Back to Journals » Cancer Management and Research » Volume 12
Long-Term Results of Concurrent Chemoradiotherapy Combined with Anti-EGFR Monoclonal Antibody Prior to Surgery in Locally Advanced Cervical Cancer: A Single-Institute Prospective Study
Authors Qing D , Wu Y, Liu X , Jiang H, Zhu C , Liu P, Dang J, Li X, Chen Z, Long X, Pang Q , Peng L, Deng S, Gu J, Zhao R, Chen C, Lu H
Received 23 September 2020
Accepted for publication 12 November 2020
Published 1 December 2020 Volume 2020:12 Pages 12309—12317
DOI https://doi.org/10.2147/CMAR.S282372
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Defeng Qing,1,* Yuying Wu,2,* Xu Liu,1,* Hailan Jiang,1,* Chaohua Zhu,1,* Pei Liu,3 Junming Dang,4 Xianglong Li,1 Zhaohong Chen,1 Xianfeng Long,1 Qiang Pang,1 Luxing Peng,1 Shan Deng,1 Junzhao Gu,1 Renfeng Zhao,2 Changyi Chen,2 Heming Lu1
1Department of Radiation Oncology, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning 530021, People’s Republic of China; 2Department of Gynecology, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning 530021, People’s Republic of China; 3Department of Radiation Oncology, Youjiang Medical University for Nationalities, Baise 533000, People’s Republic of China; 4Department of Oncology, Guangxi University of Chinese Medicine, Nanning 530001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Heming Lu
Department of Radiation Oncology, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning City 530021, People’s Republic of China
Tel +86-771-218-6504
Email [email protected]
Purpose: We aimed to evaluate the long-term survival outcomes of concurrent chemoradiotherapy (CCRT) combined with nimotuzumab followed by surgery in patients with locally advanced cervical cancer (LACC).
Patients and Methods: Patients received whole pelvic intensity-modulated radiation therapy (IMRT) and concomitantly with weekly cisplatin (40 mg/m2) or nedaplatin (30 mg/m2) and weekly nimotuzumab (200 mg). After assessment of the treatment response, patients then underwent radical surgery.
Results: Between June 2013 and July 2016, 33 patients with FIGO IB2–IIIB cervical cancer were recruited. Clinical complete response and partial response were observed in 8 (24.3%) and 23 patients (69.7%), respectively. Twenty-seven patients (81.8%) were successfully treated with radical hysterectomy and pelvic lymphadenectomy: 9 (33.3%) showed pathological complete response; 10 (37.1%) showed partial response and 8 (29.6%) presented with persistent macroscopic/microscopic residual carcinoma. For the intention-to-treat population, the median follow-up time was 53.7 months. Locoregional recurrence and distant metastases were observed in three and seven patients, respectively. The 5-year overall survival, progression-free survival, locoregional recurrence-free survival, and distant metastasis-free survival were 81.5%, 72.7%, 90.9%, and 78.3%, respectively. Both acute and late toxicities were manageable and mainly limited to grade 1 or 2.
Conclusion: Concurrent chemoradiotherapy combined with nimotuzumab followed by surgery for patients with LACC is safe and results in excellent long-term treatment outcomes. Further randomized controlled studies are warranted to confirm the findings.
Keywords: locally advanced cervical cancer, neoadjuvant chemotherapy, intensity-modulated radiotherapy, anti-EGFR monoclonal antibody, radical surgery
Introduction
As a notorious disease affecting women, cervical cancer is the fourth most common malignant tumor among women in the world.1 Annually, there are about 530,000 new cases diagnosed and 270,000 women die due to cervical cancer worldwide.2 Although the prognosis for early-stage cervical cancer is quite promising, over 80% of the patients present with locally advanced disease with dismal treatment outcomes.3 Overall, the prognosis of cervical cancer largely depended on stage and treatment modality. Currently, the management for patients with locally advanced cervical cancer (LACC) remains controversial. Women with LACC most commonly receive concurrent chemoradiotherapy (CCRT) in the US, while these patients are often treated with radical hysterectomy in Japan.4–6
Over the last decade, CCRT has been introduced as a strategy for LACC. Definitive CCRT consists of pelvic external beam radiotherapy, concomitant chemotherapy and intracavitary brachytherapy.7 Due to lack of brachytherapy equipment and technology in some radiation oncology centers in China, many patients cannot be routinely treated with brachytherapy. Therefore, it is reasonable to recommend that patients who undergo pelvic external beam radiotherapy and chemotherapy should be treated with radical surgery as an alternative to definitive CCRT, with an aim of achieving long-term tumor control.8 However, the results of CCRT followed by surgery are inconsistent. Several studies9–11 found that CCRT followed by radical surgery did not improve overall survival, whereas others12–15 indicated that CCRT prior to surgery could result in encouraging results, with a higher rate of pathological complete response (PCR), an acceptable long-term toxicity profile, and an improved clinical outcome. Therefore, further studies are worth exploring.
Currently, various molecular-targeted agents have been developed and used in cervical cancer. In particular, monoclonal antibodies (MoAbs) represent the majority of target therapies that have been employed in clinical settings.16 There are two monoclonal antibodies most frequently investigated as targeted therapy in cervical cancer, ie, antibody against epidermal growth factor receptor (EGFR) and antibody against vascular epithelial growth factor (VEGF). Nimotuzumab, as a recombinant humanized anti-EGFR monoclonal IgG1 antibody, has been proven to be effective in treating persistent, recurrent, or metastatic cervical cancer.17 In patients with LACC, CCRT combined with nimotuzumab was effective and safe in a definitive setting, with 3-year progression-free survival (PFS) and overall survival (OS) rates were 73.9% and 87.0%, respectively.18 However, to the best of our knowledge, there is no related study focusing on CCRT plus nimotuzumab followed by radical surgery in LACC so far. So, we designed a single-centre prospective study to evaluate the efficacy and safety of CCRT combined with nimotuzumab followed by radical surgery for LACC patients, and this time long-term results will be presented.
Patients and Methods
Eligibility Criteria
The inclusion criteria were as the following: biopsy-proven cervical cancer (squamous cell carcinoma or adenocarcinoma); International Federation of Gynecology and Obstetrics (FIGO) stage IB2-IIIB; age between 18 and 75 years; Eastern Cooperative Oncology Group (ECOG) performance status <2; adequate bone marrow, liver and, renal functions. Exclusion criteria were as the following: prior malignant cancer; pregnant or lactating women; history of radiotherapy or chemotherapy; allergy to cisplatin, nedaplatin, or nimotuzumab. All patients were required to sign a written informed consent. The study was registered with ClinicalTrials.gov (No NCT01938105) and approved by the Institutional Review Board of the People’s Hospital of Guangxi Zhuang Autonomous Region. The study complied with the Declaration of Helsinki.
Pretreatment assessments included patient’s medical history, physical examination, gynecological examination, cervical biopsy, blood cell count, liver and renal function tests, transvaginal ultrasound, chest computed tomography (CT), abdominal ultrasonography, pelvic magnetic resonance imaging (MRI) and bone emission computed tomography (ECT) scan if indicated.
Treatment Modalities
Radiotherapy
Patients were asked to empty their rectum and bladder before positioning, then drank water and suppressed the urination. Patients lay down on a CT simulator device (Somatom Sensation Open, Siemens Medical Solutions, Erlangen, Germany) in a prone/supine position with thermoplastic masks to cover the lower chest, abdomen, and pelvis. CT scan was performed from T12 vertebral to 3 cm below the ischial tuberosity. CT images were then reconstructed with a slice spacing of 4 mm, and were sent to the Pinnacle system (version 9.2, Philips Radiation Oncology Systems, Fitchburg, WI, USA).
Based on the findings of the CT images, pelvic MRI, and gynecological examination, the gross target volume (GTV) was delineated, including primary tumor and involved metastatic lymph nodes. There were three subsets of clinical target volume (CTV1, CTV2, and CTV3). CTV1 contained GTV, uterus, and cervix; CTV2 contained parametrial and paravaginal tissues, ovaries, and vagina according to the involvement; CTV3 contained common iliac, internal iliac, external iliac, anterior sacral and obturator lymphatic drainage region from L4-5 to the inferior margin of obturator.19 CTV1, CTV2, and CTV3 were expanded with 10-mm, 7-mm, and 7-mm margins to generate PTV1, PTV2, and PTV3, respectively. Accordingly, critical structures including the rectum, bladder, small bowel, the left and right femoral heads, and pelvic bone marrow were contoured.
The prescribed radiation doses to PTV1, PTV2, and PTV3 were 50–54 Gy at 1.95–2.12 Gy per fraction, 45–48.6 Gy at 1.8–1.86 Gy per fraction, and 45–48.6 Gy at 1.7–1.8 Gy per fraction, respectively, in 25–27 fractions. Five or seven coplanar radiation fields using intensity-modulated radiation therapy (IMRT) technique were designed with an Elekta Synergy Linear Accelerator (Elekta Ltd., Stockholm, Sweden). Prior to each treatment, kilovoltage cone-beam CT scan was acquired three to five times a week to facilitate the correction of setup error.
Chemotherapy
The concomitant chemotherapy regimen was administered as the following: cisplatin was given at a dose of 40 mg/m2, or nedaplatin was given at a dose of 30 mg/m2. Chemotherapy was repeated every week for a total of 5–6 cycles.
Nimotuzumab
Weekly nimotuzumab was given concurrently with IMRT by intravenous infusion at a dose of 200 mg over 60 min for 6 weeks, diluted in 250 mL normal saline solution. Once the infusion was finished, patients were required to receive radiotherapy within 60 minutes.
Surgery
Four to six weeks after the completion of CCRT, gynecological examination and pelvic MRI scan were performed to evaluate the treatment response according to the RECIST criteria (Response evaluation criteria in solid tumors). When no evidence of progressive disease or distant metastasis was found, patients then underwent radical surgery. The surgical procedure was composed of radical hysterectomy and pelvic lymphadenectomy. When the para-aortic lymph nodes were suspected with metastasis, a para-aortic lymphadenectomy should be taken into account. According to postoperative findings, three pathological responses were classified. Complete response (CR) was defined as no evidence of residual tumor; partial response (PR) was defined as the presence of atypical cells or cervical intraepithelial neoplasia; residual carcinoma (RC) was defined as the presence of persistent macroscopic and/or microscopic residual tumor.
Follow-Up
Patients were required to be followed up at a 3-month interval in the first 3 years and at a 6-month interval thereafter. Typically, the workup included physical examination, gynecological examination, laboratory studies (blood cell count, liver and renal function tests, and tumor markers), transvaginal ultrasound, chest CT, abdominal ultrasonography, pelvic MRI, and bone ECT scans when necessary. Treatment toxicities were graded according to the criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC).20
Statistical Analysis
Progression-free survival (PFS) was calculated from the date of patient recruitment to the date of disease progression or death from any cause. Overall survival (OS) was calculated from the date of patient recruitment to the date of death or the date of the last follow-up. Locoregional recurrence-free survival (LRRFS) was defined as the absence of either consistent or relapsed disease at the primary tumor site or the regional lymph nodes, and distant metastasis-free survival (DMFS) was calculated from the date of patient recruitment to the date of distant metastasis. Categorical data were presented as the number of patients and a percentage. PFS, OS, LRRFS, and DMFS rates were computed by the Kaplan–Meier method. Statistical analysis was carried out using SPSS 22.0 software for windows.
Results
Patient Characteristics
Between June 2013 and July 2016, 33 patients with LACC treated in the People’s Hospital of Guangxi Zhuang Autonomous Region were enrolled into this study. The median age was 53.7 years (range, 31–75 years). There were 6 patients (18.2%) in stage IB2, 6 patients (18.2%) in stage IIA, 17 patients (51.4%) in stage IIB, and 4 patients (12.2%) in stage IIIA–IIIB.
Twenty-eight patients (84.9%) received CCRT combined with nimotuzumab. Of them, 13 were treated with five cycles and 15 patients with six cycles of nimotuzumab. The remaining five patients received CCRT without nimotuzumab. Three patients (9.1%) completed 5 cycles of cisplatin, 26 patients (78.8%) completed 5 or more cycles of nedaplatin, and 4 patients (12.1%) received less than 5 cycles of nedaplatin due to myelosuppression. The median CCRT duration was 35 days (range, 28–49 days). The median doses to PTV1, PTV2, and PTV3 were 52.3 Gy, 47.1 Gy, and 46.9 Gy, respectively. The median time interval from CCRT completion to radical hysterectomy was 32 days (range, 20–61 days). Seventeen patients (51.6%) underwent radical hysterectomy within 5 weeks. The clinicopathological characteristics and treatment details are summarized in Table 1.
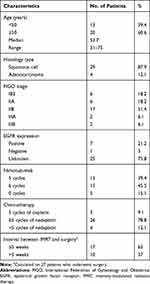 |
Table 1 Clinicopathological Characteristics and Treatment Details |
Clinical and Pathological Response
At the end of CCRT, complete response (CR) and partial response (PR) were observed in 8 (24.3%) and 23 patients (69.7%), respectively, with an overall response rate (ORR) of 94%. Only one patient (3%) showed stable disease (SD), and no patient had progressive disease (PD).
Twenty-seven patients (81.8%) were successfully treated with radical hysterectomy and pelvic lymphadenectomy. Of the remaining six patients, two refused further intervention and the other four were not amenable to surgery, and they were treated with brachytherapy instead. At the end of surgery, 9 patients (33.3%) showed a complete response (CR), and 10 patients (37.1%) showed a partial response (PR). Eight patients (29.6%) presented with persistent macroscopic/microscopic residual carcinoma (RC) (Table 2). Upon pathological evaluation, no patients were found to have ovary, oviduct, peritoneal, para-uterine, or vaginal invasion. None had positive pelvic or para-aortic lymph node involvement. All surgical margins were negative.
 |
Table 2 Clinical and Pathological Responses |
Treatment Compliance and Toxicities
The median operating time was 182 minutes (range, 85–278), and median blood loss was 514 cc (range, 100–1200). Five patients (18.5%) underwent blood transfusion.
Acute and late toxicities are summarized in Table 3. Most acute toxicities were limited to grade 1 or 2, with myelosuppression being the most frequently occurred complication. No fatal acute toxicities were observed. Late toxicities were mainly limited to grade 1. No severe long-term complication was documented.
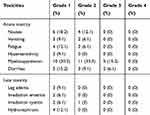 |
Table 3 Acute and Late Toxicities |
Survival Outcome
Survival analysis was performed for the intention-to-treat population. The median follow-up time was 53.7 months (range, 21.8–63.5 months). Eleven patients (33.3%) were followed for more than 5 years. By the end of the last follow-up, there were 27 patients (81.8%) still alive. Three patients (9.1%) had locoregional failure. Distant metastases were found in seven patients (21.2%), six (85.7%) of whom occurred within 3 years after the completion of treatment. Bone, chest wall, abdominal wall, and lung metastases were documented in one, one, one, and two patients, respectively. Two patients had multiple metastases to the bone, lung, mediastinal, supraclavicular lymph nodes, and retroperitoneal lymph nodes. One patient experienced both local recurrence and distant metastases.
The 5-year OS, PFS, LRRFS, and DMFS were 81.5%, 72.7%, 90.9%, and 78.3%, respectively. Distant metastasis was the main cause of treatment failure (Figure 1).
Patients who underwent CCRT plus radical surgery had a significantly higher LRRFS, compared with those who did not receive surgery (p=0.014). But no significant differences in OS and PFS were found between the two groups (Figure 2). No correlations were found between pathological response and long-term survival.
Discussion
Patients with LACC have a poor prognosis, with 5-year OS of approximately 25%.21 Over the last decade, CCRT has been introduced as a standardized management for LACC. And this strategy has not had major changes for years. CCRT has significantly improved the prognosis, with 5-year OS up to 70%, when compared with radiation therapy alone. For a considerable proportion of patients, however, locoregional recurrence and distant metastasis still remain inevitable. Due to heterogeneity within a tumor, not all tumor cells were sensitive to chemoradiotherapy; thus, persistent macroscopic and/or microscopic residual tumors may exist after chemoradiotherapy, which are considered as an important source of later recurrence. With the aim to remove these residual tumors to further achieve long-term tumor control, theoretically, neoadjuvant chemoradiotherapy followed by radical surgery would be a reasonable option.
However, the role of CCRT followed by radical surgery on LACC has long been ambiguous and controversial. Azria et al22 found that patients with bulky residual carcinoma after CCRT could not benefit from hysterectomy. Additional surgery could not only lead to poor prognosis, but also bring a higher complication rate. But this series was based on a quite small sample with only 10 patients. Lee et al11 revealed that neoadjuvant chemotherapy followed by surgery has no therapeutic advantages over CCRT alone in LACC with respect to PFS, OS, and LRRFS, whereas other studies demonstrated the benefit of additional surgery to CCRT. Ferrandina et al13 reported a long-term result of 174 patients with LACC treated with CCRT prior to surgery, pathological complete response and microscopic residual disease were observed in 76 patients (43.7%), and 48 patients (27.6%), respectively, and the 5-year PFS and OS rates were 75.5% and 77.4%, respectively. Fanfani et al15 reported on 73 patients with FIGO stage III cervical cancer undergoing radical hysterectomy after CCRT, with 3-year DFS of 68.3% and OS of 67.7%. Clinical CR and PR rates were observed in 41.1% and 54.8% of the patients, respectively.
In the present study, the long-term treatment outcome is comparable to the study by Ferrandina et al but much better than the study by Fanfani et al. The 5-year PFS, OS, LRRFS, and DMFS were 72.7%, 81.5%, 90.9%, and 78.3%, respectively. The higher OS may be attributed to the improvement in locoregional control. Our study revealed a good LRRFS, with the 5-year LRRFS of more than 90%. By the end of the last follow-up, only three (9.1%) patients had local regional recurrence. However, distant metastasis remained the main reason for the treatment failure. In addition, we also found that patients who underwent CCRT followed by surgery had a significantly higher LRRFS, compared with those who received CCRT alone (p=0.014), suggesting surgery should be considered as an indispensable ingredient for LACC patients if they are treated with CCRT without brachytherapy.
In a prospective, Phase 2 study, conducted by Ferrandina et al,23 CCRT followed by radical surgery resulted in a high rate of PCR and an encouraging local control rate. PCR was observed in 50.5% of the patients, and the 3-year local control rate and OS were 93% and 86.1%, respectively. Similarly, Mancuso et al24 reported a Phase I–II study on patients with FIGO stage II–IIIA cervical cancer who received radiation therapy concurrently with 5-fluorouracil and cisplatin plus surgery, PCR was up to 54.2% after treatment, and the 2-year LRRFS was 91.7%. Gadducci et al25 showed that pathological response to neoadjuvant chemotherapy was an independent prognostic factor for PFS and OS. Patients who harbored residual disease after treatment had a 2.8-fold higher risk of relapse and a 5.4-fold higher risk of death, compared with those who got a PCR (5-year OS 53.7% vs 96%, p<0.0001). However, a much lower PCR of 39% was also reported in the literature, as demonstrated in a study by Classe et al.25 In the present study, PCR after CCRT followed by surgery was 33.3%, which was lower than those of above-mentioned studies, and we found no correlations between pathological response and long-term survival. Direct comparison of pathological response rate between our study and others could not be feasible since different criteria were used. For example, pathological PR in our study was defined as the presence of persistent atypical cells or cervical intraepithelial neoplasia; whereas in Ferrandina’s study, it was defined as persistence of only microscopic foci (≤3 mm maximum dimension) at any site level, and PCR was defined as the absence of any residual tumor.19 It is widely recognized that cervical intraepithelial neoplasia is a non-malignant lesion and usually curable. Therefore, some of our patients classified as having pathological PR may actually achieve PCR, according to the criteria in other studies.13,23 Nevertheless, this treatment regimen in our study resulted in excellent long-term results, LRRFS in particular.
CCRT followed by surgery could also provide acceptable acute and chronic toxicity profiles. In our study, most acute and long-term toxicities were grade 1 or 2 and manageable. Grade 3 acute toxicities occurred in only seven patients. Long-term toxicities were mainly limited to grade 1. The results are consistent with other studies. Classe et al26 found that surgery after CCRT led to an acceptable morbidity. There were 18.9% of patients with grade 2 morbidity, and no treatment-related death occurred. In the current study, 87.9% of the patients were given nedaplatin as a concomitant chemotherapy regimen. Vomiting was less common, compared with cisplatin-based chemotherapy. Regarding long-term toxicities, leg edema was a common complication. Wang et al27 showed that the incidence of leg edema was higher in CCRT plus surgery combination group than in the CCRT group alone (35.29% vs 4.96%, p=0.000). Another study showed that patients with more than 31 pelvic lymph nodes resected had a higher risk of leg edema than those intacted (26% vs 16.5%, p<0.05), and the authors suggested that the elimination of circumflex iliac nodes to distal external iliac node dissection could reduce the occurrence of leg edema.28
As a monoclonal antibody, nimotuzumab has been proven to be effective and tolerable in patients with non-small-cell lung cancer, head-and-neck squamous cell carcinoma, and locally advanced esophageal squamous cell carcinoma.29–31 In patients with recurrent or metastatic cervical cancer, nimotuzumab as a second- or third-line treatment was also effective with a tolerable toxicity profile.17 A prospective study conducted by Cao et al18 showed that patients treated with the combination of nimotuzumab and CCRT had a significantly higher response rate than those who were treated with CCRT alone (87% vs 67.4%, p=0.045). The 3-year PFS and OS between the two groups were 73.9% vs 50% (p=0.042), and 87% vs 69.6% (p=0.07), respectively. The incidence and severity of adverse reactions in the nimotuzumab combination group were similar with the CCRT alone. In the current study, only three patients experienced slight skin rash, which was classified as grade 1 toxicity and certainly related to the use of nimotuzumab. No other adverse effects associated with nimotuzumab were found. However, there is no related research focusing on nimotuzumab in combination with CCRT prior to radical hysterectomy in LACC patients so far. This study was conducted in a single institution with a long-term follow-up time. The survival outcomes are superior to, or at least comparable with those from previous reports.13,15,23,24
Conclusions
Our study demonstrated that CCRT combined with nimotuzumab followed by surgery for patients with LACC is safe, feasible and effective. And this combination regimen could potentially improve the long-term survival outcomes. However, given the deficiency of any conclusion based on a single institution experience, further randomized, multi-institution, and large-sample research should be taken into account.
Data Sharing Statement
Individual participant data collected during the trial and after deidentification will be shared. Request should be directed to [email protected]. Once approved, the data will be sent through the mailbox. The data will be made available beginning 3 months and ending 12 months following article publication.
Acknowledgments
This work was supported by the Wu Jieping Medical Foundation (No 2013-428-2081), Research and Development of Appropriate Medical Technology in Guangxi (S2017082). This work was presented at the 53rd Annual Meeting of the American Society for Clinical Oncology (ASCO), Chicago, IL, USA, June 2–6, 2017; the 59th Annual Meeting of American Society for Therapeutic Radiology Oncology (ASTRO), San Diego, CA, USA, September 24–27, 2017; and the 62nd Annual Meeting of ASTRO in Poster Q&A session, November 1, 2020: https://www.redjournal.org/article/S0360-3016(20)34003-7/abstract.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Small WJ, Bacon MA, Bajaj A, et al. Cervical cancer: a global health crisis. Cancer Am Cancer Soc. 2017;123(13):2404–2412.
3. Kokka F, Bryant A, Brockbank E, Powell M, Oram D. Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer. Cochrane Database Syst Rev. 2015;4:D10260.
4. Mikami M, Aoki Y, Sakamoto M, et al. Surgical principles for managing stage IB2, IIA2, and IIB uterine cervical cancer (Bulky Tumors) in Japan: a survey of the Japanese Gynecologic Oncology Group. Int J Gynecol Cancer. 2014;24(7):1333–1340. doi:10.1097/IGC.0000000000000202
5. Saito T, Katabuchi H. Annual Report of the Committee on Gynecologic Oncology, Japan Society of Obstetrics and Gynecology: patient Annual Report for 2013 and Treatment Annual Report for 2008. J Obstet Gynaecol Res. 2016;42(9):1069–1079.
6. Matsuo K, Shimada M, Yamaguchi S, et al. Neoadjuvant chemotherapy with taxane and platinum followed by radical hysterectomy for stage IB2-IIB cervical cancer: impact of histology type on survival. J Clin Med. 2019;8(2):156. doi:10.3390/jcm8020156
7. Nag S, Erickson B, Thomadsen B, Orton C, Demanes JD, Petereit D. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;48(1):201–211. doi:10.1016/S0360-3016(00)00497-1
8. Chuang L, Kanis MJ, Miller B, Wright J, Small WJ, Creasman W. Treating locally advanced cervical cancer with concurrent chemoradiation without brachytherapy in low-resource countries. Am J Clin Oncol. 2016;39(1):92–97. doi:10.1097/COC.0000000000000222
9. Yan W, Si L, Ding Y, Qiu S, Zhang Q, Liu L. Neoadjuvant chemotherapy does not improve the prognosis and lymph node metastasis rate of locally advanced cervical squamous cell carcinoma. Medicine. 2019;98(39):e17234. doi:10.1097/MD.0000000000017234
10. Zhao H, He Y, Zhu L, et al. Effect of neoadjuvant chemotherapy followed by radical surgery for FIGO stage IB2/IIA2 cervical cancer. Medicine. 2019;98(21):e15604. doi:10.1097/MD.0000000000015604
11. Lee J, Kim TH, Kim GE, Keum KC, Kim YB. Neoadjuvant chemotherapy followed by surgery has no therapeutic advantages over concurrent chemoradiotherapy in International Federation of Gynecology and Obstetrics stage IB-IIB cervical cancer. J Gynecol Oncol. 2016;27(5):e52. doi:10.3802/jgo.2016.27.e52
12. Morice P, Uzan C, Zafrani Y, Delpech Y, Gouy S, Haie-Meder C. The role of surgery after chemoradiation therapy and brachytherapy for stage IB2/II cervical cancer. Gynecol Oncol. 2007;107(1 Suppl 1):S122–S124. doi:10.1016/j.ygyno.2007.07.015
13. Ferrandina G, Margariti PA, Smaniotto D, et al. Long-term analysis of clinical outcome and complications in locally advanced cervical cancer patients administered concomitant chemoradiation followed by radical surgery. Gynecol Oncol. 2010;119(3):404–410. doi:10.1016/j.ygyno.2010.08.004
14. Wei L, Li X, Zhang Y, et al. Individualized pelvic lymphadenectomy should follow neoadjuvant concurrent chemoradiotherapy for locally advanced cervical cancer. Medicine. 2018;97(14):e331.
15. Fanfani F, Vizza E, Landoni F, et al. Radical hysterectomy after chemoradiation in FIGO stage III cervical cancer patients versus chemoradiation and brachytherapy: complications and 3-years survival. Eur J Surg Oncol. 2016;42(10):1519–1525. doi:10.1016/j.ejso.2016.05.011
16. Bellati F, Napoletano C, Gasparri ML, et al. Monoclonal antibodies in gynecological cancer: a critical point of view. Clin Dev Immunol. 2011;2011:1–16. doi:10.1155/2011/890758
17. Cetina L, Crombet T, Jiménez-Lima R, et al. A pilot study of nimotuzumab plus single agent chemotherapy as second- or third-line treatment or more in patients with recurrent, persistent or metastatic cervical cancer. Cancer Biol Ther. 2015;16(5):684–689. doi:10.1080/15384047.2015.1026483
18. Cao Y, Deng L, Lian S, Jiang Y. Research on the efficacy of cisplatin and nimotuzumab combined with concurrent chemoradiotherapy on locally advanced cervical cancer. J BUON. 2019;24(5):2013–2019.
19. Lu H, Wu Y, Liu X, et al. A prospective study on neoadjuvant chemoradiotherapy plus anti-EGFR monoclonal antibody followed by surgery for locally advanced cervical cancer. Onco Targets Ther. 2018;11:3785–3792. doi:10.2147/OTT.S164071
20. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi:10.1016/0360-3016(95)00060-C
21. Marita A, Ordeanu C, Rancea A, Nicolae T, Nagy VM. Long-term survival following neoadjuvant chemotherapy and concomitant radiochemotherapy in locally advanced cervical cancer: results of the Oncology Institute “Prof. Dr. Ion Chiricuta” experience. J Med Life. 2018;11(1):42–50.
22. Azria E, Morice P, Haie-Meder C, et al. Results of hysterectomy in patients with bulky residual disease at the end of chemoradiotherapy for stage IB2/II cervical carcinoma. Ann Surg Oncol. 2005;12(4):332–337. doi:10.1245/ASO.2005.05.020
23. Ferrandina G, Gambacorta A, Gallotta V, et al. Chemoradiation with concomitant boosts followed by radical surgery in locally advanced cervical cancer: long-term results of the ROMA-2 prospective phase 2 study. Int J Radiat Oncol Biol Phys. 2014;90(4):778–785. doi:10.1016/j.ijrobp.2014.07.033
24. Mancuso S, Smaniotto D, Benedetti PP, et al. Phase I-II trial of preoperative chemoradiation in locally advanced cervical carcinoma. Gynecol Oncol. 2000;78(3 Pt 1):324–328. doi:10.1006/gyno.2000.5862
25. Gadducci A, Sartori E, Maggino T, et al. Pathological response on surgical samples is an independent prognostic variable for patients with Stage Ib2-IIb cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy: an Italian multicenter retrospective study (CTF Study). Gynecol Oncol. 2013;131(3):640–644. doi:10.1016/j.ygyno.2013.09.029
26. Classe JM, Rauch P, Rodier JF, et al. Surgery after concurrent chemoradiotherapy and brachytherapy for the treatment of advanced cervical cancer: morbidity and outcome: results of a multicenter study of the GCCLCC (Groupe des Chirurgiens de Centre de Lutte Contre le Cancer). Gynecol Oncol. 2006;102(3):523–529. doi:10.1016/j.ygyno.2006.01.022
27. Wang N, Li WW, Li JP, et al. Comparison of concurrent chemoradiotherapy followed by radical surgery and high-dose-rate intracavitary brachytherapy: a retrospective study of 240 patients with FIGO stage IIB cervical carcinoma. Onco Targets Ther. 2014;7:91–100. doi:10.2147/OTT.S52710
28. Todo Y, Yamazaki H, Takeshita S, et al. Close relationship between removal of circumflex iliac nodes to distal external iliac nodes and postoperative lower-extremity lymphedema in uterine corpus malignant tumors. Gynecol Oncol. 2015;139(1):160–164. doi:10.1016/j.ygyno.2015.07.003
29. Boland W, Bebb G. The emerging role of nimotuzumab in the treatment of non-small cell lung cancer. Biologics. 2010;4:289–298.
30. Basavaraj C, Sierra P, Shivu J, Melarkode R, Montero E, Nair P. Nimotuzumab with chemoradiation confers a survival advantage in treatment-naive head and neck tumors over expressing EGFR. Cancer Biol Ther. 2010;10(7):673–681. doi:10.4161/cbt.10.7.12793
31. Chen Y, Wu X, Hao D, et al. Neoadjuvant nimotuzumab plus chemoradiotherapy compared to neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma. Oncotarget. 2019;10(40):4069–4078. doi:10.18632/oncotarget.23861
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


