Back to Journals » International Medical Case Reports Journal » Volume 13
Long Term Follow-Up of Prototheca keratitis: A Case Report
Authors Minato K , Yoshikawa M , Nakanishi H , Hasegawa K
Received 29 June 2020
Accepted for publication 4 September 2020
Published 9 October 2020 Volume 2020:13 Pages 503—506
DOI https://doi.org/10.2147/IMCRJ.S268696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kazumi Minato,1 Munemitsu Yoshikawa,1 Hideo Nakanishi,1 Kaori Hasegawa2
1Department of Ophthalmology, Eye Center, Hidaka Medical Center, Toyooka Hospital, Toyooka City, Hyogo 669-5392, Japan; 2Department of Microbiology, Toyooka Hospital, Toyooka City, Hyogo 668-0065, Japan
Correspondence: Kazumi Minato
Department of Ophthalmology, Eye Center, Hidaka Medical Center, Toyooka Hospital, Hidaka-Cho Iwanaka 81, Toyooka City, Hyogo 669-5392, Japan
Tel +81-796-42-1611
Fax +81-796-42-2344
Email [email protected]
Background: Prototheca spp. are rare human pathogens, and only three cases of Prototheca keratitis have been reported. They were treated with anti-fungal drugs and surgical excision. Two of the three cases were successful, and the case of an immunocompromised patient was not successful. Thus, the best treatment of Prototheca keratitis is still undetermined, and further investigations are needed. The purpose of this report is to present our findings in a case of Prototheca keratitis that was successfully treated with topical medications without surgical excision.
Methods: This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Hidaka Medical Center, Toyooka Hospital. A written informed consent was obtained from the patient before beginning the medical treatments.
Case Report: A 75-year-old man with a history of stage 4 prostate carcinoma and bilateral limbal stem cell deficiency had undergone keratoepithelioplasty on his left eye for the deficiency. Postoperatively, a greyish-white epithelial opacity was noted on the central cornea of his left eye, and he had been treated with topical fluorometholone and oral dexamethasone together with a therapeutic contact lens. Corneal smears and contact lens swabs were positive for Prototheca spp. He required a continuous treatment with amphotericin B (AMPH-B) ointment, topical fluconazole (FLCZ), and voriconazole (VRCZ). This treatment protocol was effective, but recurrences developed when his general condition worsened.
Conclusion: Our findings indicate that Prototheca keratitis can be successfully treated but not cured with topical AMPH-B, FLCZ, and VRCZ without surgical treatment. However, recurrences can develop when the general condition of the patient worsens. Thus, continuous monitoring and treatment are necessary in cases of Prototheca keratitis.
Keywords: Prototheca spp., infection, cornea, treatment
Introduction
Prototheca spp. are achlorophyllous algae that are ubiquitous in nature and can infect the cornea of humans to cause Prototheca keratitis. The Prototheca spp. are spherical unicellular organisms that do not possess glucosamine, a specific fungal cell wall component, or muramic acid, a specific bacterial cell wall component. Lass-Flörl and Mayr investigated the in vitro effectiveness of several antimicrobials against Prototheca spp., and they reported that Prototheca spp. were sensitive to amphotericin B (AMPH-B) and had varied susceptibility to azoles. They also had varied susceptibility to a wide range of antibacterial agents, such as tetracycline, gentamicin, and amikacin.1
The Prototheca keratitis is rare, and only three cases have been reported. They were treated with triazole and polyene, anti-fungal drugs, and surgical excision.2–4 The treatment of two of three cases was successful, but of one case of an immunocompromised patient failed.
We report our findings in a case of Prototheca keratitis that was successfully treated with topical AMPH-B, fluconazole (FLCZ), and voriconazole (VRCZ). The patient was followed for 3 years, and recurrences developed when his general condition worsened.
Methods
The procedures used in this study conformed to the tenets of the Declaration of Helsinki and were approved by the Ethics Committee of Hidaka Medical Center, Toyooka Hospital. A written informed consent was obtained from the patient before beginning the medical treatments.
Case Report
A 75-year-old man with a history of stage 4 prostate carcinoma that was first diagnosed in 2008 developed an idiopathic limbal stem cell deficiency and underwent bilateral keratoepithelioplasty by a previous doctor in 2011. He was prescribed the therapeutic contact lenses postoperatively. He was referred to our clinic for further ophthalmic therapy in November 2012.
Our initial examination found that he had bilateral end-stage primary open-angle glaucoma, prior bilateral keratoepithelioplasty, and phacoemulsification with posterior chamber intraocular lens implantation in the left eye. His decimal visual acuity with Landolt broken ring chart was 0.07 in the right eye and 0.2 in the left eye, and his intraocular pressures were 13 mmHg in the right eye and 13 mmHg in the left eye. He had been treated with 0.3% topical gatifloxacin and 0.1% topical fluorometholone twice a day, topical travoprost and timolol maleate once a day, and 1% topical dorzolamide hydrochloride three times a day for glaucoma. He was also prescribed therapeutic contact lenses for both eyes to protect the corneal epithelium. Systemically, he had stage 4 prostate carcinoma and had had a cerebral infarction. He had been treated with oral anti-androgen drug and 0.1mg/day of dexamethasone. His general condition was very poor. He was examined monthly, and his ocular condition had stabilized by September 2015. His decimal visual acuity with Landolt broken ring chart was 0.2 in the right and left eyes, and his intraocular pressures were 6 mmHg in the right and left eyes in September 2015.
He visited our clinic in October 2015 with blurred vision in his left eye of two weeks duration. Our slit-lamp examination showed a greyish-white epithelial opacity in the central cornea of his left eye (Figure 1). His decimal visual acuity with Landolt broken ring chart was 0.2 in the right eye and 0.07 in the left eye, and his intraocular pressures were 14 mmHg in the right eye and 15 mmHg in the left eye. The patient was advised to stop using the therapeutic contact lens and 0.1% topical fluorometholone. Fungiflora Y staining of cultures of corneal smears and therapeutic contact lens swabs were negative for fungus but Gram-positive organisms were detected. The organisms were unicellular, spherical, and contained multiple endospores (Figure 2). We identified the organisms as Prototheca spp. based on the morphology. He was started on topical 0.3% tobramycin four times a day, but he could not continue because of irritation. So, 0.5% AMPH-B ointment was prescribed three times a day along with 0.2% topical FLCZ six times a day, and corneal surface debridement was performed four times. The keratitis improved and 4 months later a culture of the ocular surface was negative for Prototheca spp..
 |
Figure 1 Photograph of the anterior surface of the cornea of a 75-year-old man. A grayish-white epithelial opacity can be seen on the central cornea. |
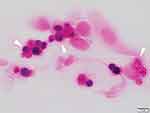 |
Figure 2 Photograph of a corneal smear with Gram staining. ×1000. Spherical Prototheca spp. organisms containing multiple endospores (morula-like form) can be seen in the corneal epithelial cells. |
We planned to continue the AMPH-B and FLCZ treatments but had to stop the eye treatments because of a worsening of his general condition. Two months later, a greyish-white epithelial opacity in the cornea of his left eye was detected again (Figure 3). We considered either continuing with the medical treatments or performing therapeutic penetrating keratoplasty (PKP) even though there was a risk of disseminating Prototheca into the intraocular space. We presented the risks of both treatment plans, and the patient declined undergoing therapeutic PKP. So, he was retreated with 0.5% AMPH-B ointment three times a day and 0.2% topical FLCZ six times a day without debridement, and the lesion did not recur. From November 2016 to April 2018, the grayish-white lesion continued to recur during periods of radiation for the recurrence of the prostate carcinoma and invasion of the urinary bladder. We continued the AMPH-B and FLCZ treatments, and the lesions were improved whenever his general condition recovered. The recurrent lesions were intermittent and remained only in the epithelial layer and did not spread into deeper layers. In April 2018, 300 mg/day of oral VRCZ was added to the treatment regimen because new grayish-white epithelial infiltrations were noted. Two months later he stopped the oral medication because he was diagnosed by his oncologist to be at the terminal stage, and he was switched topical 1% VRCZ six times a day. The infiltration was slowly resolved but he died in September 2018.
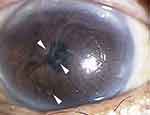 |
Figure 3 Recurrent lesions. The recurrent lesions can be seen in different areas of cornea. Spiral-shaped corneal surface is due to limbal stem cell deficiency. |
Discussion and Conclusions
Two hundred and sixty-six cases of human protothecosis have been reported up to August 2020.5 Todd et al reviewed 160 cases of human protothecosis in 2012. They reported that Prototheca spp. were low-grade pathogens in a normal host, but corticosteroid use or immunodeficiency of the patients greatly increased the susceptibility and severity of Prototheca spp. infections. Protothecosis is a chronic infection with low-grade inflammation. They reported that total excision of the lesions had a high success rate, but partial incision and drainage or simple debridement were not successful.6 Todd et al stated that intravenous AMPH-B was the most effective medication for protothecosis.7
Only three cases of Prototheca keratitis have been reported. In the first case, a Prototheca infection was detected in a patient after undergone PKP for Fuchs corneal endothelial dystrophy.2 In the second case, Prototheca keratitis and chronic endophthalmitis were found in a patient with Steven–Johnson syndrome and Boston type1 keratoprosthesis.3 The third patient had ulcerative Prototheca keratitis and was a diabetic patient post corneal trauma with no prior ocular surgery.4 The two former cases had prior treatment with systemic or topical steroids and the second case was immunodeficient.
Our case had prior treatment with oral dexamethasone for end-stage prostate carcinoma and 0.1% topical fluorometholone for limbal stem cell deficiency. We followed the chronic Prototheca keratitis treatment with 0.5% AMPH-B ointment, topical 0.2% FLCZ, topical 0.1%VRCZ and oral VRCZ. All of the antifungal drugs were effective but the Prototheca keratitis recurred when the patient’s general condition worsened. We continued treating him with topical AMPH-B and some azoles. The treatment was effective but not completely successful and recurrences occurred when the patient’s general condition worsened. The recurrent corneal lesions did not spread into deeper layers.
From our findings, we suggest that two features of Prototheca keratitis are important. First, the effectiveness of antifungal agents depended not only on the cell membranes of the Prototheca species but also on the overall immunocompetence of the patient. Neutrophils engulf the prototheca cells, and their degranulation and oxidative metabolism are necessary to kill the cells.8 Second, there is possibility that Prototheca keratitis without penetrating wound can be resolved with medical treatment. Prototheca species are not invasive, and we assumed that the recurrent lesions appeared because of the debridement at the same time as corneal smears and his limbal stem cell deficiency.
Thus, the management of Prototheca keratitis is difficult. We should consider the patient’s general condition particularly the use of steroids, presence of a penetrating wound, sensitivity to AMPH-B and azoles, the margins of the surgical excision, and the timing. In addition, complications from the long-term use of some of the medications should be considered when medical treatments are used.
In summary, we present our findings of a case of Prototheca keratitis that was successfully treated with AMPH-B ointment, topical FLCZ, and topical VRCZ without surgical excision. The lesion was improved but he was not cured by the treatment. The efficacy of AMPH-B and azoles depended on the general condition of the patient.
Informed Consent
A signed written informed consent for publication of clinical details and clinical images was obtained from the spouse of the patient.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20:230–242. doi:10.1128/CMR.00032-06
2. Solky AC, Laver NM, Williams J. Prototheca wickerhamii infection of a corneal graft. Cornea. 2011;30:1173–1175. doi:10.1097/ICO.0b013e3182011f28
3. Ng J, Minckler D, Walsh TJ, et al. An intractable case of prototheca keratitis and chronic endophthalmitis in Stevens-Johnson syndrome with Boston type1 keratoprosthesis. Cornea. 2016;35:1257–1260. doi:10.1097/ICO.0000000000000949
4. Narayanan N, Vaidehi D, Dhanurekha L, et al. Unusual ulcerative keratitis caused by Prototheca wickerhamii in a diabetic patient. Indian J Ophthalmol. 2018;66:311–314.
5. National Library of Medicine.U.S. Available from: http://www.pubmed.gov.
6. Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up and review of previously published cases. Med Mycol. 2012;50:673–689. doi:10.3109/13693786.2012.677862
7. Todd JR, Matsumoto T, Ueno R, et al. Medical phycology2017. Med Mycol. 2018;56:188–204. doi:10.1093/mmy/myx162
8. Leimann B, Monteiro P, Lazera M, et al. Protothecosis. Med Mycol. 2004;42:95–106. doi:10.1080/13695780310001653653
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
