study using a 0–10 numerical rating scale.
Results: Of 435 patients achieving an optimal dose of buprenorphine buccal film who commenced long-term treatment, 158 (36.3%) completed 48 weeks of treatment. Treatment-related adverse events occurred in 116 patients (22.9%) during the titration phase and 61 patients (14.0%) during the long-term treatment phase, and adverse events leading to discontinuation of treatment occurred in 14 (2.8%) and 14 (3.2%) patients, respectively. The most common adverse events were those typically associated with opioids, such as nausea, constipation, and headache. In both rollover and de novo patients, pain intensity scores remained constant at approximately 3–4 during long-term treatment, and the dose of buprenorphine buccal film remained unchanged in 86.2% of patients.
Conclusion: In appropriate patients, buprenorphine buccal film demonstrated tolerability and efficacy in the long-term management of chronic pain.
Keywords: buccal drug administration, buprenorphine, chronic pain management, long-term treatment, opioid analgesics
Introduction
Chronic pain is a significant health problem in the USA, and its effective management remains a major public health goal. The scale of the problem is highlighted by a cross-sectional internet-based survey of a representative sample of the national adult population, in which the weighted point prevalence estimate of chronic pain (defined as chronic, recurrent, or long-lasting pain of at least 6 months duration) was 30.7%.1 In this survey, the most common physician-diagnosed conditions resulting in primary chronic pain were lower back pain (3,197 responders) and osteoarthritis (2,439 responders), which accounted for 18% and 16%, respectively, of the overall prevalence.1 The high prevalence of chronic pain also equates to substantial economic costs. The total costs associated with pain (including both health care costs and indirect costs resulting from lost productivity) have been estimated to range from $560 to $635 billion annually (in 2010 dollars), which is higher than the annual costs associated with heart disease, cancer, or diabetes.2
Clinical guidelines for the management of chronic pain state that opioids may have an important role in its treatment.3,4 The efficacy of opioids has been well established, and currently, approximately 90% of patients with chronic pain receive opioid analgesics as a part of their pain management.5,6 However, the potential benefits of opioids in the management of pain may be limited by adverse events such as constipation, respiratory depression, and sedation.5 Additionally, studies have suggested that up to 29% of patients with chronic pain may be misusing or abusing opioid medications, which may limit their use.6–8
Buprenorphine is a semisynthetic opioid that may offer an alternative to μ-opioid agonists. Buprenorphine exhibits partial agonism at µ-opioid receptors9–12 while maintaining a relative potency, compared with oral morphine, of between 75:1 and 115:1.12,13 In addition to partial agonism at µ-opioid receptors, buprenorphine is a κ-opioid receptor antagonist and appears to act as a “chaperone” ligand, increasing the expression of µ-opioid receptors on cell membranes.12,14 It also has agonist activity at opioid receptor-like 1 (ORL1) receptors that confers both an additive analgesic effect (through activation of receptors at the dorsal horn) and an inhibitory effect (through activation of receptors in the brain). Activation of these receptors also leads to blockade of the rewarding effects of morphine, which suggests that ORL1 receptors may contribute to the limited tolerance observed with buprenorphine.12,15
A recently approved buccal film formulation of buprenorphine (Belbuca™; Endo Pharmaceuticals, Malvern, PA, USA) has been developed to provide flexible dose titration across a wide range of doses (up to 160 mg morphine sulfate equivalent [MSE]). This preparation uses BioErodible MucoAdhesive (BEMA®) technology, which produces a flexible, water-soluble film that adheres to the buccal mucosa and usually dissolves within 30 minutes of application.16,17 Studies have shown that this formulation has an absolute bioavailability of 46%–65%, with dose-proportional increases in systemic exposure over a 16-fold dose range.17,18
The efficacy and tolerability of buprenorphine buccal film have been demonstrated in opioid-naïve19 and opioid-experienced20 patients with moderate-to-severe chronic lower back pain. The aim of the present study (NCT01755546) was to evaluate the long-term safety and efficacy of this formulation in patients with moderate-to-severe chronic pain requiring around-the-clock opioid analgesia.
Methods
The trial was an open-label single-arm study conducted at 58 sites in the USA. It consisted of a dose titration phase lasting up to 6 weeks and a long-term treatment phase of up to 48 weeks. The study was conducted in accordance with the ethical principles of Good Clinical Practice and the Declaration of Helsinki; the protocol was approved by Schulman Associates Institutional Review Board (Cincinnati, OH, USA), and written informed consent was obtained from all patients before participation.
Patients
The study included patients with chronic lower back pain who had completed the 12-week double-blind phase of the studies as opioid-naïve19 and opioid-experienced20 patients (rollover patients), and a further cohort of newly recruited patients (de novo patients). De novo patients were required to be at least 18 years of age and to have at least a 3-month history of chronic pain, including chronic lower back pain with or without neuropathic involvement; osteoarthritis of the hip, knee, or lumbosacral spine; or peripheral neuropathic pain. These patients were required to have been receiving stable daily maintenance doses of around-the-clock opioid analgesics equivalent to ≥60 mg and ≤160 mg MSE per day for at least 4 weeks.
Rollover patients from the opioid-naïve19 and opioid-experienced20 studies were required to have been at an optimal dose of buprenorphine buccal film of ≥300 µg every 12 hours (q12h) during the open-label titration phase of these studies. All patients (de novo and rollover) were required to have average pain intensity scores of ≤5, measured on a 0–10 numerical rating scale (NRS) (0 indicates “no pain” and 10 indicates “pain as bad as you can imagine”), at their last two consecutive visits during the titration phase.
Exclusion criteria included clinically significant conditions that could affect the patient’s safety or the validity of the study findings; pain due to acute spinal cord compression, cauda equina compression, acute nerve root compression, meningitis, or discitis; current cancer-related pain or chemotherapy in the past 6 months; surgical procedures for pain relief within 3 months or nerve/plexus blockade within 28 days before screening; and current or past substance abuse.
Treatment and assessments
Prior to the dose titration period, de novo patients underwent an analgesic taper phase of up to 4 weeks, during which average pain intensity was recorded on an NRS, as described earlier, every 4–8 days. De novo patients with a total daily opioid dose of >30 mg oral MSE had their dose tapered down to a maximum of 30 mg oral MSE per day (excluding rescue medication), after which they entered the dose titration phase. During the taper phase, a Clinical Opiate Withdrawal Scale (COWS) assessment21 was performed at each clinic visit to ensure that patients were not experiencing clinically significant opioid withdrawal symptoms. Patients showing moderate or higher opioid withdrawal symptoms (COWS score ≥1321) were treated appropriately and reentered in the taper phase after recovery; patients showing subsequent moderate withdrawal were excluded from the study.
Patients entering the study from the two previous buprenorphine buccal film trials immediately discontinued all previous opioid and nonopioid analgesic therapy and entered the titration phase. Rollover patients were permitted to take hydrocodone/acetaminophen (HC/APAP; one to two tablets every 6 hours, maximum of eight tablets) on Day 1 (screening), but the first dose of buprenorphine buccal film was not given for at least 12 hours after the last dose of previous study medication.
At the start of the dose titration phase, all patients, both de novo and rollover, received buprenorphine buccal film 150 µg q12h, regardless of previous dose. Subsequent doses could be increased to a maximum of 900 µg q12h, depending on tolerability and the need for supplemental analgesia. During this period, patients recorded their use of buprenorphine buccal film and rescue medication (HC/APAP) on paper diaries. COWS assessments were performed three times during the titration period, and patients with COWS scores ≥13 were withdrawn from the study.
The optimal dose of buprenorphine buccal film during the titration phase was defined as the dose that the patient found satisfactory for both pain relief and tolerability, either without the need for rescue medication or with no more than two tablets of HC/APAP per day. Patients who received their optimal dose for at least 7 days, and had taken no more than two tablets of rescue medication per day for >3 days, were eligible to enter the long-term treatment phase. Buprenorphine buccal film and HC/APAP use were calculated by counting the number of film pouches and HC/APAP tablets dispensed and returned at each study visit; paper diary entries used by patients to record their daily use of buprenorphine buccal film and HC/APAP tablets were also used to aid in the identification of any discrepancies. During the treatment phase, the efficacy and tolerability of buprenorphine buccal film were assessed approximately every 4 weeks, and doses were adjusted according to analgesic efficacy and tolerability; patients requiring doses below 150 µg q12h or above 900 µg q12h were withdrawn from the study. Efficacy was assessed by measurement of pain scores on an NRS, as described earlier, whereas safety and tolerability were assessed by reporting of adverse events throughout the study, clinical laboratory evaluations, measurement of vital signs, electrocardiograms, and physical examinations.
All other opioid analgesics were prohibited during the titration and long-term treatment phases. Acetylsalicylic acid, nonsteroidal anti-inflammatory drugs, cyclooxygenase-2 inhibitors, and “adjuvant analgesics” such as antidepressants were permitted provided they had been prescribed for chronic pain at least 21 days before the start of the study. Patients received instruction in the placement of the buccal film and were advised not to eat or drink for at least 30 minutes, or until the film had dissolved. Use of study medication was calculated at each assessment using drug accounting procedures.
Statistical analysis
A sample size of approximately 380 patients was chosen to obtain long-term safety data within the selected dose range. A statistical power calculation was not performed.
Safety data were analyzed in the safety population, which included all patients who received at least one dose of buprenorphine buccal film. Efficacy data were analyzed in the efficacy population, which included all patients in the safety population who recorded at least one postdosing average pain intensity score during the titration or long-term treatment phases. Both safety and efficacy data were summarized using descriptive statistics only, and no formal statistical test of significance was performed. Statistical analyses were performed using SAS® version 9.3 software (SAS Institute, Cary, NC, USA).
Results
Patient disposition and demographics
Disposition of patients through the study is shown in Figure 1. A total of 506 patients (445 rollover patients and 61 de novo patients) entered the titration phase, of whom 438 completed this phase and 68 discontinued. Reasons for discontinuation during the titration phase included lack of efficacy (n=19; 3.8%), occurrence of adverse events (n=12; 2.4%), and other (n=20; 4.0%) for other reasons. A total of 435 patients achieved an optimal dose of buprenorphine buccal film and entered the long-term treatment phase. Overall, 158 patients (36.3%) completed the long-term phase; the most common reasons for withdrawal of 277 patients during this phase were the sponsor’s decision to close the study (n=141; 32.4%), withdrawal by the patient (n=36; 8.3%), loss to follow-up (n=21; 4.8%), and adverse events (n=17; 3.9%).
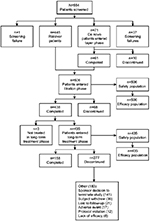  | Figure 1 Patient disposition. |
Demographic characteristics of the patients participating in the titration and long-term phases are summarized in Table 1. The mean age of the patients was 52 years, and approximately 55% were females. The majority of patients (>98%) had chronic low back pain.
Of 435 patients who entered the long-term treatment phase, 52 (12.0%) reached an optimal dose of 300 μg, 45 (10.3%) reached an optimal dose of 450 μg, 141 (32.4%) reached an optimal dose of 600 μg, 62 (14.3%) reached an optimal dose of 750 μg, and 135 (31.0%) reached an optimal dose of 900 μg. These doses remained unchanged during the long-term treatment phase in 86.2% of patients. Adherence to treatment was generally good throughout the study. Overall, 80%–110% adherence was recorded in 495 patients (97.8%) during the titration phase and 406 patients (93.3%) during the long-term treatment phase.
Long-term safety
Buprenorphine buccal film was well tolerated during the study. Adverse events were reported by 218 patients (43.1%) during the titration phase and 235 (54.0%) during the long-term treatment phase. Adverse events that were considered to be treatment-related occurred in 116 patients (22.9%) and 61 patients (14.0%), respectively, and adverse events leading to discontinuation of treatment occurred in 14 (2.8%) and 14 (3.2%) patients, respectively. The most commonly reported adverse events (occurring in ≥3% of patients in either phase) are summarized in Table 2. The most common adverse events during the titration phase were nausea (10.3% of patients), constipation (5.9%), and headache (3.6%). During the long-term treatment phase, the most commonly reported adverse events included vomiting (5.1%), upper respiratory tract infections (4.8%), back pain (3.7%), diarrhea (3.4%), nasopharyngitis (3.2%), urinary tract infection (3.0%), and falls (3.0%). The majority of adverse events were mild or moderate in severity; severe adverse events occurred in 11 patients (2.2%) during the titration phase and 26 patients (6.0%) during the long-term treatment phase.
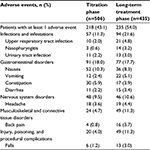  | Table 2 Adverse events occurring in ≥3% of patients in either phase |
Serious adverse events were reported in three patients (0.6%) during the titration phase and 16 (3.7%) during the long-term treatment phase. All of these events were considered to be unrelated, or unlikely to be related, to study medication. There were no clinically important changes in laboratory tests, vital signs, physical examination findings, or COWS scores throughout the study.
Long-term efficacy
Mean NRS scores during the titration and long-term treatment phases are shown in Figure 2. In de novo patients, the mean NRS score decreased from 7 to 3 during the titration phase and stabilized at 3–4 during the long-term treatment phase. In rollover patients, the mean NRS score at titration phase baseline was 4, and this increased briefly to 6 because of a rapid decrease in dose at the start of dose titration, before stabilizing at 3 during the long-term treatment phase. The mean (standard deviation) use of rescue medication decreased from 3.0 (2.1) tablets per day during the titration phase to 1.1 (3.7) tablets per day during the long-term treatment phase.
Discussion
This open-label study has shown that buprenorphine HCl buccal film can be safely and effectively titrated to an optimal dose in both treatment-naive and previously treated patients and that its effect on chronic pain is durable and maintained over a 48-week period. This is the first report demonstrating the long-term safety and efficacy of buprenorphine buccal film, a novel formulation of buprenorphine, that provides flexible dose titration across a wide range of doses using BEMA® technology.16,17 These findings complement and extend those of the previous studies in opioid-naïve19 and opioid-experienced20 patients.
Buprenorphine buccal film was previously evaluated in 749 opioid-naïve patients in a 12-week double-blind, placebo-controlled trial with an open-label titration phase.19 At the end of the double-blind phase, the mean change from baseline in pain intensity scores was observed to be higher in the placebo group, and buprenorphine buccal film had a higher percentage of responders at both ≥30% (63% vs 47% for placebo) and ≥50% (41% vs 33%) pain reduction. As expected, rescue medication use was more common in the placebo group (40% vs 31% for buprenorphine buccal film at Week 12). The most frequently reported adverse events were consistent with opioids, including nausea, constipation, and vomiting. There were no cases of respiratory depression reported.
Results were similarly observed during a previous randomized, double-blind, placebo-controlled withdrawal study evaluating buprenorphine buccal film in 815 opioid-experienced patients. Patients taking placebo demonstrated higher changes in pain intensity scores at the end of the double-blind phase than patients administered buprenorphine buccal film (1.92 vs 0.88, respectively). As shown with opioid-naïve patients, buprenorphine buccal film was observed to have more responders: 64.2% vs 30.6% at ≥30% pain reduction and 39.5% vs 16.9% at ≥50% pain reduction. Significant differences favoring buprenorphine buccal film were also observed for rescue medication use and patient-reported outcomes. Common adverse events included nausea, constipation, vomiting, headache, dizziness, and somnolence.20
Buprenorphine buccal film was well tolerated in this study. The principal adverse events were those typically associated with µ-opioid receptor agonists, notably nausea, constipation, and headache. Constipation is one of the most common adverse events associated with opioids: one systematic review of opioid use in patients with chronic pain, which included 34 trials with more than 5,500 patients, reported an average prevalence of 15% (95% confidence interval 14%–16%), although the prevalence in individual trials ranged from 0% to 71%.22 By contrast, in the present study, the incidence of constipation during the long-term treatment phase was 3.9%, consistent with rates previously reported in large longitudinal or pooled randomized trials with buprenorphine (1%–5%).12 Another adverse event that can significantly limit opioid dose titration is respiratory depression, which has been reported to occur in 1%–11% of patients receiving systemic or spinal opioids.12 However, in contrast to other opioids, buprenorphine has a dose-ceiling effect on respiratory depression,12,23,24 and hence respiratory depression might be expected to be less common. Indeed, no adverse events relating to respiratory depression were found in the present study.
The pharmacologic profile of buprenorphine differs from that of full µ-opioid receptor agonists in a number of aspects, which may confer an improved benefit–risk profile.12,25 The risk of withdrawal symptoms might be expected to be lower with buprenorphine than with other opioids because of the prolonged receptor binding of buprenorphine and the ability of buprenorphine to reduce central sensitization.10,12 This is supported by the finding in the present study of no clinically significant changes in COWS scores after dose tapering in the de novo opioid-experienced patients. Additionally, the lack of withdrawal symptoms observed during the titration phase for rollover patients is significant because an analgesic taper was not performed. In some patients, the decrease in buprenorphine dose was as high as 1,500 μg/d.20 A previous study showed that patients with chronic pain who were receiving 80–220 mg/d oral MSE could be successfully switched to buccal buprenorphine with no increased risk of withdrawal symptoms or loss of analgesia.26 Other studies have shown that withdrawal symptoms are significantly lower with buprenorphine than with morphine27 and that neither tolerance nor refractory effects occur when patients are switched from high-dose morphine to transdermal buprenorphine.28 The low levels of both tolerance and dependence seen with buprenorphine, compared with other µ-opioid receptor agonists, may be due partly to the prolonged binding of buprenorphine to µ-opioid receptors and partly to activation of ORL1 receptors.12,15
Although limited by the open-label dosing and uncontrolled design of this study, the results show that buprenorphine buccal film had sustained efficacy throughout the 48-week evaluation period. In addition, the degree to which these findings can be generalized to other chronic pain settings is unknown. However, in animal studies, buprenorphine has been shown to provide effective analgesia in various models of both chronic and acute pain, including inflammatory and neuropathic pain.9 Furthermore, numerous clinical trials have demonstrated efficacy with buprenorphine in different chronic pain settings, including neuropathic pain, mixed nociceptive and neuropathic pain, and chronic cancer pain.12,29
In conclusion, this study has shown that buprenorphine buccal film is effective and well tolerated in the long-term management of chronic pain.
Acknowledgments
Technical editorial and medical writing assistance was provided by Synchrony Medical Communications, LLC, West Chester, PA, USA. Funding for this support was provided by Endo Pharmaceuticals Inc.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
Dr Hale reports serving as a consultant to Endo Pharmaceuticals Inc. and receiving research funding from Endo Pharmaceuticals Inc. At the time the study was conducted, Dr Urdaneta was an employee of Endo Pharmaceuticals Inc. Drs Kirby and Xiang are employees of Endo Pharmaceuticals Inc. Dr Rauck reports serving as a consultant for BioDelivery Sciences International, Inc., Boston Scientific Corporation, Endo Pharmaceuticals Inc., and Pfizer Inc. and receiving research funding from AstraZeneca, BioDelivery Sciences International, Inc., Biogen, Boston Scientific Corporation, Endo Pharmaceuticals Inc., Nektar Therapeutics, and Pfizer Inc. The authors report no other conflicts of interest in this work.
References
Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230–1239. | ||
Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. | ||
Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. | ||
Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain – United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. | ||
Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–S120. | ||
Manchikanti L, Damron KS, McManus CD, Barnhill RC. Patterns of illicit drug use and opioid abuse in patients with chronic pain at initial evaluation: a prospective, observational study. Pain Physician. 2004;7(4):431–437. | ||
Palmer RE, Carrell DS, Cronkite D, et al. The prevalence of problem opioid use in patients receiving chronic opioid therapy: computer-assisted review of electronic health record clinical notes. Pain. 2015;156(7):1208–1214. | ||
Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569–576. | ||
Christoph T, Kogel B, Schiene K, Meen M, De VJ, Friderichs E. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol. 2005;507(1–3):87–98. | ||
Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274(1):361–372. | ||
Koppert W, Ihmsen H, Korber N, et al. Different profiles of buprenorphine-induced analgesia and antihyperalgesia in a human pain model. Pain. 2005;118(1–2):15–22. | ||
Davis MP. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol. 2012;10(6):209–219. | ||
Sittl R, Likar R, Nautrup BP. Equipotent doses of transdermal fentanyl and transdermal buprenorphine in patients with cancer and noncancer pain: results of a retrospective cohort study. Clin Ther. 2005;27(2):225–237. | ||
Zaki PA, Keith DE Jr, Brine GA, Carroll FI, Evans CJ. Ligand-induced changes in surface mu-opioid receptor number: relationship to G protein activation? J Pharmacol Exp Ther. 2000;292(3):1127–1134. | ||
Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol. 2004;2(4):395–402. | ||
Biodelivery Sciences International. Technology platform. 2016. http://www.bdsi.com/Technology_Platforms.aspx. Accessed April 1, 2016. | ||
Belbuca™ (buprenorphine) buccal film, CIII [package insert]. Malvern, PA: Endo Pharmaceuticals Inc.; 2015. | ||
Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358–369. | ||
Rauck RL, Potts J, Xiang Q, Tzanis E, Finn A. Efficacy and tolerability of buccal buprenorphine in opioid-naive patients with moderate to severe chronic low back pain. Postgrad Med. 2016;128(1):1–11. | ||
Gimbel J, Spierings EL, Katz N, Xiang Q, Tzanis E, Finn A. Efficacy and tolerability of buccal buprenorphine in opioid-experienced patients with moderate to severe chronic low back pain: results of a phase 3, enriched enrollment, randomized withdrawal study. Pain. 2016;157(11):2517–2526. | ||
Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35(2):253–259. | ||
Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005;7(5):R1046–R1051. | ||
Dahan A, Yassen A, Bijl H, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94(6):825–834. | ||
Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth. 2006;96(5):627–632. | ||
Pergolizzi J, Aloisi AM, Dahan A, et al. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract. 2010;10(5):428–450. | ||
Webster L, Gruener D, Kirby T, Xiang Q, Tzanis E, Finn A. Evaluation of the tolerability of switching patients on chronic full mu-opioid agonist therapy to buccal buprenorphine. Pain Med. Epub February 25, 2016. doi: 10.1093/pm/pnv110. | ||
Tompkins DA, Smith MT, Mintzer MZ, Campbell CM, Strain EC. A double-blind, within subject comparison of spontaneous opioid withdrawal from buprenorphine versus morphine. J Pharmacol Exp Ther. 2014;348(2):217–226. | ||
Freye E, Anderson-Hillemacher A, Ritzdorf I, Levy JV. Opioid rotation from high-dose morphine to transdermal buprenorphine (Transtec®) in chronic pain patients. Pain Pract. 2007;7(2):123–129. | ||
Wolff RF, Aune D, Truyers C, et al. Systematic review of efficacy and safety of buprenorphine versus fentanyl or morphine in patients with chronic moderate to severe pain. Curr Med Res Opin. 2012;28(5):833–845. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


