Back to Journals » OncoTargets and Therapy » Volume 13
Long Non-Coding RNAs in Drug Resistance of Breast Cancer
Authors Du T, Shi Y, Xu S, Wan X, Sun H, Liu B
Received 24 March 2020
Accepted for publication 12 June 2020
Published 21 July 2020 Volume 2020:13 Pages 7075—7087
DOI https://doi.org/10.2147/OTT.S255226
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Tonghua Du, Ying Shi, Shengnan Xu, Xiaoyu Wan, Haiyin Sun, Bin Liu
Department of Breast Surgery, The Second Hospital of Jilin University, Changchun, Jilin, People’s Republic of China
Correspondence: Bin Liu
Department of Breast Surgery, The Second Hospital of Jilin University, No. 218 Ziqiang Street, Changchun, Jilin 130041, People’s Republic of China
Tel +86 13844823515
Email [email protected]
Abstract: Breast cancer (BC) is the most common cancer and the leading cause of death in women. Advances in early diagnosis and therapeutic strategies have decreased the mortality of BC and improved the prognosis of patients to some extent. However, the development of drug resistance has limited the success rate of systemic therapies. Long non-coding RNAs (lncRNAs) are involved in drug resistance in BC via various mechanisms, which contribute to a complex regulatory network. In this review, we summarize the latest findings on the mechanisms underlying drug resistance modulated by lncRNAs in BC. In addition, we discuss the potential clinical applications of lncRNAs as targeted molecular therapy against drug resistance in BC.
Keywords: long non-coding RNA, lncRNA, drug resistance, breast cancer, mechanism
Introduction
Breast cancer (BC) is the second most commonly diagnosed malignant cancer and the fifth most common cause of cancer-related death worldwide.1 Among women, BC is the most common cancer (24.2% of the total cases) with the highest mortality rate (15.0% of the total cancer deaths) worldwide.1 Advances in early diagnosis and therapeutic strategies have decreased the BC death rate and improved the prognosis of patients to some extent.2 However, recurrence and metastasis occur in almost 35% of BC patients, and are generally associated with the development of resistance to chemotherapy, endocrine therapy, and radiotherapy.3,4 The frequent development of drug resistance in BC patients severely limits the efficacy of therapy and affects the prognosis of BC patients. The mechanisms underlying drug resistance are complex and some have been elucidated, such as drug efflux, DNA damage, drug target modulation, apoptosis dysfunction, and increased proliferation among others.5–8 Reversing drug resistance to overcome the adverse effects and improve the efficacy of drug therapies in BC remains challenging because of the complex mechanisms involved.
Advances in human genome sequencing technology have revealed that only 2% of human genes encode proteins. Genes that are transcribed into RNA without the ability to encode proteins are called non-coding RNAs (ncRNAs).9 Although ncRNAs were considered “junk DNA” in past decades, emerging evidence indicates that ncRNAs play important roles in epigenetics, transcription, post-transcriptional processes, and translation.10 ncRNAs modulate cell growth, proliferation, apoptosis, metastasis, epithelial–mesenchymal transition (EMT), and angiogenesis via a variety of mechanisms in many diseases including cancer.11–13
Long non-coding RNAs (lncRNAs), which consist of more than 200 nucleotides, are a common type of ncRNA.14 LncRNAs are transcribed by RNA polymerase Ⅱ, lack open reading frames, and are localized in both the cell nucleus and cytoplasm.15 Dysregulated lncRNAs are involved in drug resistance in numerous cancer cells and tissues, such as hepatocellular carcinoma, gastric cancer, colorectal cancer, and cervical cancer among others.16–19 In recent years, an increasing number of studies have demonstrated the functional role of lncRNAs in drug resistance in BC. LncRNAs sponge miRNAs as competing endogenous RNAs (ceRNAs), induce resistance in sensitive cells via exosomes, activate the EMT process, and modulate cell apoptosis and the cell cycle directly, thereby regulating the response of BC cells to chemotherapy, endocrine therapy, and molecular targeted therapy.20,21
In this review, we summarize the characteristics of lncRNAs associated with drug resistance in BC and describe the potential underlying mechanisms briefly. The purpose of studies is to identify therapeutic targets to reverse drug resistance or improve the efficacy of BC treatment.
Multidrug Resistance and Single Drug Resistance
Drug resistance is classified into multidrug resistance (MDR) and single drug resistance. MDR refers to the resistance of cancer cells to a variety of anticancer drugs with different structures and functions.22 An important mechanism underlying MDR is the activity of drug efflux pumps, which rely on energy-dependent transporters. These transporters, which are located on the cell membrane, are proteins that control the entry or exit of multiple drugs from cells.23 These molecular pumps remove drugs from cells and lead to MDR. The ATP-dependent binding cassette (ABC) transporters are a family of molecules that mediate drug efflux, and include ABCB1 (P-glycoprotein, multidrug resistance 1/MDR1) and multidrug resistance associated protein 1 (MRP1, ABCC1) among others.24 Another mechanism underlying MDR is the induction of apoptosis and autophagy in cancer cells treated with anti-cancer drugs.11 Many other mechanisms, including DNA damage repair,27 resistance dissemination by exosomes,28 ceRNAs,29 and modification of cancer stem cells (CSCs)30 regulate drug resistance in BC.
In addition to lncRNAs involved in MDR, various lncRNAs related to single anti-cancer drug resistance have been identified. Those drugs, which are applicated during chemotherapy, endocrine therapy and molecular targeted therapy, include DOX/Adriamycin (ADR), 5-FU, cisplatin (DDP/CDDP), paclitaxel (PTX), tamoxifen (TAM), trastuzumab (TZB), epirubicin, and docetaxel (DOC). LncRNAs related to MDR or single drug resistance in BC contribute to a complex regulatory network of drug resistance.
LncRNAs Involved in Multidrug Resistance
In recent years, numerous studies have reported the relationship between lncRNAs and MDR in BC. Most lncRNAs are upregulated in BC cells and tissues and promote MDR by modulating cell apoptosis, inducing the EMT process, and targeting classic signaling pathways (Table 1). LncRNA NEAT1 is upregulated in cisR (cisplatin resistance) and taxR (taxol resistance) MDA-MB-231 cells.29 Knockdown of NEAT1 downregulates drug transporter genes, including ATP7A and ATP7B, and the stemness marker SOX2 in BC cells, leading to the reversal of CDDP and PTX resistance.29 NEAT1 also affects cell proliferation and apoptosis by inhibiting the expression of cyclin D1 and E1 and upregulating cleaved caspase-3.29 Another study reported that NEAT1 inhibits miR-211 and upregulates its downstream target HMGA2, thereby inducing the EMT process and 5-FU resistance in BC cells.30 LncRNA LINP1 promotes TAM, DOX, and 5-FU resistance in BC cells by decreasing cell apoptosis.31,32 LINP1 inhibits caspase-8 and caspase-9 in DOX treated cells or caspase-9 and Bax in 5-FU treated cells.31,32 The lncRNA linc00518 is upregulated in MCF-7 cells.33 Linc00518 acts as a sponge for miR-199a to inhibit MRP1 and reverse MDR in BC cells, including resistance to ADR, vincristine (VCR), and PTX.33 The lncRNA HOTAIR is upregulated in BC cells, and silencing of HOTAIR promotes cell apoptosis by targeting caspase-3, Bcl-2, and Bax. It also decreases the expression of the classic MDR resistance proteins MDR1, MRP1, and ABCB1 to reduce DOX and TZB resistance by inhibiting the PI3K/Akt/mTOR signaling pathway.34,35 Another study reported that HOTAIR activates ligand-independent estrogen receptor (ER) to promote TAM resistance.36 Some other lncRNAs, including H19,37–44 CRALA,45 and UCA1,46–51 are also upregulated in BC cells and tissues and promote MDR via different mechanisms.
 |
Table 1 LncRNAs Involved in MDR of Breast Cancer |
Certain lncRNAs play opposite roles in drug resistance in BC by sponging miRNAs or by regulating MDR related proteins. LncRNA GAS5 is downregulated in BC cells, and overexpression of GAS5 reverses the PTX resistance of BC cells by inducing cell apoptosis through the miR-378a-5p/SUFU signaling pathway.52 GAS5 deceases TAM resistance in MCF-7R cells by inhibiting PTEN via sponging miR-222.53 Reduced GAS5 in BC cells can also activate mTOR and suppress PTEN to confer TZB resistance.54 Knockdown of lncRNA BC032585 upregulates the expression of MDR1 to promote resistance to DOX and PTX in BC cells.55 Enhanced lncRNA LINC00968 inhibits the Wnt2/β-catenin signaling pathway and the EMT process to reverse MDR in BC cells, including resistance to ADR, Taxol, and VCR.56
LncRNAs Involved in Single Drug Resistance
LncRNAs in Chemotherapy Resistance
To prevent recurrence and metastasis of BC after surgery, chemotherapy has been widely used in clinical treatment. Chemotherapy increases the disease-free survival and overall survival rates of postoperative BC patients.57 Chemotherapy drugs, including anthracycline family, taxanes, and platinum drugs are used extensively for treating BC. LncRNAs involved in chemotherapy resistance are summarized in Table 2.
 |
Table 2 LncRNAs Involved in Chemotherapy Resistance in Breast Cancer |
Doxorubicin/Adriamycin
As a member of the anthracycline family, DOX/ADR are used as the first-line chemotherapy drugs for cancers including BC. DOX/ADR increase cytotoxicity by limiting DNA replication, thereby causing the death of cancer cells.58 The relationship between DOX/ADR and BC has been reported extensively. LncRNA LINC00668, which is upregulated in BC cells, promotes DOX resistance by targeting staphylococcal nuclease domain-containing 1.59 LncRNA BORG reduces DNA damage to promote DOX resistance in BC cells by activating the NF-κB signaling pathway.60 Knockdown of linc00152 reverses DOX resistance in MCF-7/ADR cells.61 LncRNA ARA is upregulated in BC cells, and knockdown of ARA reverses ADR resistance via the MAPK signaling pathway and by modulating cell cycle progression.62
LncRNA PTENP1 is downregulated in BC cells, and increased PTENP1 sponges miR-20a to promote PTEN expression via the PI3K/Akt signaling pathway to reverse ADR resistance in BC cells.63 Low lncRNA MEG3 expression promotes DOX resistance by reducing cell apoptosis via the Bax/Bcl-2 axis in BC cells.64
Epirubicin
Epirubicin is the isomer of ADR, and studies have reported on epirubicin resistance in BC cells. LncRNA NONHSAT101069, a novel overexpressed lncRNA in MCF-7 and MCF-7/ADR cells, acts as a ceRNA by sponging miR-129-5p to target the downstream protein Twist1 and promote epirubicin resistance in BC cells.29 Zheng et al reported that lncRNA SPRY4-IT1 is overexpressed and promotes epirubicin resistance in MCF-7 and MDA-MB-231 cells.65
Taxanes
Taxanes are classic and effective cytotoxic drugs and include the traditional PTX and DOC, as well as the novel cabazitaxel and abraxane.66 PTX and DOC resistance in BC has been reported, whereas resistance to the two novel drugs has not been identified. LncRNA FTH1P3 is upregulated in MCF-7/PTX cells.67 FTH1P3 promotes ABCB1 protein expression by acting as a sponge for miR-206 to enhance PTX resistance in BC cells.67 LncRNA CASC2 is upregulated in BC cells and negatively regulates miR-18a-5p, thereby activating cyclin dependent kinase 19 (CDK19) to enhance PTX resistance in BC cells.68 LncRNA LINC00511 interacts directly with miR-29c and inhibits its expression, which upregulates the expression of cyclin dependent kinase 6 (CDK6) and suppresses PTX-induced cytotoxicity.69 LncRNA MAPT-AS1 is upregulated in BC cells, and knockdown of MAPT-AS1 decreases the stability of MAPT mRNA to induce PTX resistance.70 LncRNA RP11-770J1.3 confers PTX resistance to MCF-7 cells by increasing transmembrane protein 25 (TMEM25) and MRP, BCRP, and MDR1/P-gp.71
LncRNA EPB41L4A-AS2 is downregulated in DOC-resistant BC cells, and low expression of EPB41LA4-AS2 upregulates ABCB1 and promotes DOC resistance in BC cells.72
Cisplatin
Cisplatin is a commonly used anticancer agent that is lethal to target DNAs in cancer cells.73 LncRNA SNHG15 is upregulated in MCF-7/DDP and MDA-MB-231/DDP cells, and knockdown of SNHG15 increases DDP sensitivity in BC cells by sponging miR-381.74 LncRNA HCP5 is upregulated in MDA-MB-231/DDP cells and promotes DDP resistance by inhibiting the expression of PTEN; this effect has been observed in TNBC xenografts in vivo.75
5-Fluorouracil
5-FU is commonly used in the treatment of gastrointestinal cancer.76 Liang et al showed that lncRNA PRLB sponges miR-4766-5p to enhance the expression of sirtuin 1 and promote 5-FU resistance in BC cells.77
LncRNAs in Endocrine Therapy Resistance
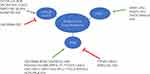
Gene expression profiling led to the classification of BC into four molecular subtypes according to the expression levels of ER, progesterone receptor and human growth factor receptor type 2 (HER2): luminal A (50%), luminal B (20%), HER2 (15–20%), and TNBC (15–20%) subtypes.78 LncRNAs play different roles in drug resistance in different BC subtypes (Figure 1). The luminal A and luminal B subtypes, which account for approximately 70% of BC cases, are positive for ERα expression.79 The tumorigenesis of ERα-positive BC is determined by estrogen signaling.80 Therefore, targeting ERα using selective ER modulators or selective ER downregulation to block estrogen action is an effective strategy for the treatment of BC. LncRNAs involved in endocrine therapy resistance are summarized in Table 3.
 |
Table 3 LncRNAs Involved in Endocrine Therapy Resistance of Breast Cancer |
Tamoxifen
TAM is the most widely used antiestrogen drug and it has shown good results as long-term adjuvant therapy in the past decades.80 However, the curative effect of TAM is limited by the acquisition of drug resistance. LncRNAs play a role in the resistance to TAM. LncRNA CYTOR promotes TAM resistance by increasing serum response factor (SRF) and activating the MAPK signaling pathway by sponging miR-125a-5p in MCF-7/TAM cells.81 Sun et al identified a novel lncRNA named LOL (lncRNA of luminal) that is upregulated in BC and luminal BC. Knockdown of LOL reverses TAM resistance in TamR MCF-7 cells by sponging let-7 miRNAs.82 LncRNA DSCAM-AS1 promotes TAM resistance via the miR-137/EPS8 axis or by modulating GREB1, a target of ER. It promotes cell reproduction and suppresses cell apoptosis by acting as a ceRNA of miR-137 and increasing EPS8 expression.83,84 Overexpression of lncRNA TMPO-AS1 activates estrogen signaling to promote TAM resistance in BC cells.85 Downregulated lncRNA ROR inhibits the EMT process by upregulating miR-205 expression and preventing ZEB1 and ZEB2 expression, thereby reversing TAM resistance in MDA-MB-231 cells.86 Knockout of ROR regulates dual specificity phosphatase 7 and activates the MAPK/ERK signaling pathway, which decreases TAM resistance.87 Li et al reported that the induction of cell autophagy is another mechanism by which ROR reverses TAM resistance.88 Knockdown of lncRNA CCAT2 promotes cell apoptosis and enhances TAM resistance in BC cells.89 LncRNA BCAR4 targets ERBB2 and ERBB3 and promotes TAM resistance.90 Low expression of lncRNA ADAMTS9-AS2 decreases proliferation and apoptosis in MCF-7R cells by increasing miR-130a-5p and preventing PTEN expression, thereby increasing TAM resistance.91
LncRNA LINC00894-002 is expressed at low levels in MCF-7/TamR cells, which could be related to the downregulation of ESR1. LINC00894-002 downregulation contributes to TAM resistance via the miR-200/TGF-β2/ZEB1 signaling pathway.92
Aromatase Inhibitors
Aromatase inhibitors (AIs) play an important role in the treatment of ERα-positive BC. Aromatase is the rate-limiting enzyme in the estrogen biosynthesis pathway, and inhibiting aromatase decreases the circulating levels of estrogen to suppress the proliferation of ERα-positive BC.93 Three AIs approved by the US Food and Drug Administration (FDA) are used in the clinical treatment of BC, including steroidal AIs (exemestane) and nonsteroidal AIs (anastrozole and letrozole).94 However, the relationship between lncRNAs and resistance to AIs has not been reported to date.
LncRNAs in Molecular Targeted Therapy Resistance
The HER2 subtype, which accounts for 15–20% of BC cases, is associated with a higher risk of metastasis and a worse prognosis than the luminal A and luminal B subtypes.95 The therapeutic efficacy of chemotherapy and anti-estrogen therapy is low for the HER2 subtype, which shows a certain degree of drug resistance.20 Therefore, molecular targeted therapy against HER2 is a promising strategy to improve the prognosis of patents with the HER2 subtype. LncRNAs involved in molecular targeted therapy resistance are summarized in Table 4.
 |
Table 4 LncRNAs Involved in Molecular Targeted Therapy Resistance of Breast Cancer |
Trastuzumab
TZB, a monoclonal antibody that targets and blocks the activity of HER2, has improved the prognosis of patients with the HER subtype.96 However, TZB resistance can develop during targeted therapy. Studies have reported a relationship between lncRNAs and TZB resistance in BC. LncRNA AFAP1-AS1 targets AU-binding factor 1 (AUF1) and activates the translation of ERBB2, leading to TZB resistance in BC cells.97 AGAP2-AS1 decreases the cytotoxicity of TZB via being incorporated into exosomes, and promotes TZB resistance in BC.98 AGAP2-AS1 inhibits cell apoptosis and promotes TZB resistance in BC cells by upregulating MyD88, which results from the enrichment of H3K27ac at the promoter region of MyD88, and activating the NF-κB signaling pathway.99 LncRNA TINCR is upregulated in HER-2+ BC cells; it sponges miR-125b and targets Snail-1, thereby promoting TZB resistance and the associated EMT process in BC cells.100 LncRNA SNHG14 modulates H3K27 acetylation, which upregulates PABPC1 expression and activates the Nrf2 signaling pathway, increasing TZB resistance in BC cells.101 SNHG14 also promotes TZB resistance through its incorporation into exosomes and transport to sensitive BC cells. Knockdown of SNHG14 increases cytotoxicity and cell apoptosis via the Bcl-2/Bax signaling pathway to reverse TZB resistance.102 LncRNA ATB upregulates ZEB1 and ZNF-217 by competitively binding to miR-200c and promotes EMT to induce TZB resistance in BC cells.103
Other Targeted Drugs
In addition to TZB, other molecular targeted drugs are currently used in the treatment of HER2-positive BC, such as lapatinib and pertuzumab. Lapatinib is a tyrosine kinase inhibitor that targets HER2 and epithelial growth factor receptor. Pertuzumab is another monoclonal antibody that targets HER2 directly.104 Although these two molecular targeted drugs have improved the prognosis of HER2 subtype BC patients, they are associated with drug resistance. Studies show that miR-16 and miR-630 reverse lapatinib resistance in BC.105,106 However, there are no studies assessing the relationship between lncRNAs and lapatinib resistance or pertuzumab resistance in BC.
Mechanisms Underlying LncRNA-Mediated Drug Resistance
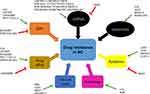
LncRNAs modulate drug resistance via various mechanisms. They act as ceRNAs for specific miRNAs and alter the expression of targets, regulate resistance through transport via exosomes, modulate cell apoptosis or autophagy, regulate drug efflux via ABC transporters, activate the EMT process, and target common signaling pathways. Acting as ceRNAs and regulating via exosomes are two of the most important ways of action. An increasing number of studies have demonstrated the functional role of lncRNAs in drug resistance in BC via these two ways.
CeRNAs
MiRNAs recruit the RNA-induced silencing complex and bind to miRNA response elements to repress translation,107 whereas lncRNAs bind competitively to miRNAs and suppress their silencing effect, acting as a “molecular sponge”.108 CeRNAs play an important role in many biological processes associated with cancer including drug resistance. Linc00518 acts as a sponge for miR-199a and reverses MDR in BC.41 UCA1 sponges miR-18a and increases TAM resistance.46,47 Overexpressed GAS5 sponges miR-378a-5p, miR-222, and miR-21, thereby reversing drug resistance in BC.52–54 SNHG15 increases the sensitivity of BC cells to DDP by sponging miR-381.74 CASC2 is upregulated in BC cells and sponges miR-18a-5p to enhance PTX resistance.68 TINCR sponges miR-125b and promotes TZB resistance in HER-2+ BC cells.100 Other lncRNAs such as FTH1P3,67 LINC00511,69 ATB,103 CYTOR,81 DSCAM-AS1,83,84 ROR,86 ADAMTS9-AS2,91 LINC00894-002,92 NONHSAT101069,29 and PRLB77 sponge specific miRNAs to regulate drug resistance in BC.
Exosomes
Exosomes are extracellular vesicles that are constantly released into the microenvironment by various types of cells including cancer cells.109 Communication between cancer cells can be mediated by exosomes through the transport of lipids, proteins, and nucleic acids including lncRNAs. This process affects cell growth, immune responses, and drug resistance in target cells, and has therefore attracted increasing attention.110 LncRNA H19 is incorporated into exosomes and promotes DOX resistance in sensitive BC cells.34 LncRNA UCA1 promotes TAM resistance in BC via transport by exosomes.51 AGAP2-AS1 is incorporated into exosomes in an hnRNPA2B1-dependent manner, thereby decreasing TZB-induced cytotoxicity and promoting TZB resistance in BC cells.98 SNHG14 promotes TZB resistance in sensitive BC cells via exosomes.102
In addition to ceRNAs and exosomes, lncRNAs can regulate drug resistance in BC via cellular or molecular targets. All those mechanisms constitute a complicated regulatory network (Figure 2).
Apoptosis
Apoptosis is a type of programmed cell death that serves a biological function under normal conditions. There are two pathways of apoptosis, the intrinsic and the extrinsic pathways, which converge to activate effector caspases such as caspase 3 and caspase 7.111 The intrinsic pathway, which is mediated by the Bcl-2 family, leads to mitochondrial outer membrane permeabilization and the release of cytochrome c into the cytoplasm. The extrinsic pathway is mediated by the tumor necrosis factor receptor family and causes the activation of caspase 8.111 An unbalanced relationship between pro-apoptosis factors and anti-apoptosis factors leads to dysregulated apoptosis, which is associated with many diseases including cancer, and is involved in the sensitivity of cancer cells to drugs.112 LINP1 decreases cell apoptosis by inhibiting caspase-8 and caspase-9 in DOX-resistant cells or by suppressing caspase-9 and Bax in 5-FU-resistant cells, thereby increasing DOX and 5-FU resistance.26 Low MEG3 expression promotes DOX resistance by reducing cell apoptosis via the Bax/Bcl-2 axis in BC cells.64 Other lncRNAs, including UCA1,48 SNHG14,102 and CCAT289 also alter BC cell sensitivity to drugs via apoptosis.
Autophagy
Autophagy is a homeostatic process in cells in which intracellular proteins and organelles are delivered to lysosomes and degraded for reuse or to generate new macromolecules.113 Autophagy is critical for cell survival under stress conditions. Recent studies suggest that lncRNAs affect drug resistance by regulating cell autophagy in BC. H19 and ROR induce cell autophagy and reverse TAM resistance in BC cells.39,88
The Cell Cycle
The mammalian cell cycle is controlled by cyclin-dependent kinases (CDKs) and certain signaling pathways, such as the RB and p53 pathways, are related to CDKs. CDKs and their associated pathways regulate exit and entry to the different phases of the cell cycle to control cell cycle progression.114 LncRNAs are involved in the regulation of the cell cycle by modulating critical cell cycle regulators. Deregulation of lncRNAs can lead to cell cycle arrest and promote tumorigenesis.115 Knockdown of NEAT1 results in cell cycle arrest at G1 phase via targeting cyclin E1 and D1 to induce apoptosis of cisR and taxR BC cells.31 High expression of UCA1 promotes TAM resistance by decreasing cell apoptosis and arresting the cell cycle at the G2/M phase.48 LINC00511 upregulates the expression of CDK6 and decreases PTX cytotoxicity.69 ARA reverses ADR resistance by modulating the cell cycle in BC.62
Drug Efflux
Certain lncRNAs promote MDR by activating drug efflux pumps via targeting ABC transporters in BC. H19 targets MDR1 and increases DOX resistance.25 HOTAIR downregulates the expression of MDR1, MRP1, and ABCB1 and decreases DOX and TZB resistance.43 Knockdown of BC032585 increases MDR1 and promotes DOX and PTX resistance.55 LINC00968 targets MRP1 to reverse MDR.56 RP11-770J1.3 confers PTX resistance by increasing MRP, BCRP and P-gp.71 Low expression of EPB41LA4-AS2 promotes DOC resistance by upregulating ABCB1.72
EMT
The EMT process is a cell biological program that converts epithelial cells, which connects cells, to mesenchymal cells, which show higher motility and invasiveness.116 The EMT process is activated during malignant progression, which leads to the completion of many steps of the invasion-metastasis cascade in cancer cells and results in cancer metastasis, relapse, and MDR.117 Some lncRNAs activate the EMT process to promote drug resistance in BC. Knockdown of linc00152 reverses DOX resistance by suppressing cell viability, invasion, migration, and EMT.61 Knockdown of LOL prevents EMT and reverses TAM resistance by sponging let-7 miRNAs.82
Conclusion
Drug resistance limits the clinical treatment of BC, which has prompted extensive investigation into the underlying mechanisms. Dysregulated lncRNAs play important roles in the regulation of drug resistance in BC through complex mechanisms. LncRNAs show potential as diagnostic, prognostic, and/or predictive biomarkers for clinical use because they are present in body fluids and exosomes.20 An effective and individualized treatment strategy based on these lncRNAs could be designed to predict the response to treatment in BC, which could prevent the use of ineffective therapies that might lead to severe side-effects.
Targeted molecular therapies are designed to bind specific targets to alter cell functions, which always act as adjuvant therapies after operation or with classical therapies.118 Many methods have been developed to target a particular ncRNA, which is depended on the expression pattern of miRNAs or lncRNAs, including antisense oligonucleotides (antagomiRs/ASOs), miRNA ablation via viral, liposomal, and nanoparticles.119 Some new gene editing technology also show the possibility of potential application, such as CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats – associated nuclease-9).120 CRISPR/Cas9 has been studied extensively in hepatocellular carcinoma and ovarian cancer, while few studies report the use of this technology in lncRNA editing in BC. The insertions and deletions induced by CRISPR/Cas9 system seem not susceptible in lncRNAs.121
In addition to lncRNAs, the other two main ncRNAs (miRNAs and circRNAs) are also involved in drug resistance in many cancers including BC. For instance, miR-181a promotes ADR resistance in BC by inhibiting cell apoptosis via Bcl-2.122 Overexpression of circRNA 0025202 reverses TAM resistance by sponging miR-182-5p in BC.123 Therefore, targeted drugs based on these selective dysregulated ncRNAs might be a novel strategy for future clinical application.
Most studies have proved the relationship between drug resistance in BC cells and ncRNAs. Few of these researches revealed this in vivo or even in clinical treatment. Injecting anti-miR-21 oligonucleotides into xenograft mouse model inhibits the growth of breast tumor.124 Silence of miR-21 reverses topotecan, taxol, and TZB resistance in BC cells.125 Therefore, classical chemotherapy combined with anti-miR-21 treatment may reduce drug resistance in BC patients.
A miRNA-targeted drug, miravirsen,126 is currently being tested for the treatment of hepatitis C virus (HCV) infection in a phase Ⅱ clinical trial. However, miravirsen is the first and only targeted drug based on ncRNAs developed to date. There are no reports describing lncRNA- or circRNA-based targeted drugs for the treatment of cancer.
Although lncRNAs provide a possible new research direction to elucidate the mechanism of drug resistance in BC, therapies based on lncRNAs have not been applied to the clinical treatment of BC. Treatments based on lncRNAs are associated with the same limitations as treatment based on small interfering RNAs, such as chemical modification and issues related to the delivery of lncRNAs.22 However, there are additional limitations specific to the clinical application of lncRNAs. The safety of targeted therapies, which is based on biological technologies, is an area that must be studied thoroughly. Therapies involving RNA interference were shown to be toxic in preclinical mouse models.127 The development of mutations is another problem that warrants investigation. Whether these biological markers can maintain their function continually, in other words, whether the effect is persistent, affects the efficacy of treatment. Another potential problem is the cost to patients. Collecting and detecting lncRNAs, as well as gene detection, are performed by real-time PCR and next-generation sequencing. Both technologies require trained professional technicians and precise and expensive instruments, which may lead to a significant finical burden to patients. Another challenge about lncRNA research is that many lncRNA species lack conservation.128 Many lncRNAs related with human cancer do not exhibit sequence conservation in mammalian, which limit the pre-clinical mouse studies for lncRNA-based target therapy. To date due to these complexity and challenge, few studies have investigated the efficacy of lncRNAs as therapeutic targets in BC patients.
Although lncRNAs play an important role in drug resistance in BC via various mechanisms, the specific efficiency of target therapies based on different mechanisms remain unclear. Select an appropriate target therapy can help patients get better efficacy and prognosis. However, there are few studies and animal experiments on this area, let alone clinical application. On the other hand, as lncRNAs exit in blood and body fluid of patients, collecting and testing lncRNAs may be feasible. Maybe in the future, lncRNAs can be used to predict the efficacy of different therapies to select an appreciate and sensitive target therapy for BC patients. More studies are needed to compare the efficacy of target therapy based on different mechanisms, to design an individual therapeutic plan in different BC patients.
In conclusion, studies indicate that lncRNAs play a significant role in drug resistance in BC. LncRNAs show potential as biomarkers to predict the response to treatment or reverse drug resistance in the future. Up to date, molecular targeted drugs based on lncRNAs remain to be developed. More extensive and comprehensive studies, especially clinical studies, are needed to elucidate the mechanisms of drug resistance and develop therapies based on lncRNAs.
Acknowledgments
This study was supported by the Department of Breast Surgery, the Second Hospital of Jilin University partially.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi:10.3322/caac.21583
3. Sharma R, Sharma R, Khaket TP, et al. Breast cancer metastasis: putative therapeutic role of vascular cell adhesion molecule-1. Cell Oncol (Dordr). 2017;40(3):199–208. doi:10.1007/s13402-017-0324-x
4. Ellis LM, Hicklin DJ. Resistance to targeted therapies: refining anticancer therapy in the era of molecular oncology. Clin Cancer Res. 2009;15(24):7471–7478. doi:10.1158/1078-0432.CCR-09-1070
5. Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462–472. doi:10.1016/j.ccell.2015.02.015
6. Zheng T, Wang J, Chen X, Liu L. Role of microRNA in anticancer drug resistance. Int J Cancer. 2010;126(1):2–10. doi:10.1002/ijc.24782
7. Ayers D, Vandesompele J. Influence of microRNAs and long non-coding RNAs in cancer chemoresistance. Genes (Basel). 2017;8(3):95. doi:10.3390/genes8030095
8. Mulrane L, McGee SF, Gallagher WM, O’Connor DP. miRNA dysregulation in breast cancer. Cancer Res. 2013;73(22):6554–6562. doi:10.1158/0008-5472.CAN-13-1841
9. Kapranov P, Cheng J, Dike S, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316(5830):1484–1488. doi:10.1126/science.1138341
10. Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157(1):77–94. doi:10.1016/j.cell.2014.03.008
11. Klinge CM. Non-coding RNAs in breast cancer: intracellular and intercellular communication. Noncoding RNA. 2018;4(4):40.
12. Previdi MC, Carotenuto P, Zito D, Pandolfo R, Braconi C. Noncoding RNAs as novel biomarkers in pancreatic cancer: what do we know? Future Oncol. 2017;13(5):443–453. doi:10.2217/fon-2016-0253
13. Riquelme I, Letelier P, Riffo-Campos AL, Brebi P, Roa JC. Emerging role of miRNAs in the drug resistance of gastric cancer. Int J Mol Sci. 2016;17(3):424. doi:10.3390/ijms17030424
14. Sahu A, Singhal U, Chinnaiyan AM. Long noncoding RNAs in cancer: from function to translation. Trends Cancer. 2015;1(2):93–109. doi:10.1016/j.trecan.2015.08.010
15. Geisler S, Coller J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14(11):699–712. doi:10.1038/nrm3679
16. Ding B, Lou W, Xu L, Fan W. Non-coding RNA in drug resistance of hepatocellular carcinoma. Biosci Rep. 2018;38(5). doi:10.1042/BSR20180915
17. Chen C, Tang X, Liu Y, Zhu J, Liu J. Induction/reversal of drug resistance in gastric cancer by non-coding RNAs (Review). Int J Oncol. 2019;54(5):1511–1524. doi:10.3892/ijo.2019.4751
18. Fanale D, Castiglia M, Bazan V, Russo A. Involvement of non-coding RNAs in Chemo- and radioresistance of colorectal cancer. Adv Exp Med Biol. 2016;937:207–228.
19. Mao BD, Xu P, Xu P, et al. LINC00511 knockdown prevents cervical cancer cell proliferation and reduces resistance to paclitaxel. J Biosci. 2019;44(2). doi:10.1007/s12038-019-9851-0
20. Pecero ML, Salvador-Bofill J, Molina-Pinelo S. Long non-coding RNAs as monitoring tools and therapeutic targets in breast cancer. Cell Oncol (Dordr). 2019;42(1):1–12. doi:10.1007/s13402-018-0412-6
21. Malhotra A, Jain M, Prakash H, Vasquez KM, Jain A. The regulatory roles of long non-coding RNAs in the development of chemoresistance in breast cancer. Oncotarget. 2017;8(66):110671–110684. doi:10.18632/oncotarget.22577
22. Dehghanzadeh R, Jadidi-Niaragh F, Gharibi T, Yousefi M. MicroRNA-induced drug resistance in gastric cancer. Biomed Pharmacother. 2015;74:191–199. doi:10.1016/j.biopha.2015.08.009
23. Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11(4):265–283. doi:10.1016/S0928-0987(00)00114-7
24. Lage H. Gene therapeutic approaches to overcome ABCB1-mediated drug resistance. Recent Results Cancer Res. 2016;209:87–94.
25. Lin C-T. Suppression of gene amplification and chromosomal DNA integration by the DNA mismatch repair system. Nucleic Acids Res. 2001;29(16):3304–3310. doi:10.1093/nar/29.16.3304
26. Yu DD, Wu Y, Zhang XH, et al. Exosomes from adriamycin-resistant breast cancer cells transmit drug resistance partly by delivering miR-222. Tumour Biol. 2016;37(3):3227–3235. doi:10.1007/s13277-015-4161-0
27. Yao N, Fu Y, Chen L, et al. Long non-coding RNA NONHSAT101069 promotes epirubicin resistance, migration, and invasion of breast cancer cells through NONHSAT101069/miR-129-5p/Twist1 axis. Oncogene. 2019;38(47):7216–7233. doi:10.1038/s41388-019-0904-5
28. Zugazagoitia J, Guedes C, Ponce S, et al. Current challenges in cancer treatment. Clin Ther. 2016;38(7):1551–1566. doi:10.1016/j.clinthera.2016.03.026
29. Shin VY, Chen J, Cheuk IW, et al. Long non-coding RNA NEAT1 confers oncogenic role in triple-negative breast cancer through modulating chemoresistance and cancer stemness. Cell Death Dis. 2019;10(4):270. doi:10.1038/s41419-019-1513-5
30. Li X, Wang S, Li Z, et al. The lncRNA NEAT1 facilitates cell growth and invasion via the miR-211/HMGA2 axis in breast cancer. Int J Biol Macromol. 2017;105(Pt 1):346–353. doi:10.1016/j.ijbiomac.2017.07.053
31. Liang Y, Li Y, Song X, et al. Long noncoding RNA LINP1 acts as an oncogene and promotes chemoresistance in breast cancer. Cancer Biol Ther. 2018;19(2):120–131. doi:10.1080/15384047.2017.1394543
32. Ma T, Liang Y, Li Y, et al. LncRNA LINP1 confers tamoxifen resistance and negatively regulated by ER signaling in breast cancer. Cell Signal. 2020;68:109536. doi:10.1016/j.cellsig.2020.109536
33. Chang L, Hu Z, Zhou Z, Zhang H. Linc00518 contributes to multidrug resistance through regulating the mir-199a/MRP1 axis in breast cancer. Cell Physiol Biochem. 2018;48(1):16–28. doi:10.1159/000491659
34. Li Z, Qian J, Li J, Zhu C. Knockdown of lncRNA-HOTAIR downregulates the drug-resistance of breast cancer cells to doxorubicin via the PI3K/AKT/mTOR signaling pathway. Exp Ther Med. 2019;18(1):435–442. doi:10.3892/etm.2019.7629
35. Chen T, Liu Z, Zeng W, Huang T. Down-regulation of long non-coding RNA HOTAIR sensitizes breast cancer to trastuzumab. Sci Rep. 2019;9(1):19881. doi:10.1038/s41598-019-53699-w
36. Xue X, Yang YA, Zhang A, et al. LncRNA HOTAIR enhances ER signaling and confers tamoxifen resistance in breast cancer. Oncogene. 2016;35(21):2746–2755. doi:10.1038/onc.2015.340
37. Zhu QN, Wang G, Guo Y, et al. LncRNA H19 is a major mediator of doxorubicin chemoresistance in breast cancer cells through a cullin4A-MDR1 pathway. Oncotarget. 2017;8(54):91990–92003.
38. Wang X, Pei X, Guo G, et al. Exosome-mediated transfer of long noncoding RNA H19 induces doxorubicin resistance in breast cancer. J Cell Physiol. 2020.
39. Sun Z, Zhang C, Wang T, et al. Correlation between long non-coding RNAs (lncRNAs) H19 expression and trastuzumab resistance in breast cancer. J Cancer Res Ther. 2019;15(4):933–940. doi:10.4103/jcrt.JCRT_208_19
40. Han J, Han B, Wu X, et al. Knockdown of lncRNA H19 restores chemo-sensitivity in paclitaxel-resistant triple-negative breast cancer through triggering apoptosis and regulating Akt signaling pathway. Toxicol Appl Pharmacol. 2018;359:55–61. doi:10.1016/j.taap.2018.09.018
41. Si X, Zang R, Zhang E, et al. LncRNA H19 confers chemoresistance in ERalpha-positive breast cancer through epigenetic silencing of the pro-apoptotic gene BIK. Oncotarget. 2016;7(49):81452–81462. doi:10.18632/oncotarget.13263
42. Gao H, Hao G, Sun Y, Li L, Wang Y. Long noncoding RNA H19 mediated the chemosensitivity of breast cancer cells via Wnt pathway and EMT process. Onco Targets Ther. 2018;11:8001–8012. doi:10.2147/OTT.S172379
43. Wang J, Xie S, Yang J, et al. The long noncoding RNA H19 promotes tamoxifen resistance in breast cancer via autophagy. J Hematol Oncol. 2019;12(1):81. doi:10.1186/s13045-019-0747-0
44. Basak P, Chatterjee S, Bhat V, et al. Long non-coding RNA H19 acts as an estrogen receptor modulator that is required for endocrine therapy resistance in ER+ breast cancer cells. Cell Physiol Biochem. 2018;51(4):1518–1532. doi:10.1159/000495643
45. Li Y, Wang B, Lai H, et al. Long non-coding RNA CRALA is associated with poor response to chemotherapy in primary breast cancer. Thorac Cancer. 2017;8(6):582–591. doi:10.1111/1759-7714.12487
46. Zhu HY, Bai WD, Ye XM, Yang AG, Jia LT. Long non-coding RNA UCA1 desensitizes breast cancer cells to trastuzumab by impeding miR-18a repression of Yes-associated protein 1. Biochem Biophys Res Commun. 2018;496(4):1308–1313. doi:10.1016/j.bbrc.2018.02.006
47. Li X, Wu Y, Liu A, Tang X. Long non-coding RNA UCA1 enhances tamoxifen resistance in breast cancer cells through a miR-18a-HIF1alpha feedback regulatory loop. Tumour Biol. 2016;37(11):14733–14743. doi:10.1007/s13277-016-5348-8
48. Li Z, Yu D, Li H, Lv Y, Li S. Long noncoding RNA UCA1 confers tamoxifen resistance in breast cancer endocrinotherapy through regulation of the EZH2/p21 axis and the PI3K/AKT signaling pathway. Int J Oncol. 2019;54(3):1033–1042. doi:10.3892/ijo.2019.4679
49. Wu C, Luo J. Long non-coding RNA (lncRNA) urothelial carcinoma-associated 1 (UCA1) enhances tamoxifen resistance in breast cancer cells via inhibiting mTOR signaling pathway. Med Sci Monit. 2016;22:3860–3867. doi:10.12659/MSM.900689
50. Liu H, Wang G, Yang L, et al. Knockdown of Long Non-Coding RNA UCA1 increases the tamoxifen sensitivity of breast cancer cells through inhibition of wnt/beta-catenin pathway. PLoS One. 2016;11(12):e0168406. doi:10.1371/journal.pone.0168406
51. Xu CG, Yang MF, Ren YQ, Wu CH, Wang LQ. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells. Eur Rev Med Pharmacol Sci. 2016;20(20):4362–4368.
52. Zheng S, Li M, Miao K, Xu H. lncRNA GAS5-promoted apoptosis in triple-negative breast cancer by targeting miR-378a-5p/SUFU signaling. J Cell Biochem. 2020;121(3):2225–2235. doi:10.1002/jcb.29445
53. Gu J, Wang Y, Wang X, et al. Downregulation of lncRNA GAS5 confers tamoxifen resistance by activating miR-222 in breast cancer. Cancer Lett. 2018;434:1–10. doi:10.1016/j.canlet.2018.06.039
54. Li W, Zhai L, Wang H, et al. Downregulation of LncRNA GAS5 causes trastuzumab resistance in breast cancer. Oncotarget. 2016;7(19):27778–27786. doi:10.18632/oncotarget.8413
55. Zeng Y, Wang G, Zhou CF, et al. LncRNA profile study reveals a three-LncRNA signature associated with the pathological complete response following neoadjuvant chemotherapy in breast cancer. Front Pharmacol. 2019;10:574. doi:10.3389/fphar.2019.00574
56. Xiu DH, Liu GF, Yu SN, et al. Long non-coding RNA LINC00968 attenuates drug resistance of breast cancer cells through inhibiting the Wnt2/beta-catenin signaling pathway by regulating WNT2. J Exp Clin Cancer Res. 2019;38(1):94. doi:10.1186/s13046-019-1100-8
57. Sledge GW, Mamounas EP, Hortobagyi GN, et al. Past, present, and future challenges in breast cancer treatment. J Clin Oncol. 2014;32(19):1979–1986. doi:10.1200/JCO.2014.55.4139
58. Kalyanaraman B. Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: have we been barking up the wrong tree? Redox Biol. 2020;29:101394. doi:10.1016/j.redox.2019.101394
59. Qian W, Zhu Y, Wu M, et al. Linc00668 promotes invasion and stem cell-like properties of breast cancer cells by interaction with SND1. Front Oncol. 2020;10:88. doi:10.3389/fonc.2020.00088
60. Gooding AJ, Zhang B, Gunawardane L, et al. The lncRNA BORG facilitates the survival and chemoresistance of triple-negative breast cancers. Oncogene. 2019;38(12):2020–2041. doi:10.1038/s41388-018-0586-4
61. Hu XL, Wang J, He W, Zhao P, WQ W. Down-regulation of lncRNA Linc00152 suppressed cell viability, invasion, migration, and epithelial to mesenchymal transition, and reversed chemo-resistance in breast cancer cells. Eur Rev Med Pharmacol Sci. 2018;22(10):3074–3084. doi:10.26355/eurrev_201805_15067
62. Jiang M, Huang O, Xie Z, et al. A novel long non-coding RNA-ARA: adriamycin resistance-associated. Biochem Pharmacol. 2014;87(2):254–283. doi:10.1016/j.bcp.2013.10.020
63. Gao X, Qin T, Mao J, et al. PTENP1/miR-20a/PTEN axis contributes to breast cancer progression by regulating PTEN via PI3K/AKT pathway. J Exp Clin Cancer Res. 2019;38(1):256. doi:10.1186/s13046-019-1260-6
64. Deocesano-Pereira C, Machado RAC, De Jesus-ferreira HC, et al. Functional impact of the long non-coding RNA MEG3 deletion by CRISPR/Cas9 in the human triple negative metastatic Hs578T cancer cell line. Oncol Lett. 2019;18(6):5941–5951. doi:10.3892/ol.2019.10969
65. Zheng A, Zhang L, Song X, Jin F. Clinical significance of SPRY4-IT1 in efficacy and survival prediction in breast cancer patients undergoing neoadjuvant chemotherapy. Histol Histopathol. 2019;18175.
66. Yared JA, Tkaczuk KH. Update on taxane development: new analogs and new formulations. Drug Des Devel Ther. 2012;6:371–384. doi:10.2147/DDDT.S28997
67. Wang R, Zhang T, Yang Z, Jiang C, Seng J. Long non-coding RNA FTH1P3 activates paclitaxel resistance in breast cancer through miR-206/ABCB1. J Cell Mol Med. 2018;22(9):4068–4075. doi:10.1111/jcmm.13679
68. Zheng P, Dong L, Zhang B, et al. Long noncoding RNA CASC2 promotes paclitaxel resistance in breast cancer through regulation of miR-18a-5p/CDK19. Histochem Cell Biol. 2019;152(4):281–291. doi:10.1007/s00418-019-01794-4
69. Zhang H, Zhao B, Wang X, Zhang F, Yu W. LINC00511 knockdown enhances paclitaxel cytotoxicity in breast cancer via regulating miR-29c/CDK6 axis. Life Sci. 2019;228:135–144. doi:10.1016/j.lfs.2019.04.063
70. Pan Y, Pan Y, Cheng Y, et al. Knockdown of LncRNA MAPT-AS1 inhibites proliferation and migration and sensitizes cancer cells to paclitaxel by regulating MAPT expression in ER-negative breast cancers. Cell Biosci. 2018;8(1):7. doi:10.1186/s13578-018-0207-5
71. Li Y, Wang Y, Wang H, et al. [Effects of lncRNA RP11-770J1.3 and TMEM25 expression on paclitaxel resistance in human breast cancer cells]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2017;46(4):364–370. Chinese
72. Huang P, Li F, Li L, et al. lncRNA profile study reveals the mRNAs and lncRNAs associated with docetaxel resistance in breast cancer cells. Sci Rep. 2018;8(1):17970. doi:10.1038/s41598-018-36231-4
73. Florea AM, Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel). 2011;3(1):1351–1371. doi:10.3390/cancers3011351
74. Mi H, Wang X, Wang F, et al. SNHG15 Contributes To Cisplatin Resistance In Breast Cancer Through Sponging miR-381. Onco Targets Ther. 2020;13:657–666. doi:10.2147/OTT.S223321
75. Wu J, Chen H, Ye M, et al. Long noncoding RNA HCP5 contributes to cisplatin resistance in human triple-negative breast cancer via regulation of PTEN expression. Biomed Pharmacother. 2019;115:108869. doi:10.1016/j.biopha.2019.108869
76. Wang M, Han D, Yuan Z, et al. Long non-coding RNA H19 confers 5-Fu resistance in colorectal cancer by promoting SIRT1-mediated autophagy. Cell Death Dis. 2018;9(12):1149. doi:10.1038/s41419-018-1187-4
77. Liang Y, Song X, Li Y, et al. A novel long non-coding RNA-PRLB acts as a tumor promoter through regulating miR-4766-5p/SIRT1 axis in breast cancer. Cell Death Dis. 2018;9(5):563. doi:10.1038/s41419-018-0582-1
78. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi:10.1038/35021093
79. Sotiriou C, Neo SY, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100(18):10393–10398. doi:10.1073/pnas.1732912100
80. Hayes EL, Lewis-Wambi JS. Mechanisms of endocrine resistance in breast cancer: an overview of the proposed roles of noncoding RNA. Breast Cancer Res. 2015;17(1):40. doi:10.1186/s13058-015-0542-y
81. Liu Y, Li M, Yu H, Piao H. lncRNA CYTOR promotes tamoxifen resistance in breast cancer cells via sponging miR125a5p. Int J Mol Med. 2020;45(2):497–509. doi:10.3892/ijmm.2019.4428
82. Sun W, Xu X, Jiang Y, et al. Transcriptome analysis of luminal breast cancer reveals a role for LOL in tumor progression and tamoxifen resistance. Int J Cancer. 2019;145(3):842–856. doi:10.1002/ijc.32185
83. Ma Y, Bu D, Long J, Chai W, Dong J. LncRNA DSCAM-AS1 acts as a sponge of miR-137 to enhance Tamoxifen resistance in breast cancer. J Cell Physiol. 2019;234(3):2880–2894. doi:10.1002/jcp.27105
84. Niknafs YS, Han S, Ma T, et al. The lncRNA landscape of breast cancer reveals a role for DSCAM-AS1 in breast cancer progression. Nat Commun. 2016;7(1):12791. doi:10.1038/ncomms12791
85. Mitobe Y, Ikeda K, Suzuki T, et al. ESR1 -Stabilizing Long Noncoding RNA TMPO-AS1 promotes hormone-refractory breast cancer progression. Mol Cell Biol. 2019;39(23):23. doi:10.1128/MCB.00261-19
86. Zhang HY, Liang F, Zhang JW, et al. Effects of long noncoding RNA-ROR on tamoxifen resistance of breast cancer cells by regulating microRNA-205. Cancer Chemother Pharmacol. 2017;79(2):327–337. doi:10.1007/s00280-016-3208-2
87. Peng WX, Huang JG, Yang L, Gong AH, Mo YY. Linc-RoR promotes MAPK/ERK signaling and confers estrogen-independent growth of breast cancer. Mol Cancer. 2017;16(1):161.
88. Li Y, Jiang B, Zhu H, et al. Inhibition of long non-coding RNA ROR reverses resistance to Tamoxifen by inducing autophagy in breast cancer. Tumour Biol. 2017;39(6):1010428317705790. doi:10.1177/1010428317705790
89. Cai Y, He J, Zhang D. [Suppression of long non-coding RNA CCAT2 improves tamoxifen-resistant breast cancer cells’ response to tamoxifen]. Mol Biol (Mosk). 2016;50(5):821–827. Russian doi:10.7868/S0026898416030046
90. Godinho MF, Sieuwerts AM, Look MP, et al. Relevance of BCAR4 in tamoxifen resistance and tumour aggressiveness of human breast cancer. Br J Cancer. 2010;103(8):1284–1291. doi:10.1038/sj.bjc.6605884
91. Shi YF, Lu H, Wang HB. Downregulated lncRNA ADAMTS9-AS2 in breast cancer enhances tamoxifen resistance by activating microRNA-130a-5p. Eur Rev Med Pharmacol Sci. 2019;23(4):1563–1573. doi:10.26355/eurrev_201902_17115
92. Zhang X, Wang M, Sun H, Zhu T, Wang X. Downregulation of LINC00894-002 contributes to tamoxifen resistance by enhancing the TGF-beta signaling pathway. Biochemistry (Mosc). 2018;83(5):603–611. doi:10.1134/S0006297918050139
93. Grizzi G, Ghidini M, Botticelli A, et al. Strategies for increasing the effectiveness of aromatase inhibitors in locally advanced breast cancer: an evidence-based review on current options. Cancer Manag Res. 2020;12:675–686. doi:10.2147/CMAR.S202965
94. Chumsri S, Howes T, Bao T, Sabnis G, Brodie A. Aromatase, aromatase inhibitors, and breast cancer. J Steroid Biochem Mol Biol. 2011;125(1–2):13–22. doi:10.1016/j.jsbmb.2011.02.001
95. Nuciforo P, Radosevic-Robin N, Ng T, Scaltriti M. Quantification of HER family receptors in breast cancer. Breast Cancer Res. 2015;17:53. doi:10.1186/s13058-015-0561-8
96. Ock CY, Lee KW, Kim JW, et al. Optimal patient selection for trastuzumab treatment in HER2-positive advanced gastric cancer. Clin Cancer Res. 2015;21(11):2520–2529. doi:10.1158/1078-0432.CCR-14-2659
97. Han M, Gu Y, Lu P, et al. Exosome-mediated lncRNA AFAP1-AS1 promotes trastuzumab resistance through binding with AUF1 and activating ERBB2 translation. Mol Cancer. 2020;19(1):26. doi:10.1186/s12943-020-1145-5
98. Zheng Z, Chen M, Xing P, Yan X, Xie B. Increased Expression of Exosomal AGAP2-AS1 (AGAP2 Antisense RNA 1) in breast cancer cells inhibits trastuzumab-induced cell cytotoxicity. Med Sci Monit. 2019;25:2211–2220. doi:10.12659/MSM.915419
99. Dong H, Wang W, Mo S, et al. SP1-induced lncRNA AGAP2-AS1 expression promotes chemoresistance of breast cancer by epigenetic regulation of MyD88. J Exp Clin Cancer Res. 2018;37(1):202. doi:10.1186/s13046-018-0875-3
100. Dong H, Hu J, Zou K, et al. Activation of LncRNA TINCR by H3K27 acetylation promotes Trastuzumab resistance and epithelial-mesenchymal transition by targeting MicroRNA-125b in breast cancer. Mol Cancer. 2019;18(1):3. doi:10.1186/s12943-018-0931-9
101. Dong H, Wang W, Mo S, et al. Long non-coding RNA SNHG14 induces trastuzumab resistance of breast cancer via regulating PABPC1 expression through H3K27 acetylation. J Cell Mol Med. 2018;22(10):4935–4947. doi:10.1111/jcmm.13758
102. Dong H, Wang W, Chen R, et al. Exosome-mediated transfer of lncRNASNHG14 promotes trastuzumab chemoresistance in breast cancer. Int J Oncol. 2018;53(3):1013–1026. doi:10.3892/ijo.2018.4467
103. Shi SJ, Wang LJ, Yu B, et al. LncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancer. Oncotarget. 2015;6(13):11652–11663. doi:10.18632/oncotarget.3457
104. Rubovszky G, Horvath Z. Recent Advances in the Neoadjuvant Treatment of Breast Cancer. J Breast Cancer. 2017;20(2):119–131. doi:10.4048/jbc.2017.20.2.119
105. Venturutti L, Cordo Russo RI, Rivas MA, et al. MiR-16 mediates trastuzumab and lapatinib response in ErbB-2-positive breast and gastric cancer via its novel targets CCNJ and FUBP1. Oncogene. 2016;35(48):6189–6202. doi:10.1038/onc.2016.151
106. Corcoran C, Rani S, Breslin S, et al. miR-630 targets IGF1R to regulate response to HER-targeting drugs and overall cancer cell progression in HER2 over-expressing breast cancer. Mol Cancer. 2014;13(1):71. doi:10.1186/1476-4598-13-71
107. Guil S, Esteller M. RNA-RNA interactions in gene regulation: the coding and noncoding players. Trends Biochem Sci. 2015;40(5):248–256. doi:10.1016/j.tibs.2015.03.001
108. Shuwen H, Qing Z, Yan Z, Xi Y. Competitive endogenous RNA in colorectal cancer: A systematic review. Gene. 2018;645:157–162. doi:10.1016/j.gene.2017.12.036
109. Yu S, Cao H, Shen B, Feng J. Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget. 2015;6(35):37151–37168. doi:10.18632/oncotarget.6022
110. Chen F, Wang N, Tan HY, et al. The functional roles of exosomes-derived long non-coding RNA in human cancer. Cancer Biol Ther. 2019;20(5):583–592. doi:10.1080/15384047.2018.1564562
111. Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69(1):217–245. doi:10.1146/annurev.biochem.69.1.217
112. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
113. Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349–364. doi:10.1038/s41580-018-0003-4
114. Kitagawa M, Kitagawa K, Kotake Y, Niida H, Ohhata T. Cell cycle regulation by long non-coding RNAs. Cell Mol Life Sci. 2013;70(24):4785–4794. doi:10.1007/s00018-013-1423-0
115. Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21(6):354–361. doi:10.1016/j.tcb.2011.04.001
116. Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018;12(4):361–373. doi:10.1007/s11684-018-0656-6
117. Shibue T, Weinberg RAEMT. CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611–629. doi:10.1038/nrclinonc.2017.44
118. Tomar D, Yadav AS, Kumar D, Bhadauriya G, Kundu GC. Non-coding RNAs as potential therapeutic targets in breast cancer. Biochim Biophys Acta Gene Regul Mech. 2019:194378.
119. Liu Z, Sall A, Yang D. MicroRNA: an emerging therapeutic target and intervention tool. Int J Mol Sci. 2008;9(6):978–999. doi:10.3390/ijms9060978
120. Fellmann C, Gowen BG, Lin PC, Doudna JA, Corn JE. Cornerstones of CRISPR-Cas in drug discovery and therapy. Nat Rev Drug Discov. 2017;16(2):89–100. doi:10.1038/nrd.2016.238
121. Lavorgna G, Vago R, Sarmini M, et al. Long non-coding RNAs as novel therapeutic targets in cancer. Pharmacol Res. 2016;110:131–138. doi:10.1016/j.phrs.2016.05.018
122. Zhu Y, Wu J, Li S, et al. The function role of miR-181a in chemosensitivity to adriamycin by targeting Bcl-2 in low-invasive breast cancer cells. Cell Physiol Biochem. 2013;32(5):1225–1237. doi:10.1159/000354521
123. Sang Y, Chen B, Song X, et al. circRNA_0025202 regulates tamoxifen sensitivity and tumor progression via regulating the miR-182-5p/FOXO3a axis in breast cancer. Mol Ther. 2019;27(9):1638–1652. doi:10.1016/j.ymthe.2019.05.011
124. Si ML, Zhu S, Wu H, et al. miR-21-mediated tumor growth. Oncogene. 2007;26(19):2799–2803. doi:10.1038/sj.onc.1210083
125. Mei M, Ren Y, Zhou X, et al. Downregulation of miR-21 enhances chemotherapeutic effect of taxol in breast carcinoma cells. Technol Cancer Res Treat. 2010;9(1):77–86. doi:10.1177/153303461000900109
126. van der Ree MH, van der Meer AJ, van Nuenen AC, et al. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment Pharmacol Ther. 2016;43(1):102–113. doi:10.1111/apt.13432
127. Boudreau RL, Martins I, Davidson BL. Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol Ther. 2009;17(1):169–175. doi:10.1038/mt.2008.231
128. Arun G, Diermeier SD, Spector DL. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol Med. 2018;24(3):257–277. doi:10.1016/j.molmed.2018.01.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.