Back to Journals » Journal of Inflammation Research » Volume 16
Long-Term Effects of Severe Burns on the Kidneys: Research Advances and Potential Therapeutic Approaches
Authors Yang G , Tan L, Yao H, Xiong Z, Wu J, Huang X
Received 16 January 2023
Accepted for publication 14 April 2023
Published 1 May 2023 Volume 2023:16 Pages 1905—1921
DOI https://doi.org/10.2147/JIR.S404983
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Guang Yang,1,2,* Lishan Tan,1,* Hua Yao,3 Zuying Xiong,1 Jun Wu,4,5 Xiaoyan Huang1
1Division of Renal Medicine, Peking University Shenzhen Hospital, Peking University, Shenzhen, 518000, People’s Republic of China; 2Department of Life Sciences, Yuncheng University, Yuncheng, 044006, People’s Republic of China; 3Guangxi Key Laboratory of Brain and Cognitive Neuroscience, Guilin Medical College, Guilin, 541004, People’s Republic of China; 4Department of Burn and Plastic Surgery, Shenzhen Institute of Translational Medicine, Shenzhen Second People’s Hospital, the First Affiliated Hospital of Shenzhen University, Shenzhen, 518035, People’s Republic of China; 5Human Histology & Embryology Section, Department of Surgery, Dentistry, Pediatrics & Gynecology, University of Verona Medical School, Verona, Venetia, 37134, Italy
*These authors contributed equally to this work
Correspondence: Xiaoyan Huang; Jun Wu, Email [email protected]; [email protected]
Abstract: Burns are a seriously underestimated form of trauma that not only damage the skin system but also cause various complications, such as acute kidney injury (AKI). Recent clinical studies have shown that the proportion of chronic kidney diseases (CKD) in burn patients after discharge is significantly higher than that in the general population, but the mechanism behind this is controversial. The traditional view is that CKD is associated with hypoperfusion, AKI, sepsis, and drugs administered in the early stages of burns. However, recent studies have shown that burns can cause long-term immune dysfunction, which is a high-risk factor for CKD. This suggests that burns affect the kidneys more than previously recognized. In other words, severe burns are not only an acute injury but also a chronic disease. Neglecting to study long-term kidney function in burn patients also results in a lack of preventive and therapeutic methods being developed. Furthermore, stem cells and their exosomes have shown excellent comprehensive therapeutic properties in the prevention and treatment of CKD, making them increasingly the focus of research attention. Their engineering strategy further improved the therapeutic performance. This review will focus on the research advances in burns on the development of CKD, illustrating the possible mechanism of burn-induced CKD and introducing potential biological treatment options and their engineering strategies.
Keywords: inflammation, acute kidney injury, chronic kidney diseases, stem cells, exosomes, sepsis, cytokine storm
Introduction
Burns are tissue injuries caused by changes in temperature, chemicals, physical factors, or radiation. According to World Health Organization (WHO), more than 180,000 people die from burns globally every year,1 and it is very difficult to quantify the number of burn victims. Burns not only injure skin tissue but also cause a variety of complications, of which kidney injury is one of the most common.2 There are three main types of kidney injury: acute kidney injury (AKI), acute kidney disease (AKD), and chronic kidney disease (CKD). AKD is a concept that has only been proposed in recent years, but past research on post-burn AKI included AKD. Among severe-burn patients, 38% develop AKI, and the average mortality rate of AKI patients is around 43%.2 In some areas, the mortality rate is as high as 80%.3 This phenomenon has been significantly reduced with the development of renal replacement therapy (RRT).4 It was considered that the physiological indicators of burn patients will return to normal when they are discharged from the hospital, so they generally will not return for follow-up. This makes long-term kidney function studies in burn patients unsustainable. However, a recent study showed that the probability of CKD in burn patients is about 2.4 times that of the normal population, and the incidence in women is higher as compared to men.5 This makes us realize that the care of burn patients should be long-term and continuous. In addition, understanding the underlying mechanisms of burn-induced CKD will help us develop relevant therapeutic approaches, which will have important implications for improving the long-term survival and quality of life of patients.
The etiology of CKD is diverse, and different pathological mechanisms require different treatment options. AKI is currently considered a main risk factor for CKD.6–9 However, there are no treatments for the progression of AKI to CKD.7,10,11 Similarly, although AKI is considered to be one of the risk factors for post-burn CKD, this has been controversial, and we also believe that other risk factors exist. Additionally, there is a lack of research into post-burn CKD, and no clear treatment plan has been proposed yet. We think that burns are a systemic injury and that the consequences of CKD may be multifaceted. Classical drug therapy may have limited efficacy. Therefore, there is an urgent need to find a more comprehensive treatment plan.
“Stem cells” is a general term for a class of cells with self-renewal and differentiation ability.12 As an emerging biologic therapy, stem cells show a more comprehensive therapeutic capability in the treatment of kidney injury compared with traditional drugs.13 Their excellent properties have led to the current booming stem cell therapy industry. Exosomes are extracellular vesicles that offer the same therapeutic properties as the cells from which they are derived, but they also represent a cell-free therapeutic approach, conferring the advantages of being non-immunogenic and non-tumorigenic.14–16 At present, researchers are continuously transforming stem cells and exosomes through engineering strategies, which improve not only the therapeutic effect but also the targeting and penetration. This also makes exosomes the most promising next-generation biologics.
Due to limited medical resources, long-term kidney function in burn patients has received little attention, and treatments have not been adequately validated. Here, we will focus on the long-term effects of burns on kidney function in the form of a literature review, discuss the possible mechanisms, and illustrate the possible value of stem cells, exosomes, and the engineering strategies involved in the prevention and treatment of post-burn CKD.
Effects of Burns on the Kidneys
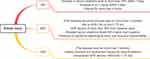
Decreased kidney function is generally divided into three types: acute renal failure (also known as AKI), chronic renal failure (also known as CKD), and the condition in between these is often referred to as AKD17 (Figure 1). They are usually distinguished by the duration of the disease. AKI was defined as abnormal kidney function with a disease duration of 7 days. AKI is divided into three stages based on severity.18 AKD presents as an abnormality in kidney function or structure, and the entire abnormality process does not last more than 3 months. CKD is defined as a disease that must last longer than 3 months. The development of CKD to an advanced stage is called end-stage kidney disease (ESKD). It turns out that burns not only cause AKI but also accelerate the progression of CKD. Since there are many studies on burns and AKI, this section will briefly describe the effects of burns on AKI and detail the research advances in burns on CKD.
Burns and AKI
There are many studies on the relationship between burns and AKI, but the results vary widely due to different criteria.3,19 We cited a meta-analysis published in 2020 for our review. This study looked at the occurrence of AKI in patients with severe burns in the intensive care unit, which pooled 33 studies and 8200 patients.2 The average incidence of AKI in these burn patients was 38%. Results vary widely from study to study with a peak incidence of 56%. It is worth noting that although the incidence of AKI in patients with mild, moderate, and severe AKI did not differ much, the mortality rate increased significantly with the severity. Approximately 12% of patients received RRT, but mortality rate remained high. Risk factors for burn-induced AKI include older age, hypertension, diabetes, burn extent and size, multiple organ failure, inhalation injury, surgery, previous chronic medical history, sepsis, mechanical ventilation, and rhabdomyolysis. In addition, AKI significantly prolonged hospital stay and increased mortality compared with non-AKI patients. Recent studies have found that burn-induced AKI is strongly associated with 90-day mortality.3,20 However, these studies focus on AKI after burns, and long-term kidney function changes are still poorly tracked.2
The causes of burn-induced AKI can be classified into two stages: early (day 0–3) or late (day 4–14)3,18 (Figure 2). Early-onset AKI is mainly caused by hypoperfusion, inflammation, release of damage-associated molecular patterns (DAMPs), hemodynamic changes, cardiac dysfunction, and hormone disorders. These risk factors lead to tubular and glomerular injury and ultimately induce AKI. Late-onset AKI is primarily caused by sepsis, fluid overload, multiple organ dysfunction syndrome (MODS), and nephrotoxic drug usage. However, some studies have pointed out that persistent inflammation is the core of AKI and is not related to decreased renal perfusion.21–23
 |
Figure 2 The causes of burn-induced AKI can be divided into two stages. |
At present, the most conventional treatment modality for burn-AKI is RRT.24 However, RRT is mainly involved in the early intervention, timing, method and cycle of RRT to improve survival and reduce CKD; however, further research is needed.24,25 In addition, many new small molecules and protein drugs have also been developed to reduce the effects of kidney-injuring molecules. For example, the C domain of insulin-like growth factor-1 nanoparticles bound to 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide (polyethylene glycol)] alleviate renal ischemia-reperfusion injury by reducing inflammation, oxidative stress and apoptosis.26 Another clinical study revealed that sodium bicarbonate Ringer’s solution reduces the incidence of AKI and the severity of kidney injuries after liver transplantation.27 However, some studies point out that some regular drugs used to treat AKI may have potential side effects. Furosemide, the most commonly used diuretic in AKI, has been found to dose-dependently increase kidney oxidative stress in patients with septic AKI.28 Post-burn AKI is closely related to sepsis, which makes the use of medications for post-burn AKI require extra caution.
Kidney Injury in Burn Patients without AKI
Another point of concern is whether kidney injury was also present in severe burn patients without detectable AKI. Although AKI includes changes in biomarkers and tissue injury, changes in kidney function in burn patients are mainly determined by blood and urine indicators. For patients in whom only body fluid biomarkers have been tested, there are cases in which biomarkers levels are normal but kidney injury has occurred. This phenomenon is also known as subclinical processes. A study based on kidney pathomorphological observation of 17 patients who died from severe burns showed that their glomeruli showed lesions of varying degrees but were relatively consistent.29 The specific manifestations are mesangial widening, mesangial cell proliferation and hypertrophy, capillary endothelial cell enlargement and cytoplasmic increase, and accumulation of neutrophils or monocytes in the lumen. It is suggested that severe burns can lead to the narrowing or occlusion of capillary loops causing glomerular ischemia, which is also called acute glomerulopathy. In addition, renal tubules showed varying degrees of degeneration, necrosis, and cast formation, and acute glomerulopathy is closely related to azotemia. Considering that renal tubular proximal epithelial cells are able to repair the injury by proliferation, whereas impairment of podocytes is usually permanent injury or loss. Therefore, in the long term, it is believed that severe burns may lead to kidney dysfunction through glomerulopathy.
AKI and CKD are consequences of changes in kidney function. These are all caused by severe kidney injury or excessive functional decline because healthy kidneys have compensatory and reserve capacity and minor injury may not cause a significant reduction in kidney function. We infer that severe burns lead to kidney injury, but not necessarily to significant changes in kidney function. In other words, severe burns can cause subclinical kidney processes. This also explains why AKI only occurs in some patients with severe burns or a history of kidney-related diseases, because the reserve capacity of the kidney is reduced in patients with severe burns or previous CKD.
Burns and Kidney Injury Biomarkers
Kidney function in burn patients is usually determined by blood and urine indicators, while biopsy can cause secondary injury and is not commonly used in burn patients. Serum creatinine (SCr) and urinary albumin/creatinine ratio (UACR) have been considered biomarkers of kidney function.30,31 Elevated values of SCr and UACR are considered to reflect changes in kidney function; however, this may not be the case for burn patients. A review published by Clark et al pointed out that burns induce changes in SCr, but the result may be difficult to predict in post-burn AKI.3 Burns can lead to decreased muscle mass, hepatic insufficiency, fluid overload, and sepsis, which all reduce the SCr values. In contrast, the SCr value may be increased by trauma, fever, and immobilization. Our unpublished study also showed that burns reduced SCr and UACR values in adenine-induced CKD mice even after 3 months of recovery. These unexpected changes may be related to alterations in muscle content, metabolism and water intake after burns.32 No in-depth studies are available. Therefore, we suggest that more research is needed on SCr and UACR as criteria for determining kidney function in burn patients, either in the early stages of burns or after hospital discharge. As for as the other biomarkers, further research is needed to determine their practical implication in burn patients.
Proteinuria testing is also a classic test for kidney function. A study of 16-year follow-up of patients with severe burns provides thought-provoking results.33 They found that proteinuria was prevalent in patients with severe burn- and sepsis-related AKI, and that the severity of proteinuria was positively correlated with peak creatinine and time to CRRT during AKI episodes. They explained that plasma from patients with severe burn sepsis was able to induce apoptosis of podocytes and renal tubular cells, thereby increasing the permeability of albumin.34,35 However, the proteinuria in these patients usually disappeared after one year. We believe that these results are significant, but we interpret them slightly differently. Renal tubular epithelial cells have a high proliferative capacity so that the injured tubules can repair themselves. Injury or loss of podocytes is usually considered permanent and hence difficult to regenerate after injury. Therefore, the disappearance of proteinuria in burn patients does not necessarily mean that the kidney is fully recovered, and the glomerular function is difficult to restore. Considering the inconsistent composition of proteinuria caused by tubular and glomerular injury,33,36 it is possible to consider the search for novel biomarkers as a diagnostic strategy for burn-related CKD.
Burns and CKD
Previous studies of burn-induced CKD have been limited by the low number of follow-up visits after discharge (Figure 3). Research related to whether burns can cause CKD has only started in the last few years. In 2009, a study of kidney dialysis in burn patients noted that few burn survivors required long-term dialysis.37 However, in 2016, a study showed that burns increase the risk of ESKD, a condition in which CKD develops into an advanced stage.5 The study, which counted burn patients in Finland between 1998 and 2011 showed an overall increased risk of ESKD following burns compared with the general population with a standardized incidence rate (SIR) of 2.40 (95% CI 1.73–3.23). The SIR was 3.11 for women (95% CI 1.66–5.32) and 1.89 for men (95% CI 1.27–2.69). Interestingly, among 43 ESKD patients, 38 were considered to have ESKD possibly unrelated to burns, and only 5 were considered to have ESKD related to burns. Their initiation of RRT ranged from 1 week to 14.8 years post-burn. The study concluded that severe burns are a high-risk factor for ESKD. They also pointed out that burns do not directly lead to ESKD but accelerate the progression of CKD to ESKD. These theories add another layer of information to what is already known—that burns can accelerate the progression of kidney failure, but this takes years or even more than a decade.
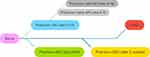 |
Figure 3 Different stages of post-burn kidney injury. |
A 2017 study followed independent risk factors for increased morbidity and mortality within 1 year in patients with burn-induced AKI.38 Burn-induced AKI was associated with the development of CKD, readmission, dialysis, and long-term mortality. In 2021, a meta-analysis revealed that burn-induced AKI+RRT patients have an increased probability of developing CKD and ESKD.39 About 35% of patients who received RRT during hospitalization required a period of RRT even if they survived. A clinical study conducted in 2021 showed that continuous RRT can alleviate kidney function decline after AKI in patients with post-burn septic shock.33 However, in the long term, these patients still experienced decreased kidney function.33 These studies have concluded that severe burns may accelerate the development of CKD through AKI. Alternatively, there are studies that take the opposite view, suggesting that post-burn CKD is not associated with AKI, which will be discussed later.
Furthermore, we could not find relevant studies, especially on the mechanism of this process. While these studies suggested that AKI is the key to accelerate the development of CKD, we believe that there could be more factors responsible for the development of CKD. We found that these studies focused more on patients with AKI following burns, while patients without AKI were rarely studied. Burns are known to have systemic and long-lasting effects on the body.40–42 CKD may be caused by more than just AKI. In addition, these studies still have insufficient follow-up time for patients. In general, kidney function gradually decreases with age.43 Young burn patients and short-term follow-up are insufficient to observe changes in kidney function. Next, this review will discuss the possible mechanism by which burns accelerate CKD progression. We hope to find some commonality in the mechanisms and further discuss potential treatments.
Potential Mechanisms of Burn-Induced CKD
The conclusion that burns cause and accelerate the development of CKD is beyond doubt. However, the reason and pathological process remain unclear. This section will focus on possible causes of CKD after burns. Since the AKI-CKD transition has been studied more, we would like to discuss more mechanisms beyond that. After all, there are too many factors of AKI-CKD transition and even less studies on burn injury.
AKI
In the 21st century, an increasing number of studies have demonstrated that AKI increases the incidence of CKD and ESKD.6–9,44–48 Many studies have explored their association, and it is believed that the reasons for AKI to CKD progression include the following points. (1) Ischemia-reperfusion may lead to epithelial cell injury and tubulointerstitial fibrosis.49,50 For example, AKI can lead to the accumulation of toxins that can cause kidney fibrosis. Indole sulfate causes post-ischemia-reperfusion kidney fibrosis by inducing epithelial mesenchymal transition, G2/M cell cycle arrest and exacerbating endoplasmic reticulum stress; however, 4-phenylbutyric acid can ameliorate this result.50 (2) There is reduced blood filtration capacity due to glomerular injury.51,52 For example, ischemia-reperfusion leads to tubular fibrosis and glomerular injury, which can be alleviated by a mineralocorticoid receptor blocker by reducing inflammation and increasing endothelial nitric oxide synthase serine 1179 phosphorylation and endothelin B receptor expression.52 (3) Capillary regeneration is a prerequisite for tissue repair/regeneration. AKI causes a decrease in capillary density, which may lead to hypoxia and an inability to carry away metabolites, which in turn leads to cellular stagnation and metabolic abnormalities, thus preventing normal tissue repair.53 (4) Additionally, AKI is often accompanied by vascular dysfunction and vascular calcification.54,55 Renal vascular injury and glomerular structure alteration can adversely affect the blood supply to tubular cells, leading to a progressive decrease in GFR.8 These studies mainly elucidate the causes of AKI-CKD transition in terms of tissue damages and structural alterations.
In addition to the above factors, AKI can also accelerate the CKD progression through hormonal regulation and functional modulation, such as inflammation, oxidative stress and dysregulation of the renin-angiotensin system, all of which affect kidney health.56–63 Animal studies have shown that AKI activates the intrarenal renin-angiotensin system (RAS), and that the blockade of RAS reduces CKD and mortality.64–68 Clinical studies have shown similar results, with RAS inhibitors reducing the risk of CKD in patients recovering from AKI.69–72 The timing of RAS inhibitor use is important, and inappropriate use may increase the risk of AKI.73 Persistent inflammation and oxidative stress after AKI drive the CKD progression by reducing tissue repair and promoting kidney tubular apoptosis.74–76 Inflammation and oxidative stress have been well studied in the AKI-CKD transition and we will not go into too much detail here.
Moreover, AKI causes various cellular dysfunctions in the kidney, including mitochondrial dysfunction, elevated oxidative stress, autophagy disturbances, cellular regulatory dysfunction, reduced tubular cell regenerative capacity, and immune cell dysregulation.7,77–83 For example, AKI disrupts mitochondrial homeostasis by increasing ROS, disrupting ultrastructure and mitochondrial-DNA, and inducing apoptosis, leading to a decrease in mitochondrial numbers and ultimately causing kidney microvascular injury, inflammation, and fibrosis.84–86 Chiara et al found that YAP1 maintains kidney function by driving tubular cell polyploidization, but this survival mechanism comes at the cost of cellular senescence, which accelerates the AKI-CKD transition.87 Inoue et al found that knockout of cellular communication network factor 2 alleviated AKI-CKD transition by reducing fibrosis and tubular epithelial cell apoptosis.88 Chen et al found that knockout of vanin-1 reduced tubular epithelial cells senescence by inhibiting RB1 expression and phosphorylation, thereby alleviating the AKI-CKD transition.89 A recent review pointed out that the metabolic process in tubular epithelial cells shifts from fatty acid β-oxidation to glycolysis due to disruptions in cellular functions and pathways.90 Although this shift increases ATP production, it also causes inflammation, lipid accumulation, and fibrosis. Thus, the AKI-CKD transition is the result of metabolic reprogramming.
Conversely, CKD is also a high-risk factor for AKI in hospitals.8 Already injured kidneys are more susceptible to risk factors; that is, kidney insufficiency patients are more prone to AKI than normal kidney patients after burns.91 For example, complications such as AKI after burns are more pronounced in patients who have undergone kidney transplantation. In addition, some clinical statistical studies have shown that the survival rate after burns is lower in the kidney insufficiency populations compared to the normal kidney function populations, but they did not explicitly state that this outcome was directly related to AKI.39,92,93 These studies suggested that AKI and CKD are mutually reinforcing. AKI contributes to the development and progression of CKD, and CKD increases the sensitivity or susceptibility to AKI. This also explains why burn patients with AKI are more likely to develop CKD and burn patients with kidney injury are more prone to AKI.
Indeed, there is disagreement on whether burn-induced AKI is the cause of CKD. Helantera et al suggested that burn-induced CKD may not be associated with AKI, and patients with preexisting kidney insufficiency are more likely to develop CKD after burns.5 However, subsequent studies have shown that burn-induced AKI patients are more likely to develop CKD.33,38,39 We agree with all these previous studies’ results, and the reason for the difference may be the statistical groups are different. Burns are a systemic injury that produce many risk factors for kidney injuries. These injuries are difficult to heal and persist for a considerable period of time. It can be explained in the following ways. First, when patients have insufficient kidney function, burns may cause AKI in the short term and accelerate the development of CKD. Second, when the patient’s kidneys are sensitive to risk factors, burns can cause kidney injury which is not easily detected in the short term because the kidneys have excess filtering capacity (renal reserve).94 Afterwards, kidney function continues to deteriorate after discharge until it is insufficient to cover the patient’s filtration needs. Third, burns cause AKI in patients with normal kidneys. When the patient is discharged from the hospital, the physiological indicators return to normal, but this does not mean that the renal injury does not exist. Later, any unexplained risk factors may accelerate the development of CKD. Therefore, we believe that burns cause kidney injury, which may or may not be detected in the short term. It can also be considered as a burn-induced subclinical process. In follow up, any risk factors that cause decreased kidney function may accelerate the development of CKD.
Aging
Age is an important factor affecting the function of different organs.43 Additionally, cell function generally decreases with aging, which is the basis of organ aging. Aging is not only a major risk factor for decreased kidney function but also a major risk factor for many diseases, such as diabetes, hypertension, and vascular disease, which are precisely high-risk factors for decreased kidney function.95,96
Some studies have found that aging is a main factor in the increased risk of CKD after kidney injury.97–99 Aging contributes to the development of CKD after AKI by affecting organ homeostasis, reducing recovery capacity, altering cytokine expression, and modulating progenitor and immune cell function. Animal experiments have shown that kidney injury produces aging-related pro-fibrotic and inflammatory factors to promote renal fibrosis and vascular damage, which may accelerate the progression of CKD.100–102 He et al suggested that young and healthy patients are mostly able to recover after experiencing AKI, whereas AKI in patients with CKD and related comorbidities is severe and refractory.8 Sato et al agreed and proposed that a single episode of AKI can lead to CKD and aging may increase the risk of progression from AKI to CKD.7 They suggested that chronic inflammation may be the main factor in the transition from AKI to CKD. Further, they emphasized that no drug has been shown to be effective to stop this progression. This makes the development of relevant therapeutic modalities more urgent.
Consistent with these theories, we believe that burns can cause more or less irreversible injury to the kidneys even when the kidneys are functioning normally.95 In healthy patients and non-severe burn patients, kidney injury can recover slowly.103 The main reason for this phenomenon is related to the proliferative capacity of the kidney cells. The proximal cells of the kidney tubular epithelium have a strong proliferative capacity and can be gradually repaired after injury. Other cells have a weaker ability to regenerate, such as podocytes. The loss or detachment of podocytes is usually considered to be permanent. As patients with mild kidney impairment become older and encounter some risk factors or face accidental injury, kidney function will further rapidly decline, eventually becoming CKD or ESKD. Therefore, we think that burns increase the incidence of AKI in people with kidney insufficiency as well as in older age. Additionally, aging aggravates AKI-induced vascular and renal injury, thereby accelerating the development of CKD and ESKD.
Persistent Inflammation
Following tissue injury, inflammatory cells accumulate at the injury site and differentiate into many different subtypes to achieve different functions.104 The proportions of these subtypes are not fixed and will change over time. For example, most macrophages polarize to the M1 subtype in the early stages of injury to clear microbes and necrotic tissue, and later to the M2 subtype to promote tissue repair.105,106 When these inflammatory cell subtypes are out of balance, they can cause abnormal tissue responses or injury. Numerous studies have confirmed that excessive or dysregulated inflammation can lead to kidney injury.30,31,107,108
Inflammation is a common response in burn patients. Severe burns can even cause systemic inflammatory response syndrome (SIRS)/cytokine storm,109 and SIRS can lead to multiple organ failure.109,110 Even if a burn patient is cured and discharged from the hospital, the inflammation can persist for months to years.40,42 There is no doubt that persistent inflammation induced by burns is a great threat to kidney health. Furthermore, inflammation appears to be a major factor in AKI and aging accelerating CKD development.7 Therefore, we think that burns accelerate the progression of CKD by causing renal injury through persistent inflammation.
Summary
At present, the incidence of post-burn AKI is not high and is associated with a high mortality rate within the first 3 weeks after burn.111 Moreover, the absence of AKI does not mean that the kidneys are not injured. Internal organ injury is known to be interconnected with kidney failure, exacerbating the failure of other organs and vice versa. Injury to any organ caused by burns can lead to decreased kidney function. According to our hypothesis, burns may accelerate CKD progression through AKI, aging, and persistent inflammation even after the patient is discharged from the hospital (Figure 4). Regardless, the long-term health of burn patients deserves attention. Therefore, considering the large population of burn patients, it is appropriate to carry out long-term kidney function tests and find relevant treatment options.
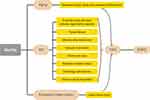 |
Figure 4 Schematic diagram of the etiology of post-burn CKD. Abbreviations: AKI, acute kidney injury; CKD, chronic kidney injury; ESKD, end-stage kidney disease. |
Stem Cells and Their Engineering Strategies in Kidney Therapy
Options for Stem Cell Therapy in Burn Patients
In fact, for either of these reasons, the end result is an increased incidence of CKD and ESKD in burn patients. The pathogenesis of CKD is complex, and it is difficult to completely cure it; therefore, we need to rely on RRT for survival in the later stages. At present, there is no effective treatment for AKI to CKD progression,7 and the treatment of burn-related CKD progression has never been studied. Risks of treatment are greater in cases of uncertain etiology such as drug toxicity and side effects of long-term dialysis. Looking for a comprehensive and safe treatment plan is the best choice. We hope to find a treatment that not only reduces inflammation and promotes tissue repair but also works as an anti-vascular aging agent. Additionally, stem cells came to our attention for their comprehensive therapeutic modality.
Stem cell therapy is a comprehensive treatment modality with proven efficacy in anti-aging, anti-inflammation, reducing kidney injury, and promoting tissue repair.13,112–114 MSCs are present in a variety of tissues, including umbilical cord, adipose, bone marrow, connective tissue, and organ stroma. They have the characteristics of pluripotent cells with the potential for self-renewal, proliferation, and multi-lineage differentiation. The most registered stem cells in clinical studies are human umbilical cord mesenchymal stem cells (HucMSCs) and bone marrow stem cells (BMSCs).115,116 Considering the specificity of burn patients, we think that allogeneic-derived MSCs are more feasible, especially HucMSCs. HucMSCs are isolated from the umbilical cord and have the advantages of low immunogenicity and avoidance of secondary injuries caused by harvesting from burn patients. Therefore, this section will discuss the possibility of treating burn patients with HucMSCs.
HucMSCs and Kidney Injury
Some studies have confirmed the role of HucMSCs in the treatment of AKI and CKD. In an ischemia-reperfusion AKI model, HucMSCs could improve kidney function in rats by reducing inflammation, scavenging free radicals, and inhibiting apoptosis.117,118 In a cisplatin-induced kidney injury study, HucMSCs promoted renal cell regeneration, eliminated inflammatory responses, inhibited apoptosis, and protected mitochondria, thereby ameliorating AKI in the early stage and alleviating the development of renal interstitial fibrosis in the later stage.119 Another similar study showed that both HucMSCs and human umbilical cord blood mononuclear cells (HcbMNCs) isolated from the human umbilical cord attenuated cisplatin-induced AKI by inhibiting high-mobility group box 1 (HMGB1) expression and reducing cell apoptosis.120 In addition, some cell experiments also provided evidence for the role of HucMSCs in the treatment of kidney injury. Xiang et al reported that HucMSCs enhanced autophagy in HK-2 cells treated with advanced oxidation protein products by inhibiting the PI3K/AKT/mTOR pathway.121
Another thing to keep in mind is the treatment period. Burn-induced persistent inflammation may typically last for years, and unfortunately stem cells have limited treatment time. Multiple injections of stem cells may be a better strategy. A study based on sepsis-induced AKI showed that compared with the no-treatment group, a single administration of HucMSCs reduced serum inflammatory factors and the ratio of intraperitoneal macrophage M1/M2 subpopulations.122 Compared with a single administration of HucMSCs, repeated administration decreased serum inflammatory factor concentrations and plasma NGAL levels and rescued sepsis-related depletion of intrarenal myeloid cell and T-cell subpopulations. Therefore, we suggest that burn patients may require long-term stem cell therapy to prevent related complications.
Perspectives for Engineered Stem Cells
Engineered stem cells are an important direction for the future development of stem cell therapy.123 Studies have shown that genetic modification of stem cells can improve the effect of MSCs in the treatment of kidney injury. Yuan et al reported that vascular endothelial growth factor (VEGF)-modified human embryonic MSCs alleviated cisplatin-induced AKI by promoting peritubular capillary angiogenesis, increasing cell proliferation, and decreasing apoptosis.124 Chen et al reported that hepatic growth factor (HGF)-modified HucMSCs ameliorated renal injury in rats with ischemia-reperfusion AKI through anti-apoptosis and anti-inflammation pathways.125
Moreover, combining exosomes with new materials to manufacture multifunctional biomaterials is also an important development direction for engineered exosomes. Park et al integrated some angiogenic factors (VEGF or angiopoietin-1) or anti-inflammatory factors (erythropoietin or α-melanocyte-stimulating hormone) into HucMSCs by genetic technology, and then made them into scaffold-free cell sheets to apply it on the surface of the decapsulated kidney.126 The results showed that the new biomaterial significantly improved renal dysfunction in AKI mice, which was superior to intravenous injection of engineered HucMSCs.
According to our experience, the most important direction of stem cell therapy for post-burn visceral injury should be to improve the therapeutic effect, rather than targeted delivery, because a severe burn is a systemic injury, not an organ-specific injury. Therefore, in burn treatment, improving the effect of stem cell therapy and evaluating its safety are the most important. Stem-cell-targeted therapy may be more suitable for some special burn patients.
Exosomes and Their Engineering Strategies in Kidney Therapy
Exosomes are small-sized vesicles secreted by cells, and they play an important role in intercellular transmembrane communication. Their efficacy comes from substrates, including nucleic acids, proteins, and metabolites. Compared with stem cells, exosomes have the advantages of low immunogenicity, non-tumorigenicity, smaller size, and stronger penetrating ability (Figure 5).123 This also clarifies whether the source of exosomes is autologous or allogeneic. Since the efficacy of exosomes is similar to that of the cells from which they are derived, stem-cell-derived exosomes are the first choice for treatment.
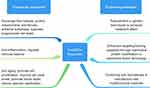 |
Figure 5 Therapeutic mechanisms of stem cells and exosomes and their engineering strategies. |
Exosomes and Kidney Injury
Several studies have demonstrated that MSC-derived extracellular vesicles (EVs) improve kidney function in several animal models of AKI and CKD, including drug-/toxin-induced nephropathy, ischemia-reperfusion injury, ureteral obstruction, renal vascular disease, and subtotal nephrectomy.127–133 For example, in an ischemic AKI study, HucMSC exosomes targeted injured renal proximal tubules by virtue of the VLA-4 and LFA-1 on exosomes surface and ameliorated tubular epithelial cell cycle arrest and apoptosis through the miR-125b-5p/p53 pathway, ultimately reducing renal injury and promoting tissue repair.134 Mechanistically, enriched miR-125b-5p suppressed p53 expression in tubular endothelial cells, leading to upregulation of CDK1/Cyclin B1 to rescue G2/M arrest and upregulation of Bcl-2/Bax ratio to inhibit Caspase-3-dependent apoptosis. In addition, numerous studies have confirmed that stem-cell-derived exosomes can reduce inflammation, reduce programmed cell death, alleviate kidney injury and promote tissue regeneration through the mRNA, miRNA, and proteins they contain.135–141 These studies are not limited to HucMSCs; however, they are mainly preclinical animal studies, and clinical data are still scarce.
Moreover, MSC exosomes have also been shown to be effective in reducing renal cell senescence. As aging progresses, accumulating gene expression errors and epigenetic perturbations affect cellular homeostasis, such as increased oxidative stress, mitochondrial dysfunction, activated programmed cell death signals, and decreased proliferation rates, leading to increased secretion of pro-fibrotic senescence-associated cytokines, which in turn accelerate fibroblast activation.142–145 If these perturbations can be regulated or corrected, they may slow down the cellular senescence process. A cellular study found that human MSC exosomes reduced murine kidney primary tubular epithelial cell senescence while reducing DNA damage and promoting cell proliferation.143 Furthermore, exosomes are involved in vascular calcification, which is one of the important causes of vascular aging and kidney disease.146,147 A study found that exogenous endothelial progenitor exosomes may ameliorate sepsis and prevent microvascular dysfunction via miR-126 delivery.148 Another study found that BMSC exosomes inhibited hyperphosphate-induced aortic calcification and alleviated CKD progression through the SIRT6/HMGB1 deacetylation pathway.149 It is important to note that exosomes are only carriers and the substrates they contain are the regulators that exert their physiological effects. The efficacy of the substrates usually depends on the source cells. Exosomes from healthy stem cells generally have positive effects, while exosomes from unhealthy cells may have reduced efficacy or even negative effects.123 Therefore, we believe that exosomes from cells used for specific medical purposes can reduce the cellular senescence process and the secretion of senescence-related cytokines by restoring cellular homeostasis, thus slowing down the tissue aging process. This suggests that the delivery of specifically enriched or knocked-out exosomes may become a therapeutic modality for the treatment of vascular calcification and CKD.
Perspectives for Engineered Exosomes
Engineered exosomes are a trend of future development. In the treatment of kidney injury, engineering technologies mainly focus on cell preconditioning and genetic modification.150–152 Further, improving the targeting stem cells is also an important engineering strategy. For example, engineered hybrid vesicles were fabricated by fusing human neutrophil membranes with HucMSC-derived small extracellular vesicles.153 This modification significantly enhanced targeting of injured kidney sites. In vivo and in vitro experiments demonstrated that the fusion vesicles ameliorated cisplatin-induced AKI by reducing inflammation, inhibiting apoptosis, promoting cell proliferation, and reducing cellular oxidative stress. In targeted therapy, our view is similar to stem cell therapy. So far, non-targeted engineered exosomes are sufficient to treat systemic injury caused by burns, and targeted engineered exosomes are more suitable for patients with severe injuries in specific parts. However, this approach assumes its use as a regular therapy to reduce post-burn persistent inflammation and general tissue injuries. In terms of precision medicine, non-targeted therapies may need further evaluation in groups with specific diseases, specific immunity, and specific complications. Stem cells and exosomes for the treatment of post-burn visceral injuries are rarely studied and cannot be discussed further here. It may be a future direction of research.
Another interesting direction is the treatment of CKD-related complications by engineering exosomes. For example, intramuscular injection of miR-26a-enriched exosomes prevented CKD-induced muscle wasting and alleviated cardiomyopathy.154 These technologies provide new therapeutic ideas for the precise treatment of various complications after burns.
Future Directions of Stem Cell and Exosomes in Post-Burn CKD Therapy
The limitation of the current application of MSCs is that they are thick, and intravenous injection may block capillaries. Although the cell volume has been reduced by optimizing the culture method,155 the effect in practical application still needs to be evaluated in the long term. Safety can only be guaranteed by further reducing the volume of stem cells. In addition, greater omentum wrapping has also been proposed.156 Stem cells immobilized in the kidney can continue to release factors for a certain period of time to treat the kidney. Therefore, finding a safe and effective drug delivery method is an important direction for future stem cell research.
Another concern with MSC therapy is its non-targeting characteristic. Most of the cells accumulate in the lungs and liver, which reduces the efficacy of the kidneys. Although burns can cause systemic chronic diseases, improving the ability of targeted therapy is undoubtedly a better choice for burn patients with specific diseases: while ensuring systemic efficacy, it can also improve the ability of targeted therapy. At present, some studies have improved the targeting ability through membrane protein modification or physical means.155 This is also an important direction for future research.
In terms of exosomes, we think that the major difficulty in clinical application lies in unifying production standards. At present, the sources of exosomes, cell culture methods and isolation methods are not uniform, so it is difficult to determine the quality control. In addition, risk factors in the postburn circulatory system require more research. A better understanding of this mechanism could provide a theoretical basis for the future development of engineered exosomes enriched with therapeutic factors.
The heterogeneity of MSCs from different sources is enormous. Although trophic factors from MSCs have been identified using multiple omics and microarray approaches there is no uniform conclusion.135 This also makes treatment outcomes more variable. Establishing a database of stem cell trophic factors will help in more targeted treatment of kidney injury diseases caused by different factors in the future.
People with renal failure often produce substances such as indoxyl sulfate that cannot be effectively removed by hemodialysis.157,158 These substances accumulate in the blood and may make stem cell therapy less effective.159 It is also necessary to find relevant solutions to improve stem cell therapy.
There are no in-depth studies on the treatment of post-burn kidney injury. Stem cells have just begun to be used in the treatment of kidney injury, and exosomes have not yet entered clinical research. As a result, the optimal timing of stem cell therapy for post-burn kidney injury is unknown. These are all worth exploring further.
Importantly, it is currently very expensive to produce exosomes and stem cells. Burn patients’ conditions require them to undergo long-term treatment, which incurs huge costs.
Conclusions
Taken together, we believe that burns cause kidney injury at an early stage, but mild injury is often masked by redundant kidney function. Afterwards, persistent inflammation further worsens kidney injury and accelerates the development of CKD until it progresses to ESKD. In other words, burns should not only be seen as an emergency but also as a chronic health problem after the patient is discharged from the hospital. Therefore, we should not only focus on kidney function changes in burn patients with AKI but also on long-term kidney function in burn patients without AKI. Early intervention and treatment are the right way to prevent CKD. Stem cells, exosomes, and their engineering strategies are extremely promising ways to improve the quality of life of burn patients in the future.
Abbreviations
AKI, acute kidney injury; AKD, acute kidney disease; BMSCs, bone marrow stem cells; CKD, chronic kidney disease; CRRT, continuous renal replacement therapy; DAMPs, damage-associated molecular patterns; EV, extracellular vesicle; ESKD, end-stage kidney disease; HucMSCs, human umbilical cord mesenchymal stem cells; HcbMNCs, human umbilical cord blood mononuclear cells; HMGB1, high-mobility group box 1; HGF, hepatic growth factor; MODS, multiple organ dysfunction syndrome; RAS, renin-angiotensin system; RRT, renal replacement therapy; SIR, standardized incidence rate; SIRS, systemic inflammatory response syndrome; VEGF, vascular endothelial growth factor.
Acknowledgments
The authors thank all the laboratory colleagues. Thanks to Ms. Fangting Zhang from the central laboratory for her kind assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by Shenzhen Postdoctoral Research Grant (50820191286), National Natural Science Foundation of China (82100726), Guangdong Basic and Applied Basic Research Foundation (2020A1515110970), Guangdong Provincial Key Clinical Specialty-Burn Surgery (2000004), Shenzhen San-Ming Project of Medicine (SZSM201812097), Shenzhen Science and Technology Innovation Commission (JCYJ20200109140412476, JCYJ20210324110403011, JCYJ20220530150412026).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. World Health Organization. Burns; 2018.
2. Folkestad T, Brurberg KG, Nordhuus KM, et al. Acute kidney injury in burn patients admitted to the intensive care unit: a systematic review and meta-analysis. Crit Care. 2020;24(1):2.
3. Clark A, Neyra JA, Madni T, et al. Acute kidney injury after burn. Burns. 2017;43(5):898–908.
4. Chen B, Zhao J, Zhang Z, et al. Clinical characteristics and risk factors for severe burns complicated by early acute kidney injury. Burns. 2020;46(5):1100–1106.
5. Helanterä I, Koljonen V, Finne P, Tukiainen E, Gissler M. The risk for end-stage renal disease is increased after burn. Burns. 2016;42(2):316–321.
6. Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol. 2013;9(2):77–85.
7. Sato Y, Yanagita M. Immune cells and inflammation in AKI to CKD progression. Am J Physiol Renal Physiol. 2018;315(6):F1501–f1512.
8. He L, Wei Q, Liu J, et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017;92(5):1071–1083.
9. Kurzhagen JT, Dellepiane S, Cantaluppi V, Rabb H. AKI: an increasingly recognized risk factor for CKD development and progression. J Nephrol. 2020;33(6):1171–1187.
10. Rayego-Mateos S, Marquez-Expósito L, Rodrigues-Diez R, et al. Molecular mechanisms of kidney injury and repair. Int J Mol Sci. 2022;23(3):1.
11. Gao L, Zhong X, Jin J, Li J, Meng XM. Potential targeted therapy and diagnosis based on novel insight into growth factors, receptors, and downstream effectors in acute kidney injury and acute kidney injury-chronic kidney disease progression. Signal Transduct Target Ther. 2020;5(1):9.
12. Wang M, Yao S, He D, et al. Type 2 diabetic mellitus inhibits skin renewal through inhibiting wnt-dependent Lgr5+ hair follicle stem cell activation in C57BL/6 mice. J Diabetes Res. 2022;2022:8938276.
13. Yun CW, Lee SH. Potential and therapeutic efficacy of cell-based therapy using mesenchymal stem cells for acute/chronic kidney disease. Int J Mol Sci. 2019;20:7.
14. Zhang Y, Wang C, Bai Z, Li P. Umbilical cord mesenchymal stem cell exosomes alleviate the progression of kidney failure by modulating inflammatory responses and oxidative stress in an ischemia-reperfusion mice model. J Biomed Nanotechnol. 2021;17(9):1874–1881.
15. Zhang Z-W, Wei P, Zhang G-J, et al. Intravenous infusion of the exosomes derived from human umbilical cord mesenchymal stem cells enhance neurological recovery after traumatic brain injury via suppressing the NF-κB pathway. Open Life Sci. 2022;17(1):189–201.
16. Cao Q, Huang C, Chen XM, Pollock CA. Mesenchymal stem cell-derived exosomes: toward cell-free therapeutic strategies in chronic kidney disease. Front Med. 2022;9:816656.
17. Levey AS. Defining AKD: the Spectrum of AKI, AKD, and CKD. Nephron. 2022;146(3):302–305.
18. Niculae A, Peride I, Tiglis M, et al. Burn-induced acute kidney injury-two-lane road: from molecular to clinical aspects. Int J Mol Sci. 2022;23:15.
19. Wu G, Xiao Y, Wang C, et al. Risk factors for acute kidney injury in patients with burn injury: a meta-analysis and systematic review. J Burn Care Res. 2017;38(5):271–282.
20. You B, Yang Z, Zhang Y, et al. Late-onset acute kidney injury is a poor prognostic sign for severe burn patients. Front Surg. 2022;9:842999.
21. Colpaert K, Hoste EA. Acute kidney injury in burns: a story of volume and inflammation. Crit Care. 2008;12(6):192.
22. Jeschke MG, Mlcak RP, Finnerty CC, et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11(4):R90.
23. Wan L, Bellomo R, Di Giantomasso D, Ronco C. The pathogenesis of septic acute renal failure. Curr Opin Crit Care. 2003;9(6):496–502.
24. Tan BK, Liew ZH, Kaushik M, Cheah AKW, Tan HK. Early initiation of renal replacement therapy among burned patients with acute kidney injury. Ann Plast Surg. 2020;84(4):375–378.
25. Su CL, Chang GH, Tsai IJ, Hsu CY, Wang IK, Chang CC. Factors impacting survival in patients with major burn-induced acute kidney injury postrenal replacement therapy: a nationwide study with 15 years follow-up in Taiwan. Ann Plast Surg. 2021;86(2S Suppl 1):S23–s29.
26. Xu M, Zhao M, Zheng D. Effect of IGF-1C domain-modified nanoparticles on renal ischemia-reperfusion injury in mice. Ren Fail. 2022;44(1):1376–1387.
27. Wu H, Meng G, Zuo C, et al. The Effects of sodium bicarbonate Ringer’s solution on acute kidney injury and the clinical outcomes after liver transplantation: a randomized controlled trial. Front Pharmacol. 2022;13:982472.
28. Silbert BI, Ho KM, Lipman J, et al. Does furosemide increase oxidative stress in acute kidney injury? Antioxid Redox Signal. 2017;26(5):221–226.
29. Shi J, Chen C, Li J, Shi T, Zhang G, Ke J. Pathological observation of kidneys in severe burn death cases. Peoples Lib Army Med J. 1983;167(1):49–52.
30. Gigliotti JC, Tin A, Pourafshar S, et al. GSTM1 deletion exaggerates kidney injury in experimental mouse models and confers the protective effect of cruciferous vegetables in mice and humans. J Am Soc Nephrol. 2020;31(1):102–116.
31. Hering L, Rahman M, Hoch H, et al. Alpha2A-adrenoceptors modulate renal sympathetic neurotransmission and protect against hypertensive kidney disease. J Am Soc Nephrol. 2020;31(4):783–798.
32. Palackic A, Suman OE, Porter C, Murton AJ, Crandall CG, Rivas E. Rehabilitative exercise training for burn injury. Sports Med. 2021;51(12):2469–2482.
33. Mariano F, De Biase C, Hollo Z, et al. Long-term preservation of renal function in septic shock burn patients requiring renal replacement therapy for acute kidney injury. J Clin Med. 2021;10:24.
34. Mariano F, Cantaluppi V, Stella M, et al. Circulating plasma factors induce tubular and glomerular alterations in septic burns patients. Crit Care. 2008;12(2):R42.
35. Oudemans-van Straaten HM. Circulating pro-apoptotic mediators in burn septic acute renal failure. Crit Care. 2008;12(2):126.
36. Wu VC, Chan CK, Chueh JS, et al. Markers of kidney tubular function deteriorate while those of kidney tubule health improve in primary aldosteronism after targeted treatments. J Am Heart Assoc. 2023;12(4):e028146.
37. Soltani A, Karsidag S, Garner W. A ten-year experience with hemodialysis in burn patients at Los Angeles County + USC Medical Center. J Burn Care Res. 2009;30(5):832–835.
38. Thalji SZ, Kothari AN, Kuo PC, Mosier MJ. Acute kidney injury in burn patients: clinically significant over the initial hospitalization and 1 year after injury: an original retrospective cohort study. Ann Surg. 2017;266(2):376–382.
39. Duan Z, Cai G, Li J, Chen F, Chen X. Meta-analysis of renal replacement therapy for burn patients: incidence rate, mortality, and renal outcome. Front Med. 2021;8:708533.
40. Mulder PPG, Vlig M, Boekema B, et al. Persistent systemic inflammation in patients with severe burn injury is accompanied by influx of immature neutrophils and shifts in T cell subsets and cytokine profiles. Front Immunol. 2020;11:621222.
41. Knuth CM, Auger C, Jeschke MG. Burn-induced hypermetabolism and skeletal muscle dysfunction. Am J Physiol Cell Physiol. 2021;321(1):C58–c71.
42. Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6(7):e21245.
43. Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. 2016;23(1):19–28.
44. Guzzi F, Cirillo L, Roperto RM, Romagnani P, Lazzeri E. Molecular mechanisms of the acute kidney injury to chronic kidney disease transition: an updated view. Int J Mol Sci. 2019;20:19.
45. Tan HL, Yap JQ, Qian Q. Acute kidney injury: tubular markers and risk for chronic kidney disease and end-stage kidney failure. Blood Purif. 2016;41(1–3):144–150.
46. Fiorentino M, Grandaliano G, Gesualdo L, Castellano G. Acute kidney injury to chronic kidney disease transition. Contrib Nephrol. 2018;193:45–54.
47. Hu MC, Shi M, Gillings N, et al. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 2017;91(5):1104–1114.
48. Heung M, Chawla LS. Predicting progression to chronic kidney disease after recovery from acute kidney injury. Curr Opin Nephrol Hypertens. 2012;21(6):628–634.
49. Shiva N, Sharma N, Kulkarni YA, Mulay SR, Gaikwad AB. Renal ischemia/reperfusion injury: an insight on in vitro and in vivo models. Life Sci. 2020;256:117860.
50. Chen JH, Chao CT, Huang JW, et al. Early elimination of uremic toxin ameliorates AKI-to-CKD transition. Clin Sci. 2021;135(23):2643–2658.
51. Cortinovis M, Perico N, Ruggenenti P, Remuzzi A, Remuzzi G. Glomerular hyperfiltration. Nat Rev Nephrol. 2022;18(7):435–451.
52. Barrera-Chimal J, Rocha L, Amador-Martínez I, et al. Delayed spironolactone administration prevents the transition from acute kidney injury to chronic kidney disease through improving renal inflammation. Nephrol Dial Transplant. 2019;34(5):794–801.
53. Polichnowski AJ. Microvascular rarefaction and hypertension in the impaired recovery and progression of kidney disease following AKI in preexisting CKD states. Am J Physiol Renal Physiol. 2018;315(6):F1513–f1518.
54. Bellomo R, Wan L, Langenberg C, Ishikawa K, May CN. Septic acute kidney injury: the glomerular arterioles. Contrib Nephrol. 2011;174:98–107.
55. Calzavacca P, May CN, Bellomo R. Glomerular haemodynamics, the renal sympathetic nervous system and sepsis-induced acute kidney injury. Nephrol Dial Transplant. 2014;29(12):2178–2184.
56. Chou YH, Chu TS, Lin SL. Role of renin-angiotensin system in acute kidney injury-chronic kidney disease transition. Nephrology. 2018;23(Suppl 4):121–125.
57. Maekawa H, Inagi R. Pathophysiological role of organelle stress/crosstalk in AKI-to-CKD Transition. Semin Nephrol. 2019;39(6):581–588.
58. Wang Z, Zhang C. From AKI to CKD: maladaptive repair and the underlying mechanisms. Int J Mol Sci. 2022;23(18):5.
59. Black LM, Lever JM, Traylor AM, et al. Divergent effects of AKI to CKD models on inflammation and fibrosis. Am J Physiol Renal Physiol. 2018;315(4):F1107–f1118.
60. Li Z, Li N. Epigenetic modification drives acute kidney injury-to-chronic kidney disease progression. Nephron. 2021;145(6):737–747.
61. Zuk A, Bonventre JV. Recent advances in acute kidney injury and its consequences and impact on chronic kidney disease. Curr Opin Nephrol Hypertens. 2019;28(4):397–405.
62. Basile DP, Collett JA. Orai1: a new therapeutic target for the acute kidney injury-to-chronic kidney disease transition. Nephron. 2022;146(3):264–267.
63. Meng X, Jin J, Lan HY. Driving role of macrophages in transition from acute kidney injury to chronic kidney disease. Chin Med J. 2022;135(7):757–766.
64. Zhang J, Rudemiller NP, Patel MB, et al. Competing actions of type 1 angiotensin II receptors expressed on T lymphocytes and kidney epithelium during cisplatin-induced AKI. J Am Soc Nephrol. 2016;27(8):2257–2264.
65. Rodríguez-Romo R, Benítez K, Barrera-Chimal J, et al. AT1 receptor antagonism before ischemia prevents the transition of acute kidney injury to chronic kidney disease. Kidney Int. 2016;89(2):363–373.
66. Barrera-Chimal J, Pérez-Villalva R, Rodríguez-Romo R, et al. Spironolactone prevents chronic kidney disease caused by ischemic acute kidney injury. Kidney Int. 2013;83(1):93–103.
67. Barrera-Chimal J, Prince S, Fadel F, et al. Sulfenic acid modification of endothelin B receptor is responsible for the benefit of a nonsteroidal mineralocorticoid receptor antagonist in renal ischemia. J Am Soc Nephrol. 2016;27(2):398–404.
68. Cheng SY, Chou YH, Liao FL, et al. Losartan reduces ensuing chronic kidney disease and mortality after acute kidney injury. Sci Rep. 2016;6:34265.
69. Cao W, Jin L, Zhou Z, et al. Overexpression of intrarenal renin-angiotensin system in human acute tubular necrosis. Kidney Blood Press Res. 2016;41(6):746–756.
70. Chen C, Yang X, Lei Y, et al. Urinary biomarkers at the time of AKI diagnosis as predictors of progression of AKI among patients with acute cardiorenal syndrome. Clin J Am Soc Nephrol. 2016;11(9):1536–1544.
71. Hsu CY, Hsu RK, Yang J, Ordonez JD, Zheng S, Go AS. Elevated BP after AKI. J Am Soc Nephrol. 2016;27(3):914–923.
72. Benedetto U, Melina G, Capuano F, et al. Preoperative angiotensin-converting enzyme inhibitors protect myocardium from ischemia during coronary artery bypass graft surgery. J Cardiovasc Med. 2008;9(11):1098–1103.
73. Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol. 2008;3(5):1266–1273.
74. Anders HJ. Immune system modulation of kidney regeneration--mechanisms and implications. Nat Rev Nephrol. 2014;10(6):347–358.
75. Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80(9):915–925.
76. Basile DP, Bonventre JV, Mehta R, et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol. 2016;27(3):687–697.
77. Jiang M, Bai M, Lei J, et al. Mitochondrial dysfunction and the AKI-to-CKD transition. Am J Physiol Renal Physiol. 2020;319(6):F1105–f1116.
78. Sato Y, Takahashi M, Yanagita M. Pathophysiology of AKI to CKD progression. Semin Nephrol. 2020;40(2):206–215.
79. Liu Z, Wang Y, Shu S, Cai J, Tang C, Dong Z. Non-coding RNAs in kidney injury and repair. Am J Physiol Cell Physiol. 2019;317(2):C177–c188.
80. De Chiara L, Conte C, Antonelli G, Lazzeri E. Tubular cell cycle response upon AKI: revising old and new paradigms to identify novel targets for CKD prevention. Int J Mol Sci. 2021;22:20.
81. Zhang X, Agborbesong E, Li X. The role of mitochondria in acute kidney injury and chronic kidney disease and its therapeutic potential. Int J Mol Sci. 2021;22:20.
82. Szeto HH. Pharmacologic approaches to improve mitochondrial function in AKI and CKD. J Am Soc Nephrol. 2017;28(10):2856–2865.
83. Cao C, Yao Y, Zeng R. Lymphocytes: versatile participants in acute kidney injury and progression to chronic kidney disease. Front Physiol. 2021;12:729084.
84. Baligand C, Qin H, True-Yasaki A, et al. Hyperpolarized (13) C magnetic resonance evaluation of renal ischemia reperfusion injury in a murine model. NMR Biomed. 2017;30:10.
85. Lan R, Geng H, Singha PK, et al. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J Am Soc Nephrol. 2016;27(11):3356–3367.
86. Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol. 2012;302(7):F853–864.
87. De Chiara L, Conte C, Semeraro R, et al. Tubular cell polyploidy protects from lethal acute kidney injury but promotes consequent chronic kidney disease. Nat Commun. 2022;13(1):5805.
88. Inoue T, Kusano T, Amano H, Nakamoto H, Okada H. Cellular communication network factor 2 (CCN2) promotes the progression of acute kidney injury to chronic kidney disease. Biochem Biophys Res Commun. 2019;517(1):96–102.
89. Chen J, Lu H, Wang X, et al. VNN1 contributes to the acute kidney injury-chronic kidney disease transition by promoting cellular senescence via affecting RB1 expression. FASEB j. 2022;36(9):e22472.
90. Zhu Z, Hu J, Chen Z, et al. Transition of acute kidney injury to chronic kidney disease: role of metabolic reprogramming. Metabolism. 2022;131:155194.
91. Zhang H, Qu W, Nazzal M, Ortiz J. Burn patients with history of kidney transplant experience increased incidence of wound infection. Burns. 2020;46(3):609–615.
92. Knowlin LT, Purcell L, Cairns BA, Charles AG. Burn injury mortality in patients with preexisting and new onset renal disease. Am J Surg. 2018;215(6):1011–1015.
93. Brusselaers N, Monstrey S, Colpaert K, Decruyenaere J, Blot SI, Hoste EA. Outcome of acute kidney injury in severe burns: a systematic review and meta-analysis. Intensive Care Med. 2010;36(6):915–925.
94. Ronco C, Bellomo R, Kellum J. Understanding renal functional reserve. Intensive Care Med. 2017;43(6):917–920.
95. Hoste EAJ, Kellum JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607–625.
96. Lin X, Jin H, Chai Y, Shou S. Cellular senescence and acute kidney injury. Pediatr Nephrol. 2022;37(12):3009–3018.
97. Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20(1):223–228.
98. Schmitt R, Cantley LG. The impact of aging on kidney repair. Am J Physiol Renal Physiol. 2008;294(6):F1265–1272.
99. Franzin R, Stasi A, Fiorentino M, et al. Inflammaging and complement system: a link between acute kidney injury and chronic graft damage. Front Immunol. 2020;11:734.
100. Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–543, 531p following 143.
101. Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82(2):172–183.
102. Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281(5):F887–899.
103. Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11(5):264–276.
104. Zhu M, Zhu M, Wu X, et al. Porcine acellular dermal matrix increases fat survival rate after fat grafting in nude mice. Aesthetic Plast Surg. 2021;2021:1.
105. Meng F, Qiu J, Chen H, et al. Dietary supplementation with N‐3 polyunsaturated fatty acid‐enriched fish oil promotes wound healing after ultraviolet B‐induced sunburn in mice. Food Sci Nutri. 2021;00:1–8.
106. Hammer A, Yang G, Friedrich J, et al. Role of the receptor Mas in macrophage-mediated inflammation in vivo. Proc Natl Acad Sci USA. 2016;113(49):14109–14114.
107. Hering L, Rahman M, Potthoff SA, Rump LC, Stegbauer J. Role of α2-adrenoceptors in hypertension: focus on renal sympathetic neurotransmitter release, inflammation, and sodium homeostasis. Front Physiol. 2020;11:566871.
108. Wang Y, Zhang H, Chen Q, et al. TNF-alpha/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif. 2020;53(6):e12829.
109. Gibson BHY, Wollenman CC, Moore-Lotridge SN, et al. Plasmin drives burn-induced systemic inflammatory response syndrome. JCI Insight. 2021;6:23.
110. Li Y, Zhang H, Chen C, et al. Biomimetic immunosuppressive exosomes that inhibit cytokine storms contribute to the alleviation of sepsis. Adv Mater. 2022;34(19):e2108476.
111. Kraft R, Herndon DN, Finnerty CC, Shahrokhi S, Jeschke MG. Occurrence of multiorgan dysfunction in pediatric burn patients: incidence and clinical outcome. Ann Surg. 2014;259(2):381–387.
112. Ohta H, Liu X, Maeda M. Autologous adipose mesenchymal stem cell administration in arteriosclerosis and potential for anti-aging application: a retrospective cohort study. Stem Cell Res Ther. 2020;11(1):538.
113. Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31–42.
114. Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15(7):1794–1804.
115. Markov A, Thangavelu L, Aravindhan S, et al. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther. 2021;12(1):192.
116. Gao J, Gao C. Development and regulation of stem cell-based therapies in China. Cell Prolif. 2022;55(8):e13217.
117. Cao H, Qian H, Xu W, et al. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett. 2010;32(5):725–732.
118. Fahmy SR, Soliman AM, El Ansary M, Elhamid SA, Mohsen H. Therapeutic efficacy of human umbilical cord mesenchymal stem cells transplantation against renal ischemia/reperfusion injury in rats. Tissue Cell. 2017;49(3):369–375.
119. Peng X, Xu H, Zhou Y, et al. Human umbilical cord mesenchymal stem cells attenuate cisplatin-induced acute and chronic renal injury. Exp Biol Med. 2013;238(8):960–970.
120. Xu Q, Yan P, Duan XJ, et al. Human umbilical cord-derived mesenchymal stem cells and human cord blood mononuclear cells protect against cisplatin-induced acute kidney injury in rat models. Exp Ther Med. 2020;20(6):145.
121. Xiang J, Jiang T, Zhang W, Xie W, Tang X, Zhang J. Human umbilical cord-derived mesenchymal stem cells enhanced HK-2 cell autophagy through MicroRNA-145 by inhibiting the PI3K/AKT/mTOR signaling pathway. Exp Cell Res. 2019;378(2):198–205.
122. Fazekas B, Alagesan S, Watson L, et al. Comparison of single and repeated dosing of anti-inflammatory human umbilical cord mesenchymal stromal cells in a mouse model of polymicrobial sepsis. Stem Cell Rev Rep. 2022;18(4):1444–1460.
123. Yang G, Waheed S, Wang C, Shekh M, Li Z, Wu J. Exosomes and their bioengineering strategies in the cutaneous wound healing and related complications: current knowledge and future perspectives. Int J Biol Sci. 2023;19(5):1430–1454.
124. Yuan L, Wu MJ, Sun HY, et al. VEGF-modified human embryonic mesenchymal stem cell implantation enhances protection against cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol. 2011;300(1):F207–218.
125. Chen Y, Qian H, Zhu W, et al. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev. 2011;20(1):103–113.
126. Park HJ, Kong MJ, Jang HJ, et al. A nonbiodegradable scaffold-free cell sheet of genome-engineered mesenchymal stem cells inhibits development of acute kidney injury. Kidney Int. 2021;99(1):117–133.
127. Hu Q, Lyon CJ, Fletcher JK, Tang W, Wan M, Hu TY. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharm Sin B. 2021;11(6):1493–1512.
128. Wan F, Yang RC, Tang YW, et al. BMSC-derived exosomes protect against kidney injury through regulating klotho in 5/6 nephrectomy rats. Eur J Med Res. 2022;27(1):118.
129. Aghajani Nargesi A, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther. 2017;8(1):273.
130. Jia H, Liu W, Zhang B, et al. HucMSC exosomes-delivered 14-3-3ζ enhanced autophagy via modulation of ATG16L in preventing cisplatin-induced acute kidney injury. Am J Transl Res. 2018;10(1):101–113.
131. Zhang R, Zhu Y, Li Y, et al. Human umbilical cord mesenchymal stem cell exosomes alleviate sepsis-associated acute kidney injury via regulating microRNA-146b expression. Biotechnol Lett. 2020;42(4):669–679.
132. Zhou Y, Xu H, Xu W, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4(2):34.
133. Huang J, Cao H, Cui B, et al. Mesenchymal stem cells-derived exosomes ameliorate ischemia/reperfusion induced acute kidney injury in a porcine model. Front Cell Dev Biol. 2022;10:899869.
134. Cao JY, Wang B, Tang TT, et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics. 2021;11(11):5248–5266.
135. Tsuji K, Kitamura S, Wada J. Secretomes from mesenchymal stem cells against acute kidney injury: possible heterogeneity. Stem Cells Int. 2018;2018:8693137.
136. Racchetti G, Meldolesi J. Extracellular vesicles of mesenchymal stem cells: therapeutic properties discovered with extraordinary success. Biomedicines. 2021;9:6.
137. Liu L, Wu Y, Wang P, et al. PSC-MSC-derived exosomes protect against kidney fibrosis in vivo and in vitro through the SIRT6/β-catenin signaling pathway. Int J Stem Cells. 2021;14(3):310–319.
138. Zhu F, Chong Lee Shin OLS, Pei G, et al. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget. 2017;8(41):70707–70726.
139. Yang Y, Wang J, Zhang Y, Hu X, Li L, Chen P. Exosomes derived from mesenchymal stem cells ameliorate renal fibrosis via delivery of miR-186-5p. Hum Cell. 2022;35(1):83–97.
140. Alasmari WA, El-Shetry ES, Ibrahim D, et al. Mesenchymal stem-cells’ exosomes are renoprotective in postmenopausal chronic kidney injury via reducing inflammation and degeneration. Free Radic Biol Med. 2022;182:150–159.
141. Alasmari WA, Abdelfattah-Hassan A, El-Ghazali HM, et al. Exosomes derived from BM-MSCs mitigate the development of chronic kidney damage post-menopause via interfering with fibrosis and apoptosis. Biomolecules. 2022;12:5.
142. Wang D, Kang L, Chen C, et al. Loss of legumain induces premature senescence and mediates aging-related renal fibrosis. Aging Cell. 2022;21(3):e13574.
143. Liao CM, Luo T, von der Ohe J, de Juan Mora B, Schmitt R, Hass R. Human MSC-derived exosomes reduce cellular senescence in renal epithelial cells. Int J Mol Sci. 2021;22:24.
144. Dan QQ, Chen L, Shi LL, Zhou X, Wang TH, Liu H. Urine-derived mesenchymal stem cells-derived exosomes enhances survival and proliferation of aging retinal ganglion cells. BMC Mol Cell Biol. 2023;24(1):8.
145. Yang G, Chen H, Chen Q, et al. Injury-induced interleukin-1 alpha promotes Lgr5 hair follicle stem cells de novo regeneration and proliferation via regulating regenerative microenvironment in mice. Inflamm Regen. 2023;43(1):14.
146. Liu S, Zhang N. Narrative review of exosomes: novel players in vascular calcification of chronic kidney disease. Ann Palliat Med. 2021;10(12):13002–13008.
147. Qin Z, Liao R, Xiong Y, et al. A narrative review of exosomes in vascular calcification. Ann Transl Med. 2021;9(7):579.
148. Zhou Y, Li P, Goodwin AJ, et al. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol Ther. 2018;26(5):1375–1384.
149. Wei W, Guo X, Gu L, et al. Bone marrow mesenchymal stem cell exosomes suppress phosphate-induced aortic calcification via SIRT6-HMGB1 deacetylation. Stem Cell Res Ther. 2021;12(1):235.
150. Yea JH, Yoon YM, Lee JH, Yun CW, Lee SH. Exosomes isolated from melatonin-stimulated mesenchymal stem cells improve kidney function by regulating inflammation and fibrosis in a chronic kidney disease mouse model. J Tissue Eng. 2021;12:20417314211059624.
151. Jin J, Qian F, Zheng D, He W, Gong J, He Q. Mesenchymal stem cells attenuate renal fibrosis via exosomes-mediated delivery of microRNA Let-7i-5p antagomir. Int J Nanomed. 2021;16:3565–3578.
152. Liang M, Zhang D, Zheng D, He W, Jin J. Exosomes from miR-374a-5p-modified mesenchymal stem cells inhibit the progression of renal fibrosis by regulating MAPK6/MK5/YAP axis. Bioengineered. 2022;13(2):4517–4527.
153. Wu P, Tang Y, Jin C, et al. Neutrophil membrane engineered HucMSC sEVs alleviate cisplatin-induced AKI by enhancing cellular uptake and targeting. J Nanobiotechnol. 2022;20(1):353.
154. Wang B, Zhang A, Wang H, et al. miR-26a limits muscle wasting and cardiac fibrosis through exosome-mediated microRNA transfer in chronic kidney disease. Theranostics. 2019;9(7):1864–1877.
155. Mo M, Zhou Y, Li S, Wu Y. Three-dimensional culture reduces cell size by increasing vesicle excretion. Stem Cells. 2018;36(2):286–292.
156. Yang Y, Geng X, Chi K, et al. Ultrasound enhances the therapeutic potential of mesenchymal stem cells wrapped in greater omentum for aristolochic acid nephropathy. Stem Cell Res Ther. 2021;12(1):261.
157. Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25(9):1897–1907.
158. Niwa T. Removal of protein-bound uraemic toxins by haemodialysis. Blood Purif. 2013;35(Suppl 2):20–25.
159. Wang W, Liu X, Wang W, et al. The effects of indoxyl sulfate on human umbilical cord-derived mesenchymal stem cells in vitro. Cell Physiol Biochem. 2016;38(1):401–414.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.