Back to Journals » OncoTargets and Therapy » Volume 14
Long Noncoding RNA CCDC26 Promotes Thyroid Cancer Malignant Progression via miR-422a/EZH2/Sirt6 Axis
Authors Ma X, Li Y, Song Y, Xu G
Received 15 September 2020
Accepted for publication 7 April 2021
Published 11 May 2021 Volume 2021:14 Pages 3083—3094
DOI https://doi.org/10.2147/OTT.S282011
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Xiao Ma,1 Yanyan Li,2 Yuntao Song,1 Guohui Xu1
1Key Laboratory of Carcinogenesis and Translational Research, Department of Head and Neck, Peking University Cancer Hospital and Institute, Beijing, 100142, People’s Republic of China; 2Department of Cardiology, Air Force Medical Center, Beijing, 100036, People’s Republic of China
Correspondence: Xiao Ma
Key Laboratory of Carcinogenesis and Translational Research, Department of Head and Neck, Perking University Cancer Hospital and Institute, No. 52 Fucheng Road, Haidian District, Beijing, 100142, People’s Republic of China
Email [email protected]
Purpose: Long noncoding RNAs are crucial regulators in thyroid cancer progression. However, the role of lncRNA CCDC26 in thyroid cancer remains unclear. Here, we aimed to explore the effect of CCDC26 on thyroid cancer progression and the underlying mechanism.
Materials and Methods: A total of 50 clinical thyroid cancer samples were studied in patients’ samples, cultured cells, and nude mice before and after treatment using quantitative reverse transcription-PCR, CCK-8 assays, BrdU incorporation assays, Transwell assays, cell apoptosis analysis, luciferase reporter gene assay, RNA immunoprecipitation, Western blot analysis, and tumorigenicity analysis.
Results: CCDC26 expression was elevated in patients’ thyroid cancer tissues and thyroid cancer cell lines. CCDC26 depletion remarkably reduced proliferation, invasion, and migration but induced apoptosis of thyroid cancer cells. Mechanically, miR-422a mimic remarkably reduced the luciferase activity of CCDC26 transfected cells but failed to affect cells transfected with CCDC26 containing the mutated miR-422a-binding site. RNA immunoprecipitation (RIP) assays showed that CCDC26 and miR-422a preferentially interacted with Ago2, but not IgG, in the micro-ribonucleoprotein complexes (miRNPs). CCDC26 depletion enhanced miR-422a expression and MiR-422a inhibitor reversed CCDC26 knockdown-induced inhibition of thyroid cancer progression in vitro. CCDC26 upregulated EZH2 and Sirt6 expression by sponging miR-422a in thyroid cancer cells. Tumorigenicity analysis in nude mice revealed that CCDC26 contributed to thyroid tumor growth via miR-422a/EZH2/Sirt6 axis in vivo.
Conclusion: CCDC26 promotes thyroid cancer malignant progression via miR-422a/EZH2/Sirt6 axis. This finding provides new insights into the mechanism by which CCDC26 promotes malignant thyroid cancer development, advances our understanding of lncRNAs’ association with thyroid cancer, and indicates that CCDC26 and miR-422a may serve as potential targets for thyroid cancer.
Keywords: thyroid cancer, CCDC26, miR-422a, EZH2, Sirt6
Introduction
Thyroid cancer is a common endocrine tumor with severe morbidity1 and steadily growing incidence,3,4 resulting in approximately 1% of cancer-related morbidity globally.2 Although thyroid cancer displays a favorable prognosis,5 patients with aggressive metastasis exhibit considerably poor outcomes.6 Understanding the molecular mechanisms underlying thyroid cancer progression will benefit thyroid cancer diagnosis, therapy, and prognosis7,8 and is crucial and urgently required. However, the advancement in the mechanism investigation of thyroid cancer pathogenesis is still limited.
Long noncoding RNAs (lncRNAs), a type of noncoding RNAs of length > 200 nucleotides, display fundamental functions in carcinogenesis.9–11 Many lncRNAs participate in thyroid cancer modulation. For example, lncRNA FOXD2-AS1 acts as a competing endogenous factor in thyroid cancer to modulate TERT expression by targeting miR-7-5p.12 LncRNA TNRC6C-AS1 as a competing endogenous RNA mediates UNC5B to induce cell invasion, migration, and proliferation by modulating miR-129-5p in thyroid cancer.13 LncRNA DARS-AS1 targets microRNA-129 to increase thyroid cancer progression.14 Moreover, lncRNA CCDC26, as a poorly investigated lncRNA, plays crucial roles in several cancer models, such as pancreatic cancer, leukemia, and laryngeal squamous cell carcinoma.15–17 However, the effect of CCDC26 on the progression of thyroid cancer remains unclear.
MicroRNAs (miRNAs) are short noncoding RNAs with a length of approximately 20‐25 nucleotides and have significant impacts on numerous biological processes, such as cell apoptosis, proliferation, differentiation, invasion, metastasis, and tumorigenesis by interacting with lncRNAs.18,19 MiRNAs post‐transcriptionally control the expression of genes within the target mRNAs’ 3ʹ untranslated region (3ʹ UTR).20 Many investigations have revealed that miRNAs are involved in thyroid cancer progression.21 For instance, miR-597-3p represses thyroid cancer cell invasion and migration by regulating RAB23.22 MiR-335-5p restrains thyroid cancer cell metastasis and invasion by targeting ICAM-1.23 MiR-422a is crucial in cancer development24,25 and is involved in thyroid cancer modulation by interacting with lncRNAs and targeted genes.26,27 Besides, epigenetic regulators are involved in tumorigenesis and play critical roles in cancer development.28 Enhancer of zeste homolog 2 (EZH2), a histone methyltransferase, and sirtuin 6 (Sirt6), a deacetylase, are well-studied epigenetic regulators exhibiting essential roles in thyroid cancer development and targets of miRNAs.29,30 In the present study, we identified a novel function of CCDC26 in promoting thyroid cancer progression by regulating the miR-422a/EZH2/Sirt6 axis in vitro and in vivo.
Materials and Methods
Clinical Thyroid Cancer Samples
A total of 50 clinical thyroid cancer samples used in the study was obtained between August 2017 and August 2019 from Peking University Cancer Hospital and Institute. All patients were diagnosed and reviewed independently by two clinicians based on histopathological data as advanced thyroid carcinoma involving the anterior superior mediastinum and did not receive any systemic or local therapy before surgery. Among them, 32 had papillary adenocarcinoma, 6 had follicular adenocarcinoma, 6 had medullary carcinoma, and 4 had poorly differentiated carcinoma. The thyroid cancer tissues and corresponding para-neoplastic tissues obtained from the patients were snap-frozen in liquid nitrogen and stored at −80°C before further analysis. All patients provided informed consent, and this study was approved by the Ethics Committee of Peking University Cancer Hospital and Institute (No. 76HG5573). This study conformed to the experimental guidelines of the World Medical Association and the Ethics Committee of Peking University Cancer Hospital and Institute.
Cell Culture and Treatment
Normal human thyroid Nthy-ori 3–1 cell line and thyroid cancer cell lines including FTC-133, SW579, TPC-1, and 8505C were purchased from Type Culture Collection of the Chinese Academy of Sciences (Shanghai) and cultured in DMEM (Solarbio, China) containing 10% fetal bovine serum (Gibco, USA), 0.1 mg/mL streptomycin (Solarbio, China) and 100 units/mL penicillin (Solarbio, China) at 37 °C with 5% CO2. CCDC26 siRNA, CCDC26 shRNA, pcDNA3.1-CCDC26 overexpression vector, miR-422a mimic, miR-422a inhibitor, and corresponding controls were obtained from GenePharma (Shanghai, China). Cell transfection was performed by Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
Quantitative Reverse Transcription-PCR (qRT-PCR)
Total RNAs were extracted by TRIZOL (Biosntech, China). The first-strand cDNA was synthesized following the manufacturer’s instructions (Thermo, USA). qRT-PCR was carried out by applying SYBR-Green (Takara Biotechnology, Co., Lt., China). The standard control for miRNA and mRNA/lncRNA was U6 and GAPDH, respectively. Quantitative determination of RNA levels was conducted in three independent experiments. The primer sequences are as follows:
CCDC26 forward: 5ʹ-CAAAGCTGGTCCTGTGCTTG-3ʹ; CCDC26 reverse: 5ʹ-TTGAGTGTGGCA TCACTTCC-3ʹ; miR-422a forward: 5ʹ-GGCCTGGCTGGACAGA-3ʹ; miR-422a reverse: 5ʹ-GTCA CATGACAACCCAGCTT-3ʹ; EZH2 forward: 5ʹ-TTGCCTGGATGATGGGGTCT-3ʹ; EZH2 reverse: 5ʹ-CATCTTAGTGGCCAGGTACA-3ʹ; Sirt6 forward: 5ʹ-GCCTTCTTCAGCCTGGAATAC-3ʹ; Sirt6 reverse: 5ʹ-TTCTGGAGTTCCTTGGTGGAGTG-3ʹ; GAPDH forward: 5ʹ-GGGCTGCTTTTAACT CTGGT-3ʹ; GAPDH reverse: 5ʹ-GCAGGTTTTTCTAGACGG-3ʹ; U6 forward: 5ʹ-CTCGCTTCGG CAGCACATATACT-3ʹ; and U6 reverse: 5ʹ-ACGCTTCACGAATTTGCGTGTC-3ʹ.
CCK-8 Assay
Cell viability was analyzed by the CCK‐8 assays. About 5×103 cells were put into 96 wells and cultured for 12 hours and used for transfection or treatment. After 0 hours, 24 hours, 48 hours, 72 hours, and 96 hours, cells were incubated with a CCK-8 solution (KeyGEN Biotech, China) and cultured for another 2 hours at 37°C. The absorbance at 450nm was measured with an ELISA browser (Bio-Tek EL 800, USA).31
BrdU Incorporation Assay
Cell proliferation was assessed by the BrdU incorporation assays. About 2×103 cells were put into 96 wells and cultured for 12 hours and used for transfection or treatment. After 0 hours, 24 hours, 48 hours, 72 hours, and 96 hours, cell proliferation was measured by BrdU Cell Proliferation Assay Kit (Yilaisa, China).32
Colony Formation Assay
About 1×103 SW579 and TPC-1 cells were layered in 6 wells and incubated in DMEM at 37 °C. After two weeks, cells were cleaned with PBS Buffer, fixed in methanol for thirty minutes, and stained with crystal violet dye at the dose of 1%, after which the number of colonies was calculated.31
Transwell Assays
Transwell assays were applied to analyze thyroid cancer cell invasion and migration using a Transwell plate (Corning, USA) according to the manufacturer’s instruction. Briefly, the upper chambers were plated with around 1×105 cells and cultured for 48 hours. The migrated and invaded cells were solidified with 4% paraformaldehyde, stained with crystal violet, recorded and calculated.31
Cell Apoptosis Analysis
Around 2×105 cells were plated on 6-well plates. Cell apoptosis was analyzed by using the Annexin V-FITC Apoptosis Detection Kit (CST, USA) according to the manufacturer's instruction. Briefly, about 2×106 collected and washed cells were resuspended in binding buffer and dyed at 25 °C, followed by the flow cytometry analysis.33
Luciferase Reporter Gene Assay
Luciferase reporter gene assays were performed by using the Dual-luciferase Reporter Assay System (Promega, USA). Briefly, cells were treated with the miR-422a mimic, or control mimic, the vector containing CCDC26, CCDC26 mutant, EZH2, EZH2 mutant, Sirt6, and Sirt6 mutant fragment were transfected into the cells by using Lipofectamine 2000 (Invitrogen, USA), followed by the analysis of luciferase activities, in which Renilla was applied as a normalized control.31
RNA Immunoprecipitation
RNA immunoprecipitation (RIP) assays were conducted by an EZMagna RIP kit (Millipore, USA). Cells were lysed using a complete RIP lysis buffer. Cell extracts were incubated with magnetic beads conjugated to anti-AGO2 (Abcam, USA) or anti-IgG (Abcam, USA) antibodies for 6 hours, protein beads were removed, and RNA was purified and followed by qPCR analysis.34
Western Blot Analysis
Total proteins were extracted from the cells or mice tissues with RIPA buffer (CST, USA). Protein concentrations were measured by using the BCA Protein Quantification Kit (Abbkine, USA). The same amount of proteins were separated by SDS-PAGE (12% polyacrylamide gels) and transferred onto PVDF membranes (Millipore, USA). The membranes were blocked with 5% milk and incubated overnight at 4°C with the primary antibodies for EZH2 (1:1000) (Amyjet, China), Sirt6 (1:1000) (Amyjet, China), and GAPDH (1:1000) (Amyjet, China), in which GAPDH served as the control. The corresponding second antibodies (1:1000) (Amyjet, China) were used for hatching the membranes for 1 hour at room temperature, followed by the visualization by using an Odyssey CLx Infrared Imaging System.
Tumorigenicity Analysis in Nude Mice
In vivo tumor growth was analyzed in nude Balb/c mice (male, 4-week-old) that were randomly separated into four groups (n=4). To establish an in vivo tumor model, SW579 cells were treated with control shRNA (sh-NC), CCDC26 shRNA (sh-CCDC26), miR-422a inhibitor, or co-treated with CCDC26 shRNA (sh-CCDC26) and miR-422a inhibitor, respectively. Then, about 1×107 cells were subcutaneously injected into the mice. Tumor growth were measured every 7 days. After 35 days of injection, mice were sacrificed, and tumors were scaled. Tumor volume (V) was observed by measuring the length and width with calipers and calculated as measured with the method × 0.5. Ki-67 expression in tumor tissues was tested by immunohistochemical staining with the Ki67 antibody (Santa Cruz Biotechnology, USA). EZH2, Sirt6, and GAPDH protein levels in the tumor tissues were analyzed by Western blot using primer antibodies against EZH2 (1:1000) (Amyjet, China), Sirt6 (1:1000) (Amyjet, China), and GAPDH (1:1000) (Amyjet, China), respectively. All animal handling procedures were approved by Peking University Cancer Hospital and Institute Animal Welfare Committee (No. 76HG5573) and complied with the authorized Animal Care and Method Procedure published by this hospital and the World Medical Association.
Statistical Methods
Data were presented as mean ± SD, and the statistical analysis was performed by SPSS 19.0 statistical software. The unpaired Student’s t-test was applied for comparing two groups, and the one-way ANOVA was applied for comparing multiple groups. LSD test was used for subsequent analysis. P < 0.05 were considered statistically significant.
Results
CCDC26 is Upregulated in Thyroid Cancer Tissues and Cell Lines
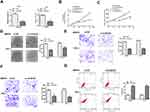
To assess the potential correlation of CCDC26 with thyroid cancer progression, we analyzed its expression in the thyroid cancer tissues and cell lines. Significantly, our data showed that CCDC26 expression was elevated in thyroid cancer tissues (n = 50) compared with the adjacent normal tissues (n = 50) (P < 0.01, (Figure 1A and B), implying that CCDC26 is associated with thyroid cancer development. In addition, CCDC26 expression was upregulated in thyroid cancer cell lines, including FTC-133 (P < 0.01), SW579 (P < 0.001), TPC-1 (P < 0.001), and 8505C (P < 0.01) compared with the normal human thyroid Nthy-ori 3–1 cells (Figure 1C). SW579 and TPC-1 cells were selected for subsequent investigations. Moreover, based on the mean CCDC26 expression, thyroid cancer tissues (n = 50) were assigned into two groups for correlation analysis between CCDC26 and thyroid cancer patients’ prognosis. We observed that a high CCDC26 level was associated with poor overall survival (OS) (P < 0.01, Figure 1D), suggesting that CCDC26 expression is negatively correlated with thyroid cancer patients’ prognosis. Taken together, these data suggest that CCDC26 is upregulated in thyroid cancer tissues and cell lines.
CCDC26 Depletion Inhibits Thyroid Cancer Progression in vitro
We further explored the effect of CCDC26 on thyroid cancer progression in vitro. To this end, CCDC26 was depleted by siRNA in SW579, and TPC-1 cells, and the knockdown efficiency was validated by qPCR (P < 0.001, Figure 2A). CCK-8 assays revealed that CCDC26 depletion remarkably reduced SW579 and TPC-1 cells’ viability (P < 0.01, Figure 2B). Similarly, BrdU incorporation assays demonstrated that SW579 and TPC-1 cell proliferation was inhibited by CCDC26 knockdown (P < 0.01, Figure 2C). Colony formation assays showed that CCDC26 knockdown decreased colony number in the cells (P < 0.01, Figure 2D). Furthermore, Transwell assays demonstrated that CCDC26 depletion impaired the invasion and migration of SW579 and TPC-1 cells (P < 0.01, Figure 2E and F). Meanwhile, CCDC26 knockdown remarkably induced apoptosis of SW579 and TPC-1 cells (P < 0.01, Figure 2G). Together, these results indicate that CCDC26 promotes thyroid cancer progression in vitro.
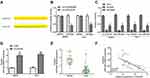
CCDC26 Serves as a Sponge of miR-422a in Thyroid Cancer Cells
Next, we identified the potential interaction between CCDC26 and miR-422a in the bioinformatic analysis using the web tool StarBase v.2.0 (Figure 3A). The miR-422a mimic remarkably reduced the luciferase activities of CCDC26 but failed to affect the CCDC26 with mutated miR-422a-binding site in SW579 and TPC-1 cells (P < 0.01, Figure 3B). RNA immunoprecipitation (RIP) assays showed that CCDC26 and miR-422a preferentially interacted with Ago2, but not IgG, in the micro-ribonucleoprotein complexes (miRNPs) of SW579 and TPC-1 cells (P < 0.01, Figure 3C). CCDC26 depletion enhanced miR-422a expression in these cells (P < 0.01, Figure 3D). Moreover, miR-422a expression was reduced in the thyroid cancer tissues (n=50) compared with the adjacent normal tissues (n=50) (P < 0.01, Figure 3E), and CCDC26 expression was negatively correlated with miR-422a in thyroid cancer tissues (n=50) (P < 0.01, Figure 3F), indicating the potential interplay of CCDC26 and miR-422a in the thyroid cancer progression. Together, CCDC26 serves as a sponge of miR-422a in thyroid cancer cells.
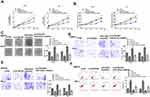
MiR-422a Inhibitor Reverses CCDC26 Knockdown-Induced Inhibition of Thyroid Cancer Progression in vitro
We then explored the role of CCDC26/miR-422a axis in thyroid cancer development in vitro. CCK-8 assays revealed that CCDC26 depletion reduced (P < 0.01) while miR-422a inhibitor enhanced (P < 0.01) the viability of SW579 and TPC-1 cells, and miR-422a inhibitor reversed the effect of CCDC26 knockdown in these two cells (P < 0.05, Figure 4A). BrdU incorporation assays showed that miR-422a inhibitor reversed CCDC26 knockdown-inhibited cell proliferation (P < 0.05, Figure 4B) and colony numbers (P < 0.05, Figure 4C) and increased CCDC26 knockdown-attenuated invasion and migration of SW579 and TPC-1 cells (P < 0.05, Figure 4D and E). Besides, CCDC26 depletion-induced apoptosis was blocked by miR-422a inhibitor (P < 0.05, Figure 4F). Together, these data suggest that miR-422a inhibitor reversed CCDC26 knockdown-induced inhibition of thyroid cancer progression in vitro.
CCDC26 Upregulates EZH2/Sirt6 Expression by Sponging miR-422a in Thyroid Cancer Cells
Next, we tried to identify miR-422a’s target genes in thyroid cancer development. Bioinformatic analysis using Targetscan (http://www.targetscan.org/vert_72/) revealed miR-422a-targeted sites in EZH2 and Sirt6 3ʹ UTR (Figure 5A). Notably, miR-422a mimic inhibited luciferase activities of wild type EZH2 and Sirt6 but failed to affect the EZH2 and Sirt6 with mutated miR-422a-binding sites in SW579 and TPC-1 cells (P < 0.01, Figure 5B). miR-422a mimic downregulated mRNA expression of EZH2 and Sirt6 (P < 0.01), and CCDC26 overexpression reversed this effect (P < 0.05, Figure 5C). Besides, CCDC26 overexpression significantly rescued the protein expression of EZH2 and Sirt6 inhibited by miR-422a mimic in these cells (P < 0.05) (Figure 5D). Furthermore, the expression of EZH2 and Sirt6 was increased in the thyroid cancer tissues (n=50) compared with the adjacent normal tissues (n=50) (P < 0.01, Figure 5E), and miR-422a expression was negatively correlated with EZH2 and Sirt6 levels in thyroid cancer tissues (n=50) (P < 0.01, Figure 5F). Taken together, these results suggest that CCDC26 upregulates EZH2/Sirt6 expression by sponging miR-422a in thyroid cancer cells.
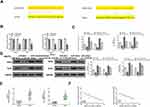 |
Figure 5 CCDC26 up-regulates EZH2/Sirt6 expression by sponging miR-422a in thyroid cancer cells. (A) The interaction of miR-422a with EZH2 and Sirt6 3ʹ UTR identified by bioinformatic analysis using Targetscan (http://www.targetscan.org/vert_72/). (B) Luciferase activities in SW579 and TPC-1 cells transfected with wild type EZH2 and Sirt6, and EZH2 and Sirt6 with mutated miR-422a-binding site determined by luciferase reporter gene assays. (C and D) SW579 and TPC-1 cells were treated with control mimic (miR-NC), miR-422a mimic, or co-treated with pcDNA3.1-CCDC26 (pc-CCDC26) overexpression vector and control mimic (miR-NC) or miR-422a mimic. (C) EZH2 and Sirt6 mRNA levels measured by qPCR. (D) Protein expression of EZH2, Sirt6, and GAPDH tested by Western blot analysis. The results of Western blot analysis were quantified by ImageJ software. (E) EZH2 and Sirt6 expression measured by qPCR in the thyroid cancer tissues (n=50) and adjacent normal tissues (n=50). (F) The correlation of EZH2 and Sirt6 with miR-422a analyzed by qPCR in thyroid cancer tissues (n=50). Data are presented as mean ± SD. Statistic significant differences were indicated: *P < 0.05, **P < 0.01, #P < 0.05. |
CCDC26 Contributes to Thyroid Tumor Growth via miR-422a/EZH2/Sirt6 Axis in vivo
We further determined the impact of CCDC26/miR-422a/EZH2/Sirt6 axis on thyroid cancer development in vivo. For this purpose, we performed tumorigenicity analysis in nude mice injected with SW579 cells, which were pretreated with control shRNA, CCDC26 shRNA, miR-422a inhibitor, or co-treated with CCDC26 shRNA and miR-422a inhibitor. Our data showed that CCDC26 depletion significantly reduced while miR-422a inhibitor enhanced the tumor growth in vivo, and miR-422a inhibitor reversed the effects of CCDC26 knockdown in the system, as demonstrated by the tumor size (Figure 6A), tumor weight (P < 0.05, Figure 6B), tumor volume (P < 0.05, Figure 6C), and Ki-67 expression in mouse tumor tissues (Figure 6D). Besides, we validated that CCDC26 knockdown-attenuated expression of EZH2 and Sirt6 was enhanced by miR-422a inhibitors in mouse tumor tissues (P < 0.05, Figure 6E). Together, these data suggest that CCDC26 contributes to thyroid tumor growth via miR-422a/EZH2/Sirt6 axis in vivo.
Discussion
Thyroid cancer serves as a prevailing endocrine neoplasm globally with increasing mobility and severe morbidity.35 However, the mechanism of thyroid cancer progression remains unclear, extremely limiting the development of biomarkers for diagnosis, prognosis, and therapeutic candidates for thyroid cancer patients.36 In recent years, lncRNAs have been well-recognized to present critical roles in thyroid cancer pathogenesis. This study identified that lncRNA CCDC26 promoted thyroid cancer malignant progression via miR-422a/EZH2/Sirt6 axis.
As a crucial regulator in cancer development, lncRNAs are recognized to participate in thyroid cancer modulation. However, the role of CCDC26 in thyroid cancer is poorly understood despite some studies have revealed the function of CCDC26 in the progression of other cancer models. It has been reported that CCDC26 downregulation promotes imatinib resistance by upregulating IGF-1R and c-KIT in gastrointestinal stromal cancers.37,38 CCDC26 depletion inhibits glioma cell migration and growth by sponging miR-203.39 CCDC26 regulates cell growth by modulating KIT expression in myeloid leukemia.40 Nevertheless, the role of CCDC26 in thyroid cancer is still unreported. In this study, we, for the first time, identified that CCDC26 expression was elevated thyroid cancer tissues and cell lines, CCDC26 depletion reduced cell proliferation, migration, and invasion and induced apoptosis of thyroid cancer cells, and CCDC26 contributed to thyroid tumor growth in vivo. These data present a novel function of CCDC26 in promoting thyroid cancer progression, providing valuable evidence for the fundamental role of lncRNAs in thyroid cancer development, which is consistent with the findings that a lncRNA can play similar roles in a variety of cancer models and how various lncRNAs work cooperatively in cancer development, although which needed to be further explored.
As a primary component of noncoding RNAs and a significant interplay factor with lncRNAs in the physiological and pathological processes, miRNAs are also involved in thyroid cancer modulation. It has been reported that miRNA-125b controls autophagy by interacting with Foxp3 in thyroid cancer.41 MiRNA-1270 regulates thyroid cancer progression through regulating SCAI.42 MiRNA-592 suppresses thyroid cancer development by downregulating NOVA1 and controlling lncRNA NEAT1.43 MiRNA-15 mediates thyroid cancer invasion, migration, and proliferation by regulating Bcl-2.44 It has been identified that miR-422a participates in thyroid cancer modulation. miR-422a is involved in iodine/MAPK1-induced thyroid cancer progression.27 SP1-mediated lncRNA LINC00313 upregulation activates thyroid cancer tumorigenesis by modulating miR-422a.26 These investigations have well proven that miRNAs are regularly involved in the modulation of thyroid cancer progression. In the present study, we identified that miR-422a expression was reduced in thyroid cancer tissues. Our mechanism investigation further demonstrated that miR-422a inhibitor reversed CCDC26 knockdown-induced inhibition of thyroid cancer progression in vitro. These data displayed an unreported role of miR-422a in thyroid cancer development and identified a new association of miR-422a with CCDC26 in thyroid cancer modulation.
As two significant epigenetic regulators, EZH2 and Sirt6 have been well-identified to participate in the modulation of thyroid cancer development. It has been reported that miR-30d and miR-25 downregulation promotes thyroid cancer progression by targeting EZH2.29 EZH2 upregulation by ERα enhances thyroid cancer cell migration and proliferation.45 In addition, Sirt6 contributes to the Warburg effect by regulating BCPAP through reactive oxygen species in papillary thyroid cancer cells.30 Sirt6/HIF-1α signaling enhances the progression of papillary thyroid carcinoma by stimulating epithelial-mesenchymal transition.46 Sirt6 is upregulated and correlated with malignancy aggressiveness by BRAF/ERK/Mcl 1 signaling in papillary thyroid cancer.47 Our data showed that miR-422a targets EZH2 and Sirt6 in thyroid cancer cells, and CCDC26 upregulated EZH2/Sirt6 expression by sponging miR-422a in modulating thyroid cancer progression. These data provide new evidence that EZH2 and Sirt6 serve as the crucial factors in thyroid cancer development and present an unreported correlation of EZH2 and Sirt6 with miR-422a and CCDC26 in tumorigenesis. The effect of miR-422a on other potential targets in thyroid cancer development will be investigated in the future.
In conclusion, we identified that CCDC26 contributes to thyroid cancer malignant progression via miR-422a/EZH2/Sirt6 axis. Our finding provides new insights into the mechanism by which CCDC26 promotes thyroid cancer malignant development and improves our understanding of the association of lncRNA with thyroid cancer. CCDC26 and miR-422a might serve as potential targets for thyroid cancer treatment.
Acknowledgments
The authors would like to express our gratitude for those who have critically reviewed this manuscript and those who gave us help during this experiment.
Funding
This work was supported by the Science Foundation of Peking University Cancer Hospital (Grant No. 202003).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Janjua N, Wreesmann VB. Aggressive differentiated thyroid cancer. Eur J Surg Oncol. 2018;44(3):367–377. doi:10.1016/j.ejso.2017.09.019
2. Sharifi A, Shojaeifard A, Soroush A, Jafari M, Abdehgah AG, Mahmoudzade H. Predictors of regional lymph node recurrence after initial thyroidectomy in patients with thyroid cancer. J Thyroid Res. 2016;2016:4127278. doi:10.1155/2016/4127278
3. Wang Y, Wang W. Increasing incidence of thyroid cancer in Shanghai, China, 1983–2007. Asia Pac J Public Health. 2015;27(2):NP223–229. doi:10.1177/1010539512436874
4. Carlberg M, Hedendahl L, Ahonen M, Koppel T, Hardell L. Increasing incidence of thyroid cancer in the Nordic countries with main focus on Swedish data. BMC Cancer. 2016;16:426. doi:10.1186/s12885-016-2429-4
5. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184–199. doi:10.1038/nrc3431
6. Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106(3):524–531. doi:10.1002/cncr.21653
7. Schmidbauer B, Menhart K, Hellwig D, Grosse J. Differentiated thyroid cancer-treatment: state of the art. Int J Mol Sci. 2017;18(6). doi:10.3390/ijms18061292
8. Naoum GE, Morkos M, Kim B, Arafat W. Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol Cancer. 2018;17(1):51. doi:10.1186/s12943-018-0786-0
9. Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–5667. doi:10.1038/onc.2017.184
10. Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407. doi:10.1016/j.cell.2018.01.011
11. Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965–3981. doi:10.1158/0008-5472.CAN-16-2634
12. Liu X, Fu Q, Li S, et al. LncRNA FOXD2-AS1 functions as a competing endogenous RNA to Regulate TERT expression by sponging miR-7-5p in thyroid cancer. Front Endocrinol (Lausanne). 2019;10:207. doi:10.3389/fendo.2019.00207
13. Hou S, Lin Q, Guan F, Lin C. LncRNA TNRC6C-AS1 regulates UNC5B in thyroid cancer to influence cell proliferation, migration, and invasion as a competing endogenous RNA of miR-129-5p. J Cell Biochem. 2018;119(10):8304–8316. doi:10.1002/jcb.26868
14. Zheng W, Tian X, Cai L, et al. LncRNA DARS-AS1 regulates microRNA-129 to promote malignant progression of thyroid cancer. Eur Rev Med Pharmacol Sci. 2019;23(23):10443–10452. doi:10.26355/eurrev_201912_19683
15. Peng W, Jiang A. Long noncoding RNA CCDC26 as a potential predictor biomarker contributes to tumorigenesis in pancreatic cancer. Biomed Pharmacother. 2016;83:712–717. doi:10.1016/j.biopha.2016.06.059
16. Izadifard M, Pashaiefar H, Yaghmaie M, et al. Expression analysis of PVT1, CCDC26, and CCAT1 long noncoding RNAs in acute myeloid leukemia patients. Genet Test Mol Biomarkers. 2018;22(10):593–598. doi:10.1089/gtmb.2018.0143
17. Li D, Wang X, Lu S, et al. Integrated analysis revealing genome-wide chromosomal copy number variation in supraglottic laryngeal squamous cell carcinoma. Oncol Lett. 2020;20(2):1201–1212. doi:10.3892/ol.2020.11653
18. Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141(4):1202–1207. doi:10.1016/j.jaci.2017.08.034
19. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi:10.1038/nrd.2016.246
20. Zhou Q, Huang SX, Zhang F, et al. MicroRNAs: a novel potential biomarker for diagnosis and therapy in patients with non-small cell lung cancer. Cell Prolif. 2017;50(6). doi:10.1111/cpr.12394.
21. Ghafouri-Fard S, Shirvani-Farsani Z, Taheri M. The role of microRNAs in the pathogenesis of thyroid cancer. Noncoding RNA Res. 2020;5(3):88–98. doi:10.1016/j.ncrna.2020.06.001
22. Wen HL, Xu ZM, Lin SY, Wen D, Xie JP. miR-597-3p inhibits invasion and migration of thyroid carcinoma SW579 cell by targeting RAB23. Endokrynol Pol. 2020. doi:10.5603/EP.a2020.0041
23. Luo L, Xia L, Zha B, et al. miR-335-5p targeting ICAM-1 inhibits invasion and metastasis of thyroid cancer cells. Biomed Pharmacother. 2018;106:983–990. doi:10.1016/j.biopha.2018.07.046
24. Li WQ, Zhang JP, Wang YY, Li XZ, Sun L. MicroRNA-422a functions as a tumor suppressor in non-small cell lung cancer through SULF2-mediated TGF-beta/SMAD signaling pathway. Cell Cycle. 2019;18(15):1727–1744. doi:10.1080/15384101.2019.1632135
25. Zhang X, Gao D, Fang K, Guo Z, Li L. Med19 is targeted by miR-101-3p/miR-422a and promotes breast cancer progression by regulating the EGFR/MEK/ERK signaling pathway. Cancer Lett. 2019;444:105–115. doi:10.1016/j.canlet.2018.12.008
26. Yan DG, Liu N, Chao M, Tu YY, Liu WS. SP1-induced upregulation of long noncoding RNA LINC00313 contributes to papillary thyroid cancer progression via the miR-422a. Eur Rev Med Pharmacol Sci. 2019;23(3):1134–1144. doi:10.26355/eurrev_201902_17004
27. Wang J, Yang H, Si Y, et al. Iodine promotes tumorigenesis of thyroid cancer by suppressing Mir-422a and up-regulating MAPK1. Cell Physiol Biochem. 2017;43(4):1325–1336. doi:10.1159/000481844
28. Chen Y, Hong T, Wang S, Mo J, Tian T, Zhou X. Epigenetic modification of nucleic acids: from basic studies to medical applications. Chem Soc Rev. 2017;46(10):2844–2872. doi:10.1039/C6CS00599C
29. Esposito F, Tornincasa M, Pallante P, et al. Down-regulation of the miR-25 and miR-30d contributes to the development of anaplastic thyroid carcinoma targeting the polycomb protein EZH2. J Clin Endocrinol Metab. 2012;97(5):E710–718. doi:10.1210/jc.2011-3068
30. Yu W, Yang Z, Huang R, Min Z, Ye M. SIRT6 promotes the Warburg effect of papillary thyroid cancer cell BCPAP through reactive oxygen species. Onco Targets Ther. 2019;12:2861–2868. doi:10.2147/OTT.S194256
31. Zhao W, Han T, Li B, Ma Q, Yang P, Li H. miR-552 promotes ovarian cancer progression by regulating PTEN pathway. J Ovarian Res. 2019;12(1):121. doi:10.1186/s13048-019-0589-y
32. Chen Y, Gao Y, Lian Y, Li C, Qu C, Jiang X. Overexpression of miR-221 stimulates proliferation of rat neural stem cell with activating Phosphatase and tensin homolog/protein kinase B signaling pathway. Neuroreport. 2020;31(14):1015–1023. doi:10.1097/WNR.0000000000001513
33. Zou YT, Gao JY, Wang HL, Wang Y, Wang H, Li PL. Downregulation of microRNA-630 inhibits cell proliferation and invasion and enhances chemosensitivity in human ovarian carcinoma. Genet Mol Res. 2015;14(3):8766–8777. doi:10.4238/2015.July.31.25
34. Kelly TJ, Suzuki HI, Zamudio JR, Suzuki M, Sharp PA. Sequestration of microRNA-mediated target repression by the Ago2-associated RNA-binding protein FAM120A. RNA. 2019;25(10):1291–1297. doi:10.1261/rna.071621.119
35. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. doi:10.1016/S0140-6736(16)30172-6
36. Carling T, Udelsman R. Thyroid cancer. Annu Rev Med. 2014;65:125–137. doi:10.1146/annurev-med-061512-105739
37. Yan J, Chen D, Chen X, et al. Downregulation of lncRNA CCDC26 contributes to imatinib resistance in human gastrointestinal stromal tumors through IGF-1R upregulation. Braz J Med Biol Res. 2019;52(6):e8399. doi:10.1590/1414-431x20198399
38. Cao K, Li M, Miao J, et al. CCDC26 knockdown enhances resistance of gastrointestinal stromal tumor cells to imatinib by interacting with c-KIT. Am J Transl Res. 2018;10(1):274–282.
39. Wang S, Hui Y, Li X, Jia Q. Silencing of lncRNA CCDC26 restrains the growth and migration of glioma cells in vitro and in vivo via targeting miR-203. Oncol Res. 2018;26(8):1143–1154. doi:10.3727/096504017X14965095236521
40. Hirano T, Yoshikawa R, Harada H, Harada Y, Ishida A, Yamazaki T. Long noncoding RNA, CCDC26, controls myeloid leukemia cell growth through regulation of KIT expression. Mol Cancer. 2015;14:90. doi:10.1186/s12943-015-0364-7
41. Wang S, Wu J, Ren J, et al. MicroRNA-125b interacts with Foxp3 to induce autophagy in thyroid cancer. Mol Ther. 2018;26(9):2295–2303. doi:10.1016/j.ymthe.2018.06.015
42. Yi T, Zhou X, Sang K, Zhou J, Ge L. MicroRNA-1270 modulates papillary thyroid cancer cell development by regulating SCAI. Biomed Pharmacother. 2019;109:2357–2364. doi:10.1016/j.biopha.2018.08.150
43. Luo Y, Hao T, Zhang J, Zhang M, Sun P, Wu L. MicroRNA-592 suppresses the malignant phenotypes of thyroid cancer by regulating lncRNA NEAT1 and downregulating NOVA1. Int J Mol Med. 2019;44(3):1172–1182. doi:10.3892/ijmm.2019.4278
44. Lu Z, Wu Z, Hu J, Wei W, Ma B, Wen D. MicroRNA-15 regulates the proliferation, migration and invasion of thyroid cancer cells by targeting Bcl-2. J BUON. 2019;24(5):2114–2119.
45. Xue L, Yan H, Chen Y, et al. EZH2 upregulation by ERalpha induces proliferation and migration of papillary thyroid carcinoma. BMC Cancer. 2019;19(1):1094. doi:10.1186/s12885-019-6306-9
46. Yang Z, Yu W, Huang R, Ye M, Min Z. SIRT6/HIF-1alpha axis promotes papillary thyroid cancer progression by inducing epithelial-mesenchymal transition. Cancer Cell Int. 2019;19:17. doi:10.1186/s12935-019-0730-4
47. Qu N, Hu JQ, Liu L, et al. SIRT6 is upregulated and associated with cancer aggressiveness in papillary thyroid cancer via BRAF/ERK/Mcl1 pathway. Int J Oncol. 2017;50(5):1683–1692. doi:10.3892/ijo.2017.3951
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.