Back to Journals » Cancer Management and Research » Volume 12
Long Non-Coding RNA TMPO-AS1 Promotes Cell Migration and Invasion by Sponging miR-140-5p and Inducing SOX4-Mediated EMT in Gastric Cancer
Received 24 October 2019
Accepted for publication 21 January 2020
Published 20 February 2020 Volume 2020:12 Pages 1261—1268
DOI https://doi.org/10.2147/CMAR.S235898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Yonghong Sun, Chunyao Han
Department of General Surgery, Second Hospital of Shanxi Medical University, Taiyuan City, Shanxi Province 030001, People’s Republic of China
Correspondence: Yonghong Sun
Department of General Surgery, Second Hospital of Shanxi Medical University, No. 382 Wuyi Road, Taiyuan City 030001, Shanxi Province, People’s Republic of China
Email [email protected]
Background: Mounting evidence show that long non-coding RNAs (lncRNAs) play critical roles in the progression of various human cancers, including gastric cancer (GC), a common gastrointestinal tumor. In this study, the biological functions of lncRNA TMPO-AS1 in GC were studied.
Methods: TMPO-AS1 and miR-140-5p expression levels were detected in GC tissues and cell lines by RT-qPCR analysis. Knockdown or overexpression of TMPO-AS1 was conducted to evaluate the effects of TMPO-AS1 on the malignant behaviors of GC cells. Bioinformatic prediction and dual-luciferase reporter assay were performed to investigate the direct interaction between TMPO-AS1 and miR-140-5p in GC.
Results: We observed that TMPO-AS1 was up-regulated in GC tissues, and high TMPO-AS1 expression in GC patients was closely correlated with aggressive clinicopathologic characteristics and poor overall survival. Functionally, gain- and loss-of-function studies showed that TMPO-AS1 overexpression enhanced the proliferation, migration, invasion and EMT of GC cells in vitro, whereas knockdown of TMPO-AS1 inhibited these malignant traits. Importantly, we demonstrated that TMPO-AS1 could function as a competing endogenous RNA (ceRNA) by sponging miR-140-5p in GC cells, thereby diminishing the inhibition on SOX4, an EMT regulator.
Conclusion: Our findings indicated that TMPO-AS1 promotes GC progression partly by regulating miR-140-5p/SOX4 axis, and may serve as a novel therapeutic target for GC.
Keywords: gastric cancer, long non-coding RNA TMPO-AS1, miR-140-5p, SOX4, EMT
Introduction
Gastric cancer (GC) is one of the most prevailing malignant tumors of the digestive tract worldwide. At present, surgical resection and chemoradiotherapy are the main therapeutic methods for GC;1 however, the 5-year survival rate of patients with advanced or metastatic GC is still less than 30%.2 Accordingly, identification of potential biomarkers and understanding of detailed mechanisms underlying GC is of critical importance for therapeutic benefits.
Long non-coding RNAs (lncRNAs), a large group of non-protein coding RNA transcripts with more than 200 nucleotides in length, regulate a variety of cellular processes.3 In recent years, lncRNAs are attracting great attention due to their frequent involvement in cancer biology.4 Among many cancer-related lncRNAs, TMPO antisense transcript 1 (TMPO-AS1) was currently identified as an oncogene in many human cancers, including prostate cancer, non-small cell lung cancer and cervical cancer.5–7 In this study, we aimed to investigate the potential regulatory functions of TMPO-AS1 in GC and to further elucidate the underlying mechanisms.
Materials and Methods
Patients and Tissue Samples
One hundred and five pairs of tumor tissues and adjacent normal stomach mucosa tissues (from the margin of tumor tissues ≥6 cm) were collected from GC patients who underwent radical gastrectomy at Second Hospital of Shanxi Medical University (Taiyuan City, China). The clinicopathologic characteristics of the patients are presented in Table 1. All patients did not receive radiotherapy and chemotherapy before surgery. The specimens were frozen and stored at −80°C for further use. This study was approved by the Ethics Committee of Second Hospital of Shanxi Medical University in accordance with the Helsinki Declaration. All subjects were informed of the study and signed consent forms before surgery.
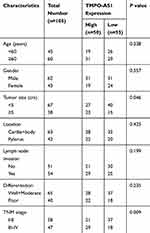 |
Table 1 Correlation Between the Clinicopathologic Characteristics and TMPO-AS1 Expression in GC |
Cell Culture and Transfection
Four GC cell lines (HGC-27, SGC-7901, BGC-823 and AGS) and one normal human gastric mucosa cell line GES-1 were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). These cell lines were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) and 1% penicillin-streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
si-TMPO-AS1, pcDNA3.1-TMPO-AS1, miR-140-5p mimics, the scrambled oligonucleotides (NC) and empty pcDNA3.1 vector were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China). To perform transfection, cells were cultured to about 70–80% confluence. Then, Lipofectamine 3000 Transfection Reagent (Invitrogen) was used. After 48 h, the transfection efficiency was validated by RT-qPCR analysis.
RNA Extraction and RT-qPCR Analysis
Total RNA was extracted from cells or tissue specimens using TRIzol reagent (Invitrogen), and then reverse-transcribed into cDNA by the PrimeScript RT reagent Kit (TaKaRa, Dalian, China). PCR amplifications were performed using a SYBR Green PCR Kit (TaKaRa) on an ABI PRISM 7300 Sequence Detection system (Applied Biosystems, Foster City, CA, USA). Quantification of lncRNA and miRNA was performed by using 2−ΔΔCt method.8 We used GAPDH or U6 snRNA as an internal reference.
Protein Extraction and Western Blot Analysis
Cells were lysed in RIPA lysis buffer (Beyotime, Shanghai, China). Proteins were separated by SDS-PAGE and transferred electrophoretically onto PVDF membranes (Millipore, Billerica, MA, USA). Next, the membrane was blocked in 5% non-fat milk at room temperature for 2 h, and then incubated with the primary antibodies at 4°C overnight, followed by the secondary antibody for 1 h at 37°C. Finally, the blots were detected by the Immobilon ECL substrate kit (Millipore). GAPDH was utilized as an internal control.
MTT Assay
Cells were seeded into 96‑well plates at a density of 3000 cells/well. MTT (20 µL; Sigma-Aldrich, St. Louis, MO, USA) was added to each well at indicated time points, and the plates were cultured for an additional 4 h. Then, the supernatant was abandoned, and DMSO (100 µL/well; Sigma-Aldrich) was added to each well to dissolve the formazan crystals. The absorbance was measured at the wavelength of 570 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Wound Healing Assay
Cells (5×104 cells/well) were seeded into a six-well plate. When cells grew to confluence at 90%, an artificial wound was created with a sterile pipette tip. Scratch photo was taken at 0 h and 48 h by a light microscope.
Transwell Invasion Assay
Cells suspended in 200 μL serum-free medium were added into the upper chamber of Matrigel-coated transwell inserts (8 μm pore size; BD Biosciences, San Jose, CA, USA). The bottom chamber was filled with 500 μL medium containing 10% FBS as a chemoattractant. After incubation for 48 h, the cells on the lower side were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet solution. Images were captured using a light microscope, and cells in five random fields were counted.
Dual-Luciferase Reporter Assay
The fragment of TMPO-AS1 or SOX4 mRNA containing the predicted miR-140-5p-binding sites was synthesized and inserted into the psiCHECK-2 luciferase reporter vector (Promega, Madison, WI, USA). We co-transfected miR-140-5p mimics or NC with the reporter plasmid into HEK293T cells using Lipofectamine 3000 Transfection Reagent. After 48 h, the cells were collected, and the luciferase activity was measured with the Dual-luciferase Reporter assay system (Promega).
Statistical Analysis
All statistical analyses were carried out using GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). The experimental results are presented as the mean ± standard deviation (SD). Differences between two or more groups were compared using Student’s t‑test or one‑way analysis of variance, respectively. The Kaplan–Meier method was used to depict the overall survival (OS) curves, and the difference between groups was estimated using the log-rank test. P < 0.05 was considered to be statistically significant.
Results
TMPO-AS1 Is Upregulated in GC Tissues and Predicts a Poor Prognosis
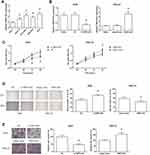
We collected 105 pairs of GC tissues and adjacent normal tissues, and the results of RT-qPCR analysis showed that TMPO-AS1 expression in GC tissues was significantly higher than in adjacent normal tissues (Figure 1A). According to the median TMPO-AS1 expression, these patients were allocated into the high expression group (n=50) and low expression group (n=55). We noticed that a high level of TMPO-AS1 was positively correlated with larger tumor size (P=0.046) and advanced TNM stage (P=0.009) of GC patients (Table 1). Moreover, survival analysis using the Kaplan–Meier method demonstrated that high TMPO-AS1 expression level was closely associated with poor overall survival of GC patients (P=0.010; Figure 1B).
TMPO-AS1 Promotes GC Cell Proliferation, Migration and Invasion
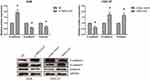
We also found that the expression levels of TMPO-AS1 were remarkably increased in a panel of GC cell lines (HGC-27, SGC-7901, BGC-823 and AGS) compared with normal GES-1 cells (Figure 2A). AGS and HGC-27 cells were selected for further analysis. We then carried out loss-of-function and gain-of-function assays to evaluate the functional roles of TMPO-AS1 in GC. We confirmed that TMPO-AS1 expression was significantly silenced by si-TMPO-AS1 in AGS cells, and TMPO-AS1 was obviously overexpressed in HGC-27 cells by pcDNA3.1-TMPO-AS1 (Figure 2B). MTT assay showed that si-TMPO-AS1 could suppress the proliferation of AGS cells, while the proliferation of TMPO-AS1-overexpressing HGC-27 cells was accelerated (Figure 2C). Wound healing assay demonstrated that the scratch wound closure in AGS cells was retarded by TMPO-AS1 knockdown (Figure 2D), and as shown in Figure 2E, TMPO-AS1 knockdown reduced the number of invaded AGS cells. On the contrary, the migration and invasion abilities of HGC-27 cells were remarkably enhanced by TMPO-AS1 overexpression.
TMPO-AS1 Activates EMT in GC Cells
The expression levels of EMT-related proteins were determined by Western blot analysis. As shown in Figure 3, TMPO-AS1 knockdown markedly increased the expression of the epithelial marker E-cadherin, and decreased the expression of the mesenchymal markers N-cadherin and Vimentin in AGS cells. On the other hand, when TMPO-AS1 was overexpressed in HGC-27 cells, we observed the opposite results.
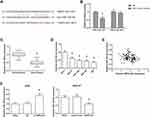
TMPO-AS1 Serves as a ceRNA by Sponging miR-140-5p in GC
We further observed that TMPO-AS1 might possess a complementary sequence to miR-140-5p by bioinformatics analysis through Starbase database (http://starbase.sysu.edu.cn/index.php) (Figure 4A). Then, dual-luciferase reporter assay was performed to validate the prediction, and the results showed that the luciferase activity of TMPO-AS1-WT was notably decreased under the cotransfection with miR-140-5p mimics in HEK293T cells (Figure 4B). Figure 4C and D showed that miR-140-5p was significantly downregulated in GC tissues and cell lines. Besides, miR-140-5p expression was negatively associated with TMPO-AS1 expression in GC tissues (r=−0.217, P=0.026; Figure 4E). We also found that miR-140-5p expression was increased in AGS cells after TMPO-AS1 knockdown, while was decreased when TMPO-AS1 was overexpressed in HGC-27 cells (Figure 4F).
miR-140-5p Reverses the Biological Effects of TMPO-AS1 on GC Cells
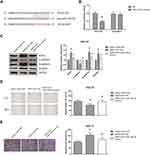
We then predicted the potential target of miR-140-5p in GC. Through Targetscan database (http://www.targetscan.org/vert_71/), we noticed that the 3ʹ-UTR of SOX4 mRNA contains the binding sites of miR-140-5p (Figure 5A). Besides, miR-140-5p mimics markedly reduced the luciferase activity of SOX4-WT but not SOX4-MUT in HEK293T cells (Figure 5B). As shown in Figure 5C, the effects of TMPO-AS1 on SOX4 expression and EMT in HGC-27 cells were blocked by cotransfection with miR-140-5p mimics. Consistently, the increased migration and invasion abilities of TMPO-AS1-overexpressing HGC-27 cells were also reversed by miR-140-5p restoration (Figure 5D and E).
Discussion
GC is a genetic disease involving multiple changes in the genome, and at present, more and more lncRNAs are demonstrated to be deregulated in GC.9 For example, Wang et al indicated that lncRNA CASC19 is associated with the progression and prognosis of advanced GC,10 while Jiao et al reported that lncRNA MEG3 suppresses GC cell growth, invasion and migration.11 These lncRNAs may be promising candidate diagnostic biomarkers, prognostic indicators and therapeutic targets in GC.
In this study, we identified a novel GC-related lncRNA TMPO-AS1, which was overexpressed in GC samples and predicted poor prognosis of GC patients. Functional experiments further revealed that TMPO-AS1 overexpression enhanced the proliferation, migration and invasion of GC cells in vitro, whereas TMPO-AS1 knockdown had the opposite effects, indicating the oncogenic role of TMPO-AS1 in GC. Metastasis is a poor prognostic factor for cancer patients. EMT is a critical step for GC cells to obtain migration and invasion abilities, and EMT regulation can provide potential targets for GC therapy.12 This study also verified TMPO-AS1 as a positive regulator of EMT in GC.
One of the popular action mechanisms of lncRNAs is to serve as a competing endogenous RNA (ceRNA) to regulate the expression and function of target genes by binding and sequestering with miRNAs.13 Among many miRNAs, miR-140-5p was selected as a candidate for further analysis since several publications have reported its tumor-suppressive role in GC,14–16 and in this study, we confirmed that TMPO-AS1 negatively regulated miR-140-5p expression in GC cells, and the oncogenic effects of TMPO-AS1 overexpression on GC cells were partially reversed by miR-140-5p restoration. MiRNAs exert their functions by negative regulation of their target genes,17 and this study further showed that miR-140-5p directly targets SOX4, a master mediator of EMT in GC cells.18,19
In conclusion, this study, for the first time, verified the oncogenic role of TMPO-AS1 in GC. We confirm that TMPO-AS1 promotes GC progression partly by acting as a ceRNA to sponge miR-140-5p, thereby inducing SOX4-mediated EMT. We believe that TMPO-AS1 may serve as a novel therapeutic target for GC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–730. doi:10.1056/NEJMoa010187
2. Sun W, Yan L. Gastric cancer: current and evolving treatment landscape. Chin J Cancer. 2016;35(1):83. doi:10.1186/s40880-016-0147-6
3. Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi:10.1146/annurev-biochem-051410-092902
4. Rao A, Rajkumar T, Mani S. Perspectives of long non-coding RNAs in cancer. Mol Biol Rep. 2017;44(2):203–218. doi:10.1007/s11033-017-4103-6
5. Huang W, Su X, Yan W, et al. Overexpression of AR-regulated lncRNA TMPO-AS1 correlates with tumor progression and poor prognosis in prostate cancer. Prostate. 2018;78(16):1248–1261. doi:10.1002/pros.v78.16
6. Qin Z, Zheng X, Fang Y. Long noncoding RNA TMPO-AS1 promotes progression of non-small cell lung cancer through regulating its natural antisense transcript TMPO. Biochem Biophys Res Commun. 2019;516(2):486–493. doi:10.1016/j.bbrc.2019.06.088
7. Yang J, Liang B, Hou S. TMPO-AS1 promotes cervical cancer progression by upregulating RAB14 via sponging miR-577. J Gene Med. 2019;21(11):e3125.
8. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
9. Zhao J, Liu Y, Huang G, Cui P, Zhang W, Zhang Y. Long non-coding RNAs in gastric cancer: versatile mechanisms and potential for clinical translation. Am J Cancer Res. 2015;5(3):907–927.
10. Wang WJ, Guo CA, Li R, et al. Long non-coding RNA CASC19 is associated with the progression and prognosis of advanced gastric cancer. Aging. 2019;11(15):5829–5847. doi:10.18632/aging.102190
11. Jiao J, Zhang S. Long noncoding RNA MEG3 suppresses gastric carcinoma cell growth, invasion and migration via EMT regulation. Mol Med Rep. 2019;20(3):2685–2693. doi:10.3892/mmr.2019.10515
12. Peng Z, Wang CX, Fang EH, Wang GB, Tong Q. Role of epithelial-mesenchymal transition in gastric cancer initiation and progression. World J Gastroenterol. 2014;20(18):5403–5410. doi:10.3748/wjg.v20.i18.5403
13. Sanchez-Mejias A, Tay Y.Competing endogenous RNA networks: tying the essential knots for cancer biology and therapeutics. J Hematol Oncol.2015;8:30. doi:10.1186/s13045-015-0129-1
14. Fang Z, Yin S, Sun R, et al. miR-140-5p suppresses the proliferation, migration and invasion of gastric cancer by regulating YES1. Mol Cancer. 2017;16(1):139. doi:10.1186/s12943-017-0708-6
15. Cha Y, He Y, Ouyang K, Xiong H, Li J, Yuan X. MicroRNA-140-5p suppresses cell proliferation and invasion in gastric cancer by targeting WNT1 in the WNT/beta-catenin signaling pathway. Oncol Lett. 2018;16(5):6369–6376. doi:10.3892/ol.2018.9480
16. Wu K, Zou J, Lin C, Jie ZG. MicroRNA-140-5p inhibits cell proliferation, migration and promotes cell apoptosis in gastric cancer through the negative regulation of THY1-mediated Notch signaling. Biosci Rep. 2019;39:7. doi:10.1042/BSR20181434
17. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi:10.1016/j.cell.2009.01.002
18. Peng X, Liu G, Peng H, Chen A, Zha L, Wang Z. SOX4 contributes to TGF-beta-induced epithelial-mesenchymal transition and stem cell characteristics of gastric cancer cells. Genes Dis. 2018;5(1):49–61. doi:10.1016/j.gendis.2017.12.005
19. Pang L, Li B, Zheng B, Niu L, Ge L. miR-138 inhibits gastric cancer growth by suppressing SOX4. Oncol Rep. 2017;38(2):1295–1302. doi:10.3892/or.2017.5745
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.