Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Long-Term Outcomes and Risk Factors for Mortality in a Cohort of HIV-Infected Children Receiving Antiretroviral Therapy in Vietnam
Authors Nguyen RN , Ton QC, Luong MH, Le LHL
Received 6 October 2020
Accepted for publication 10 November 2020
Published 24 November 2020 Volume 2020:12 Pages 779—787
DOI https://doi.org/10.2147/HIV.S284868
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Rang Ngoc Nguyen,1,2 Quang Chanh Ton,2 My Huong Luong,2 Ly Ha Lien Le2
1Department of Pediatrics, Can Tho Univesity of Medicine and Pharmacy, Can Tho, Vietnam; 2Women and Children Hospital of An Giang, An Giang, Vietnam
Correspondence: Rang Ngoc Nguyen Tel +84 913106404
Email [email protected]
Background: Management of HIV-infected children on a long-term basis is a challenge in resource-limited countries. The aim of this study is to evaluate the long-term outcome and identify the risk factors for mortality in a cohort of children with antiretroviral therapy (ART) in Vietnam.
Patients and Methods: A retrospective cohort study was conducted in children aged 0– 15 years, seen at the outpatient clinic of the Women and Children Hospital of An Giang, Vietnam, from August 2006 to May 2019. Cox proportional-hazard models were used to determine factors associated with mortality.
Results: A total of 266 HIV-infected children were on ART. During 1545 child-years of follow-up (median follow-up was 5.8 years), 28 (10.5%) children died yielding a mortality rate of 1.8 death per 100 child-years. By multivariate analysis, World Health Organization clinical stage 3 or 4 (AHR; 7.86, 95% CI; 1.02– 60.3, P= 0.047), tuberculosis (TB) co-infection (AHR; 6.26, 95% CI; 2.50– 15.64, P= 0.001) and having severe immunosuppression before ART (AHR; 11.73, 95% CI; 1.52– 90.4, P= 0.018) were independent factors for mortality in these children.
Conclusion: Antiretroviral therapy has reduced mortality in HIV-infected children in resource-limited settings. Independent risk factors for mortality were advanced clinical stage (3 or 4), TB co-infection and severe immunosuppression. Early investigation and treatment of TB co-infection allow early ART initiation which may improve outcomes in our settings.
Keywords: antiretroviral therapy, children, tuberculosis, mortality
Introduction
HIV is a major public health issue in the world. It is estimated that about 37.9 million people worldwide are living with HIV/AIDS. Of these, 1.7 million were children under 15 years old by the end of 2018.1 In Vietnam, about 5400 children are living with HIV/AIDS.2
Several studies suggest that early Antiretroviral therapy (ART) benefits include slowing disease progression, increased immunity, reduced long-term neurologic sequelae, and reduced mortality.3,4 In response to the HIV epidemic, in 2005 the Ministry of Health of Vietnam issued guidelines for HIV/AIDS diagnosis and treatment and set up Outpatients Clinics (OPCs) in all provinces to provide free care and treatment to patients.5
An Giang is a southwestern province in the Mekong Delta of Vietnam and shares a border with Cambodia. The HIV prevalence of this province is about 0.6% and ranks fifth in the number of HIV infections in Vietnam.6 The pediatric outpatient clinic (pOPC) of the Women and Children Hospital of An Giang has initiated ART for HIV-infected children since 2006. This pOPC is funded by PEPFAR and Vietnam’s national HIV prevention and control program.
ART programs for HIV-infected children in Asian countries have been shown to be effective, with mortality rates ranging from 1.9 child-years to 4.9 child-years.7–11 The common risk factors for mortality were a low CD4 percentage, WHO clinical stage 3 or 4 and malnutrition.8–10,12 In a cohort of 266 HIV-infected children with suspected tuberculosis (TB) in four countries (Burkina Faso, Cambodia, Cameroon, and Vietnam), Marcy et al found that TB, young age, CD4 less than 10%, miliary features, and elevated serum transaminases were all independent predictors of mortality.13
In Vietnam, by the end of 2011, there were 3200 Vietnamese children with HIV/AIDS receiving ART; However, there were a few reports on ART outcomes among HIV-infected children14–16 In a study of 86 children on ART in Hanoi, Vietnam, Pham et al reported that the success rate (plasma HIV-1 viral load <1000 copies/mL) was 79.1% after 2-year follow-up.14 As far as this author knows, no studies to evaluate long-term ART outcomes for infected-HIV children have been conducted in Vietnam. The purpose of this study is to evaluate the long-term outcome and to identify the risk factors for mortality in a cohort of children receiving ART at one provincial OPC in Vietnam.
Patients and Methods
Study Design, Setting and Participants
This retrospective cohort study was conducted from August 2006 to May 2019, at the Women and Children Hospital of An Giang in the Mekong Delta of Vietnam. This hospital serves a catchment of about 560,000 children under 16 years of age, out of the 2.2 million population An Giang province. This province had the fifth-highest prevalence of HIV infection (0.6%) in Vietnam (Vietnam Ministry of Health, unpublished).
This study included children 2 months to 15 years of age diagnosed with HIV-1 infection, who were enrolled at pOPC and subsequently receiving ART. Children who did not receive ART were excluded from the analysis.
Laboratory tests were conducted at the central laboratory (Pasteur Institute in Ho Chi Minh city). HIV diagnosis was based on two HIV antibody tests for children≥ 18 months old or a double polymerase chain reaction (PCR) to detect HIV-1 proviral DNA for children less than 18 months old. The CD4+ T-cell count was tested on BD FACSCount™ (Becton Dickinson, USA) and was performed at An Giang General Hospital.
Data Collection
Data were extracted from the patient medical records kept at the pOPC at the end of the study. The study variables recorded twice at enrolment and at the end of the study included age, gender, WHO clinical stage, nutritional status, immunosuppression status (CD4 cell count, viral load), anemia (hemoglobinemia), abnormal liver enzymes (AST, ALT), TB co-infection and ART regimens. Recording outcome variables included lost to follow-up (LTFU), transfer out and mortality. Data were double-checked by a second reviewer for quality and accuracy.
Patient Care and Management
Before starting ART, children underwent a general examination including history, physical examination, chest X-ray and performing necessary laboratory tests (full blood count, liver enzymes, and CD4 count). HIV viral load testing was not routinely performed unless the patient does not respond to therapy. Comprehensive counseling for caregivers about ART adherence and side effects was provided.
ART initiation for HIV-infected children was indicated based on the child’s clinical stage and CD4 percentage according to the World Health Organization (WHO) guideline criteria in 2006 and the HIV diagnosis and treatment guidelines of the Ministry of Health in Vietnam.17,18 During the study period, the national guideline of HIV/AIDS treatment in Vietnam was changed from version 2006 to version 2015 (ART was given to all HIV-infected children ≥ 5 years of age with CD4 cell count ≤500 cells/mm,3 regardless of WHO clinical stage) and version 2017 (ART should be initiated in all HIV-infected children, regardless of CD4 cell count and WHO clinical stage) according to the WHO consolidated guidelines on the use of ARV drugs for treating and preventing HIV infection.19,20
Co-trimoxazole prophylaxis was given to all patients regardless of CD4 percentage levels. Besides, children under 2 years of age with HIV-tuberculosis co-infection received ART regardless of the CD4 counts. The first-line ART regimen was stavudine (D4T) or zidovudine (AZT) plus lamivudine (3TC) plus nevirapine (NVP) or efavirenz (EFZ). In patients with severe anemia (hemoglobin less than 8g/dL), AZT is replaced by D4T or abacavir (ABC). The lopinavir/ritonavir (LPV/r) was given to children already receiving NVP to prevent mother-to-child transmission of HIV (since 2010). For patients with combined TB infection, the first-line ART was started 2–8 weeks after anti-tuberculous therapy. A combination of three nucleoside reverse transcriptase inhibitors (AZT + 3TC + ABC) was prescribed for those weighing less than 10 kg and/or for those less than 3 years old; a combination of three antiretroviral drugs (AZT or D4T + 3TC + EFZ) was provided for children weighing ≥ 10 kg and/or for those ≥ 3 years of age. The patients with clinical, immunological or virological failures will be switched to second-line regimens (containing LPV/r).
Follow-Up
Children were followed at 2 weeks, at 1 month, and then at monthly intervals after ART initiation for the first 9 months and once per quarter thereafter. Tracing of LTFU patients was done by phone calls or direct visits (before 2016). At every appointment weight and height, WHO clinical stage, symptoms and clinical signs were recorded. Information regarding adherence, adverse drug effects, and intercurrent illness was obtained by interview. Follow-up hematologic and biochemical tests for liver function were performed 1 month after ART initiation and every 6 months thereafter. The CD4 cell profile was performed every 6 months from ART initiation or before switching to second-line therapy, and the plasma HIV-1 RNA level was performed when clinical or immunological failure was suspected. Clinical response to ART was assessed by a gain in weight and height and decreased frequency of hospitalization. Immunologic response was measured by a change in CD4 cell count and CD4%, whereas virologic response was assessed by change in plasma HIV-1 RNA levels from baseline. An undetectable viral load was defined as <200 copies/mL.
Operational Definitions
- Severe immunosuppression was defined when CD4 percentage is <25% in children less than 11 months, < 20% for children 12–35 months, < 15% for children 36–59 months, and < 15% or absolute CD4 count < 200 cells/mm3 for children ≥59 months.21
- Severe anemia was defined as a hemoglobin concentration value less than 8.0 g/dL
- Severe malnutrition was defined when the weight for age Z-score (WAZ) less than −3 standard deviations (SD) in children less than or equal to 10 years of age (using the WHO Anthro 3.0 software) or the BMI-for-age Z-score (BMIZ) less than −3SD in children older than 10 years of age (using the WHO Anthro plus software)22,23
- The abnormal liver enzymes were defined as an alanine transaminase (ALT) or aspartate transaminase (AST) higher than 43 U/liter (1.25 times the upper limit of normal)24
- Tuberculosis was diagnosed before or after ART which was based on the following criteria: (1) patients with positive sputum or gastric aspirates for acid-fast bacilli (AFB) by GeneXpert MTB/RIF assay; (2) or patients with negative sputum for AFB; however, they had a persistent cough and/or fever for more than 2 weeks history of contact with TB patients, failure to thrive, failure of antibiotics for a pulmonary infection and radiographic signs compatible with active pulmonary TB.
Lost to Follow-Up, Transfer-Out and Deaths
- Lost to follow up (LTFU) was defined when the patient did not attend OPC for 3 consecutive months.
- Transfer-out when patients were transferred to other pOPC for continuing management or to move to adult OPC when children were over 15 years of age.
- Death: patient death were confirmed from hospital records. The cause of death was ascertained by the attending physician or by reports from family members.
All LTFU and transfer-out were censored on the date of their last visit. Death events were defined as all-cause deaths occurring after ART initiation but before the May 31, 2019 end of the study period.
Ethical Issues
Informed consent was taken from all parents and caregivers at enrolment. Approval for this study was obtained from the Science and Technology Board of the Women and Children Hospital of An Giang (reference No: 31b-QĐBVSN). The study was conducted in accordance with the Declaration of Helsinki. Patient confidentiality was assured.
Statistical Analysis
Categorical variables were expressed as numbers and percentages, continuous variables were expressed as median and interquartile range. Continuous variables were compared using the Mann–Whitney test, and categorical variables were compared using Pearson’s Chi-square test or Fischer’s exact test.
Cox proportional hazards models were used to identify factors (age, gender, baseline CD4 cell count, baseline WHO clinical stage, severe malnutrition, treatment for active TB, and ART regimens). Hazard ratios (HRs) and 95% confidence intervals (CI) were calculated. Multivariate Cox models were constructed by clinically significant variables. All analyzes were conducted using software SPSS 20.0 and a two-tailed P value of 0.05 was considered significant.
Results
Between August 2006 and May 2019, this pOPC has enrolled 303 children, of whom 266 HIV-1 infected children who started ART were included in this analysis. After 13 years of follow-up, 154 children were still retained in care. (Figure 1)
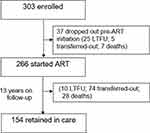 |
Figure 1 Schematic diagram of the follow-up of the children over 13 years of ART. |
Baselines Characteristics of HIV-Infected Children on ART
At the initiation of ART, there were 266 children aged between 0.2 and 15.8 years. The median age and the interquartile range (IQR) were 6.1 (3.5–9.0) years. Most children (80%) were 3 years of age or older. Males accounted for 50.4%
At the beginning of ART, the median CD4 count and CD4% were 386 cells/mm3 and 13.1%, respectively. 55.7% children had severe immunosuppression (SIS). The median hemoglobin was 11.4 g/dL. The median AST/ALT was 38/26 U/L. The median weight-for-height Z score (WHZ), weight-for-age Z score (WAZ), height-for-age Z score (HAZ), and the body mass index Z score (BMIZ) were −0.22 (IQR: −1.2; 0.56), −2.50 (IQR: −3.65; −1.48), −3.17 (IQR: −4.10; −1.96) and −1.04 (IQR: −2.33; −0.15), respectively (Table 1)
 |
Table 1 Baseline Characteristics of HIV-Infected Children on ART at Initiation and at the End of Study |
At the end of the study, all parameters were improved. Median CD4 count increased from 386 to 734 cells (P< 0.001), only 2 (1.5%) patients had SIS. Median hemoglobin level increased from 11.4 to 12.8 g/dL (P< 0.001), median AST/ALT decreased from 38/26 to 22/15 U/L (P< 0.001). All growth parameters (WAZ, HAZ and BMIZ) except WHZ were improved (Table 1)
The first line ART regimen was nevirapine-based (56.4%), efavirenz-based (16.9%), Lopinavir/r-based (16.2%) and ABC-based (10.5%). There were 29 (10.9%) patients switching to second line ART (containing Lopinavir/r) because of clinical (n=2), immunological (n=2) or virological failures (n=25). During follow-up, 74 (27.8%) were transferred out (transferred-out rate: 4.9 per 100 child-years), and 10 (3.8%) were LTFU (rate 0.6 per 100 child-years).
Treatment Outcomes
At the end of the study, 238 (89.4%) children were still alive. The viral load testing was performed in 166 children, 147 (88.5%) of those achieved a viral suppression level below
200 copies/mL of HIV-1 RNA. During 1545 child-years of follow-up (median follow-up was 5.8 years), 28 out of 266 patients receiving ART died yielding a mortality rate of 1.8 death per 100 child-years. There were 10 deaths (35.7%) within 3 months, 10 deaths (35.7%) within one year and 8 deaths after one year of follow-up. Half of the patients died in 2006–2007 when the pOPC was first established. Eleven (39%) patients died before 2012 and the remaining 3 (11%) patients died after 2012.
Cause of Deaths
Among 28 children who died, the median time from enrolment study to death was 0.5 years (IQR: 0.1–1.8 years). Causes of deaths were available for 25 children and included TB (n=11), severe malnutrition (n=5), pneumonia (n=4), sepsis (n=2) and encephalitis (n=2). Three children died at home with an unknown cause.
Risk Factors for Mortality
There were 55 (20.7%) children less than 3 years old, 134 (50.4%) of them were male. 36 (13.5%) patients were on TB treatment, of which 22 were diagnosed before ART and 14 were diagnosed after ART. There were 164 (61.7%) children with severe HIV disease (WHO clinical stages 3 or 4), 140 (55.7%) children having SIS and 68 (25.6%) children with severe malnutrition (WAZ <-3SD or BMIZ <-3SD) (Table 2).
 |
Table 2 Predictors of Mortality in 266 HIV-Infected Children on ART in Univariate and Multivariate Analyses in Cox Proportional Hazards Models, an Giang, Vietnam (2006–2019) |
In the univariate Cox regression analysis, the risk of mortality was more in HIV-infected children with TB co-infection (CHR; 8.02, 95% CI; 3.82–16.87, P< 0.001); severe malnutrition (CHR; 2.79, 95% CI; 1.33–5.88, P= 0.007); children with WHO clinical stage 3 or 4 (CHR; 17.85, 95% CI; 2.42–131.3, P= 0.005) and children with SIS (CHR; 23.62, 95% CI; 3.21–173.8, P= 0.001). The risk of mortality was not dependent on age, gender, and ART regimens. In the multivariate Cox regression, children with WHO clinical stage 3 or 4 (AHR; 7.86, 95% CI; 1.02–60.3, P= 0.047), SIS (AHR; 11.73, 95% CI; 1.52–90.4, P=0.018) and those with having TB co-infection (AHR; 6.26, 95% CI; 2.50–15.64, P=0.001) were significantly associated with a higher risk of mortality (Table 2).
Discussion
This study collected data starting in 2006 with a follow-up until 2019. To our knowledge, this is the first report to present the mortality rate and the risk factors for mortality from a cohort of HIV-infected children receiving ART in one provincial OPC in Vietnam.
A total of 266 children has initiated ART at a median (interquartile range) age of 6.1 (3.5–9.0) years. The median duration of ART was 5.8 years with 1545 child-years of follow-up. There were 28 (10.5%) deaths, yielding a mortality rate of 1.8 death per 100 child-years. Mortality was highest in the first three months after ART commencement and was frequently caused by tuberculosis (TB), pneumonia and severe malnutrition. In survivors, the viral suppression rate at the end of the study was 88.5%. The independent predictors of mortality in this cohort were advanced disease stage (WHO clinical stage 3 or 4), TB co-infection and severe immunosuppression (SIS).
In the current study, no association was found between age, gender and mortality rate in children on ART. This is in contrast with previous studies which suggested that girls25 and children with young age were at risk of mortality.25–29
The mortality rate in our study is lower than the rates of 2.25, 3.0 4.0, 4.7, and 8.4 per 100 child-years reported in Malawi, Lesotho, and Swaziland,30 in Nigeria,31 in Ethiopia,27 in South Africa,25 and in Kenya,32 respectively. It is also lower than the rate in other reports from in Asian countries such as China (2.31 per 100 child-years),9 Malaysia (2.86 per 100 child-years)8 and India (4.4–4.9 per 100 person-years).10 The outcome of this study is similar to that of Lumbiganon et al7 who studied 2280 children in 5 countries in the Asia-Pacific region, including Vietnam. However, our study has a longer duration of follow-up and lower LTFU rates. The mortality rate in this cohort was higher than those in recent studies in Nigeria33 and in Thailand,34 which reported 1.0 death and 1.3 deaths per 100 child-years, respectively. The outcome in these reports was better probably because their patients were followed up and treated at the referral centers with more facilities than at our pOPC.
Our facility in An Giang province had the highest prevalence of TB (252 cases per 100,000 in 2004) and ranked fifth-highest HIV infection in Vietnam.6 This may explain a high incidence of TB infections (13.5%) in HIV-infected children in this cohort and TB co-infection was significantly associated with a high risk of mortality. This finding was consistent with one study in Thailand, Lolekha et al35 which found that HIV-infected TB children were 6.9 times more likely to die than negative-TB children. Also, in the study by Mwiru et al36 in Myanmar, a history of TB infections was found to be associated with an increased risk of mortality (RR, 1.55; 95% CI, 1.09–2.18). A recent study in South Africa showed that TB infection combined with HIV increased the risk of death by up to 76%37
In one study with a large cohort from 6 Asian countries, Mu et al38 have demonstrated that TB co-infection increased the risk of virologic failure in HIV-infected children and adolescents. In another cohort data from Mozambique and Uganda, Costernaro et al39 revealed that HIV-patients with clinical stage 4 and TB co-infection were significantly associated with treatment failure. In HIV-infected adult patients, Assefa et al40 also found that incident TB increased the risk of immunological failure in patients on ART. These may explain why HIV-infected children combined with TB have a higher death rate. Contrary to our study, one study in Myanmar with a large cohort of 1159 children with nearly half of those having TB at diagnosis, Kaung Nyunt et al41 revealed that TB co-infections were not significantly associated with higher death. In a meta-analysis of 25 cohort studies, Soeters et al42 also found that adult patients receiving TB treatment at the time of ART initiation experienced similar virologic suppression and CD4 cell count reconstitution as those not receiving TB treatment.
Severe immunosuppression is an independent risk factor for mortality in this study. In a 5-year cohort study in Thailand, Collins et al43 found that CD4 percentage was associated with death, with a 5% decrease in baseline CD4 percentage resulting in an increased risk of death by 67%. This finding was also supported by most previous studies in resource-limited countries (RLCs) and most authors suggest that a decrease in CD4 percentage below 10% was significantly associated with death in children on ART.26,31,43–48 When comparing RLCs and developed countries (DCs), Peacock-Villada et al49 found that baseline CD4% in RLCs countries (12%) was lower than that in DCs (23%). As a result, the mortality rate per 100 child-years after ART was significantly higher in RLCs than in DCs (8.0 vs 0.9, P< 0.001).
The advanced disease stage (WHO clinical stage 3 or 4) is also a predictor of mortality in our study. Children at baseline with clinical stage 3 or 4 had 7.2 times the chance of death when compared with children at clinical stage 1 or 2. The findings of this study appear to be in line with other previous studies in Asia including Vietnam, Bartlett et al12 found that more than half of children have experienced a WHO stage 3 or 4 at presentation and the advanced disease stage was associated with higher mortality.7,12 In a cohort of 1818 Chinese children with 93 deaths, Zhao et al9 found that WHO stage 3 or 4 disease increased the risk of death by 2.4 times (aHR = 2.4; 95% CI, 1.1–5.2). The advanced disease stage increased mortality risk has also been reported from many countries in Africa.27,28,46,50,51
The death rate of our study was still high due to the late presentation of patients who came to health facilities with advanced HIV disease, late diagnosis in HIV-infected children, delayed investigation of TB infection in HIV-infected children and interrupting ARV drugs supply which forced patients to have to change to other drugs. Half of the patients in our cohort died from the late presentation and delayed ART because there was no previous local HIV treatment facility until the newly established pOPC (years 2006–2007), while the remaining were diagnosed late due to delayed sending the samples to the central laboratories. After being equipped with PCR machines to diagnose HIV, GeneXpert system for TB diagnosis at our hospital and after HIV-infected children receiving early ART irrespective of their CD4 count according to the updated version (2015, 2017) issued by the National Health Ministry, the mortality of HIV-infected children in our setting has been improved. In recent years, only 3 HIV- patients died from TB co-infection and from poor adherence to ART.
The strength of this study is the low rate of LTFU and the long duration of follow-up (13 years). However, there are several limitations to our study. Firstly, this is a retrospective study and there was missing information, particularly regarding baseline viral loads and CD4 counts. Secondly, this study only conducted at one provincial OPC with a limited sample and the number of events was small (28 deaths), which may induce overfitting of the Cox model to predict risk factors for mortality. Thirdly, accurate causes of death could not be determined because an autopsy cannot be performed in our setting. Fourth, some patients with a negative test by GeneExpert assay for sputum or gastric aspirates rely on clinical grounds and pulmonary X-ray to diagnose TB. Lastly, all children who were LTFU or transferred out to other OPCs are considered as being censored.
Conclusions
Antiretroviral therapy has reduced mortality in HIV-infected children in this cohort. Independent risk factors for mortality are advanced clinical stage, TB co-infection and severe immunosuppression. Adequate equipment for HIV and TB diagnosis for point-of-care testing and early treatment according to 2016 WHO consolidated guidelines for starting all HIV-infected children on ART will improve the outcomes.20 A good outcome for the treatment of HIV-infected children at a provincial OPC in Vietnam may be a typical model for scaling up ART for children in resource-limited facilities, but additional data are needed to confirm these findings.
Acknowledgments
We thank Nguyen Thi Kieu Linh for collecting the data and Tai Huynh, MD, MMM for English editing. We also thank Pham Ngoc Dung MD and Phan Thi Xuan BSc for performing CD4 cell counts and other laboratory tests.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report that there are no conflicts of interest in this research.
References
1. UNAIDS. Global HIV & AIDS statistics-2019 fact sheet. Available from: https://www.unaids.org/en/resources/fact-sheet.
2. UNAIDS. UNAIDS data 2018; 2018. Available from: https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf.
3. Goetghebuer T, Haelterman E, Le Chenadec J, et al. European infant collaboration group. Effect of early antiretroviral therapy on the risk of AIDS/death in HIV-infected infants. AIDS. 2009;23(5):597–604. doi:10.1097/QAD.0b013e328326ca37
4. Violari A, Cotton MF, Gibb DM, et al. CHer study team. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–2244. doi:10.1056/NEJMoa0800971
5. Nguyen DB, Do NT, Shiraishi RW, et al. Outcomes of antiretroviral therapy in Vietnam: results from a national evaluation. PLoS One. 2013;8(2):e55750. doi:10.1371/journal.pone.0055750
6. Thuy TT, Shah NS, Anh MH, et al. HIV-associated TB in An Giang Province, Vietnam, 2001–2004: epidemiology and TB treatment outcomes. PLoS One. 2007;2(6):e507. doi:10.1371/journal.pone.0000507
7. Lumbiganon P, Kosalaraksa P, Bunupuradah T, et al. HIV-infected children in the Asia-Pacific region with baseline severe anemia: antiretroviral therapy and outcomes. Asian Biomed (Res Rev News). 2016;10(3):229–234.
8. Moy FS, Fahey P, Nik Yusoff NK, Razali KA, Nallusamy R, Asia Pediatric TREAT. HIV observational database (TApHOD) outcomes of human immunodeficiency virus-infected children after anti-retroviral therapy in Malaysia. J Paediatr Child Health. 2015;51(2):204–208. doi:10.1111/jpc.12712
9. Zhao Y, Li C, Sun X, et al. Mortality and treatment outcomes of China’s national pediatric antiretroviral therapy program. Clin Infect Dis. 2013;56(5):735–744. doi:10.1093/cid/cis941
10. Nimkar S, Valvi C, Kadam D, et al. Loss to follow-up and mortality among HIV-infected adolescents receiving antiretroviral therapy in Pune, India. HIV Med. 2018;19(6):395–402. doi:10.1111/hiv.12605
11. Sophan S, Meng CY, Pean P, et al. Virologic and immunologic outcomes in HIV-infected Cambodian children after 18 months of highly active Antiretroviral therapy (HAART). Southeast Asian J Trop Med Public Health. 2010;41(1):126–137.
12. Bartlett AW, Truong KH, Songtaweesin WN, et al. Characteristics, mortality and outcomes at transition for adolescents with perinatal HIV infection in Asia. AIDS. 2018;32(12):1689–1697. doi:10.1097/QAD.0000000000001883
13. Marcy O, Tejiokem M, Msellati P, et al. Mortality and its determinants in antiretroviral treatment-naive HIV-infected children with suspected tuberculosis: an observational cohort study. Lancet HIV. 2018;5(2):e87–e95. doi:10.1016/S2352-3018(17)30206-0
14. Pham HV, Ishizaki A, Nguyen LV, et al. Two-year outcome of first-line antiretroviral therapy among HIV-1 vertically-infected children in Hanoi, Vietnam. Int J STD AIDS. 2015;26(11):821–830. doi:10.1177/0956462414556328
15. Dang MD, Nguyen DM, Tran HB, et al. Clinical characteristics of pediatric HIV-1 patients treated with first-line antiretroviral therapy in Vietnam: a nested case-control study. Int J Public Health. 2017;62(Suppl 1):113–119. doi:10.1007/s00038-016-0937-2
16. Dang VPL, Pham VH, Dinh TT, Le TH, Nguyen VL, Vu TP. Growth in children infected with HIV receiving anti-retroviral therapy in Vietnam. Pediatr Int. 2019;61(4):369–374. doi:10.1111/ped.13800
17. WHO. Antiretroviral therapy for HIV infection in adult and adolescents; 2006. Available from: https://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf.
18. Vietnam Ministry of Health. Guidelines for HIV/AIDS Diagnosis and Treatment. Hanoi: Vietnam Ministry of Health; 2017.
19. Vietnam Ministry of Health. Guidelines for HIV/AIDS Diagnosis and Treatment. Hanoi: Vietnam Ministry of Health; 2015.
20. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach.
21. WHO. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva, Switzerland: WHO Library Cataloging-in-Publication Data; 2007. Available from: https://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf?ua=1.
22. WHO. Nutrition landscape information system (NLIS); 2010. Available from: https://www.who.int/nutrition/nlis_interpretation_guide.pdf.
23. De Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. doi:10.2471/BLT.07.043497
24. Dusingize JC, Hoover DR, Shi Q, et al. Association of abnormal liver function parameters with HIV serostatus and CD4 count in antiretroviral-naive Rwandan women. AIDS Res Hum Retroviruses. 2015;31(7):723–730. doi:10.1089/aid.2014.0170
25. Zanoni BC, Phungula T, Zanoni HM, France H, Feeney ME. Risk factors associated with increased mortality among HIV infected children initiating antiretroviral therapy (ART) in South Africa. PLoS One. 2011;6(7):e22706. doi:10.1371/journal.pone.0022706
26. Gebremedhin A, Gebremariam S, Haile F, Weldearegawi B, Decotelli C. Predictors of mortality among HIV infected children on anti-retroviral therapy in Mekelle Hospital, Northern Ethiopia: a retrospective cohort study. BMC Public Health. 2013;6(13):1047. doi:10.1186/1471-2458-13-1047
27. Koye DN, Ayele TA, Zeleke BM. Predictors of mortality among children on antiretroviral therapy at a referral hospital, Northwest Ethiopia: a retrospective follow up study. BMC Pediatr. 2012;12(1):161. doi:10.1186/1471-2431-12-161
28. Mutanga JN, Mutembo S, Ezeamama AE, et al. Long-term survival outcomes of HIV infected children receiving antiretroviral therapy: an observational study from Zambia (2003–2015). BMC Public Health. 2019;19(1):115. doi:10.1186/s12889-019-6444-7
29. Shabangu P, Beke A, Manda S, Mthethwa N, Mthethwa N. Predictors of survival among HIV-positive children on ART in Swaziland. Afr J AIDS Res. 2017;16(4):335–343. doi:10.2989/16085906.2017.1386219
30. Kabue MM, Buck WC, Wanless SR, et al. Mortality and clinical outcomes in HIV-infected children on antiretroviral therapy in Malawi, Lesotho, and Swaziland. Pediatrics. 2012;130(3):e591–9. doi:10.1542/peds.2011-1187
31. Anigilaje EA, Aderibigbe SA. Mortality in a cohort of HIV-infected children: a 2-month outcome of antiretroviral therapy in Makurdi, Nigeria. Adv Med. 2018;9:6409134.
32. Wamalwa DC, Obimbo EM, Farquhar C, et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatr. 2010;18(10):33.
33. Ebonyi AO, Oguche S, Meloni ST, et al. Predictors of mortality in a clinic cohort of HIV-1 infected children initiated on antiretroviral therapy in Jos, Nigeria. J AIDS Clin Res. 2014;5(12):403.
34. Phongsamart W, Hansudewechakul R, Bunupuradah T, et al. Long-term outcomes of HIV-infected children in Thailand: the Thailand pediatric HIV observational database. Int J Infect Dis. 2014;22:19–24. doi:10.1016/j.ijid.2013.12.011
35. Lolekha R, Anuwatnonthakate A, Nateniyom S, et al. Childhood TB epidemiology and treatment outcomes in Thailand: a TB active surveillance network, 2004 to 2006. BMC Infect Dis. 2008;18(8):94. doi:10.1186/1471-2334-8-94
36. Mwiru RS, Spiegelman D, Duggan C, et al. Nutritional status and other baseline predictors of mortality among HIV-infected children initiating antiretroviral therapy in Tanzania. J Int Assoc Provid AIDS Care. 2015;14(2):172–179. doi:10.1177/2325957413500852
37. Bassett IV, Chetty S, Wang B, et al. Loss to follow-up and mortality among HIV-infected people co-infected with TB at ART initiation in Durban, South Africa. J Acquir Immune Defic Syndr. 2012;59(1):25–30. doi:10.1097/QAI.0b013e31823d3aba
38. Mu W, Bartlett AW, Bunupuradah T, et al. Early and late virologic failure after virologic suppression in HIV-infected asian children and adolescents. J Acquir Immune Defic Syndr. 2019;80(3):308–315. doi:10.1097/QAI.0000000000001921
39. Costenaro P, Penazzato M, Lundin R, et al. Predictors of treatment failure in hiv-positive children receiving combination antiretroviral therapy: cohort data from Mozambique and Uganda. J Pediatric Infect Dis Soc. 2015;4(1):39–48. doi:10.1093/jpids/piu032
40. Assefa A, Gelaw B, Getnet G, Yitayew G. The effect of incident tuberculosis on immunological response of HIV patients on highly active antiretroviral therapy at the university of Gondar hospital, northwest Ethiopia: a retrospective follow-up study. BMC Infect Dis. 2014;14:468.
41. Kaung Nyunt KK, Han WW, Satyanarayana S, et al. Factors associated with death and loss to follow-up in children on antiretroviral care in mingalardon specialist hospital, Myanmar, 2006–2016. PLoS One. 2018;13(4):e0195435. doi:10.1371/journal.pone.0195435
42. Soeters HM, Napravnik S, Patel MR, Eron JJ
43. Collins IJ, Jourdain G, Hansudewechakul R, et al. Long-term survival of HIV-infected children receiving antiretroviral therapy in Thailand: a 5-year observational cohort study. Clin Infect Dis. 2010;51(12):1449–1457. doi:10.1086/657401
44. Anaky MF, Duvignac J, Wemin L, et al. Scaling up antiretroviral therapy for HIV-infected children in Côte d’Ivoire: determinants of survival and loss to programme. Bull World Health Organ. 2010;88(7):490–499. doi:10.2471/BLT.09.068015
45. Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298(16):1888–1899. doi:10.1001/jama.298.16.1888
46. Bong CN, Yu JK, Chiang HC, et al. Risk factors for early mortality in children on adult fixed-dose combination antiretroviral treatment in a central hospital in Malawi. AIDS. 2007;21(13):1805–1810. doi:10.1097/QAD.0b013e3282c3a9e4
47. Fenner L, Brinkhof MW, Keiser O, et al. International epidemiologic databases to evaluate AIDS in Southern Africa. Early mortality and loss to follow-up in HIV-infected children starting antiretroviral therapy in Southern Africa. J Acquir Immune Defic Syndr. 2010;54(5):524–532. doi:10.1097/QAI.0b013e3181e0c4cf
48. Musoke PM, Mudiope P, Barlow-Mosha LN, et al. Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort study. BMC Pediatr. 2010;10(1):56. doi:10.1186/1471-2431-10-56
49. Peacock-Villada E, Richardson BA, John-Stewart GC, John-Stewart GC . Post-HAART outcomes in pediatric populations: comparison of resource-limited and developed countries. Pediatrics. 2011;127(2):e423–41. doi:10.1542/peds.2009-2701
50. Biru M, Hallström I, Lundqvist P, Jerene D, Paraskevis D. Rates and predictors of attrition among children on antiretroviral therapy in Ethiopia: a prospective cohort study. PLoS One. 2018;13(2):e0189777. doi:10.1371/journal.pone.0189777
51. Ebissa G, Deyessa N, Biadgilign S. Predictors of early mortality in a cohort of HIV-infected children receiving highly active antiretroviral treatment in public hospitals in Ethiopia. AIDS Care. 2015;27(6):723–730. doi:10.1080/09540121.2014.997180
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
