Back to Journals » OncoTargets and Therapy » Volume 12
Long Non-Coding RNA STARD13-AS Suppresses Cell Proliferation And Metastasis In Colorectal Cancer
Authors Yang B, Zhou SN, Tan JN , Huang J, Chen ZT, Zhong GY, Han FH
Received 27 May 2019
Accepted for publication 11 October 2019
Published 6 November 2019 Volume 2019:12 Pages 9309—9318
DOI https://doi.org/10.2147/OTT.S217094
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Bin Yang,1,* Sheng-Ning Zhou,1,* Jia-Nan Tan,1 Jing Huang,1 Zhi-Tao Chen,1 Guang-Yu Zhong,1 Fang-Hai Han1,2
1Department of Gastrointestinal Surgery, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, Guangdong 510120, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-Sen University, Guangzhou, Guangdong 510120, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fang-Hai Han
Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Department of Gastrointestinal Surgery, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, 107 Yan Jiang West Road, Guangzhou, Guangdong 510120, People’s Republic of China
Tel +86-20-81332020
Fax +86-20-81332532
Email [email protected]
Background: Dysregulation of long non-coding RNAs (lncRNAs) is closely related with the progression of cancer in humans. The functional and regulatory roles of lncRNAs in colorectal cancer (CRC) are still largely unclear. The purpose of this study is to explore the function of lncRNA STARD13-AS in CRC.
Methods: The bioinformatics tool “GEPIA” was used to predict the potential expression of STARD13-AS in CRC. qRT-PCR was used to evaluate the relative expression level of STARD13-AS in CRC cells lines and tissues samples. The functional involvement of STARD13-AS in the CRC cells was assessed using MTT assay, flow cytometry, and Transwell assay. The expression levels of cyclin D, cyclin E, E-cadherin, N-cadherin, and vimentin were assessed using Western blot.
Results: Bioinformatics prediction and qRT-PCR results showed that STARD13-AS expression was decreased in CRC tissues. Patients with low STARD13-AS expression exhibited distant and lymphatic metastasis as well as enhancement in tumor size. STARD13-AS expression was downregulated in CRC cell lines compared to normal human colon mucosal epithelial cell line NCM460 and STARD13-AS expression in SW620 and LoVo cell lines was lowest. Moreover, we observed that while STARD13-AS overexpression suppressed the cell cycle, proliferation, migration, and invasion, while promoted apoptosis both in LoVo and SW620 cells. In addition, STARD13-AS overexpression inhibited Cyclin E, Cyclin D, N-cadherin and vimentin expression, and promoted E-cadherin expression both in LoVo and SW620 cells.
Conclusion: Expression of STARD13-AS suppresses cell proliferation and metastasis in CRC, suggesting that STARD13-AS might act as a potential target for CRC treatment.
Keywords: LncRNA STARD13-AS, colorectal cancer, proliferation, metastasis
Introduction
Colorectal cancer (CRC), the third most common form of malignant cancer, is one of the leading causes of cancer-related deaths around the world.1 There has been a rise in its incidence and mortality in recent years.2 According to recent cancer statistics from China, there are approximately 3.8 million newly diagnosed CRC patients and nearly 0.2 million deaths related to CRC.3 Currently, curative surgery and post-operative adjuvant chemotherapy are the most common therapeutic strategies for CRC treatment.4 Despite significant advances in the diagnosis and treatment of CRC over recent decades, the overall 5-year survival rate of CRC remains unsatisfactory due to post-surgical recurrence and metastasis.5 Thus, it is necessary to conduct a large-scale study to obtain a better understanding of the underlying molecular mechanisms for the pathogenesis of CRC. This may help in the developing new therapeutic strategies to improve the patient outcomes.
Recent studies have demonstrated that approximately 98% of all human genome transcripts consist of non-coding RNA (ncRNA), which are involved in regulating a variety of cellular and biological processes such as embryo development, immunoregulation, and tumor development.6–8 Long non-coding RNAs (lncRNAs), members of the ncRNA family, consist of more than 200 nucleotides with limited or no protein coding capacity.9 Although lncRNAs were previously regarded as “transcriptional noise”, they have since been demonstrated to play a key role in various types of biological processes, such as cell proliferation, migration, and invasion.10 Dysregulation of lncRNAs are closely related with the progression of human cancers.11 Increasing number of research reports have associated certain lncRNAs, such as lncRNAs TUG112 and CASC213 with cell proliferation and metastasis in CRC. However, the role of most lncRNAs are still largely unknown. Therefore, there is an urgent need to verify the cancer-related lncRNAs and their potential molecular mechanisms in cancers.
LncRNA STARD13-AS is a 645 bp ncRNA that is located on chromosome 13q13.1. The biological functions and correlation of STARD13-AS in CRC still mostly unknown. The purpose of this study is to explore the function of STARD13-AS in CRC. In order to do this, we first evaluated the expression of STARD13-AS in CRC tissues and cell lines and then, analyzed the relationship between STARD13-AS expression and clinicopathologic characteristics of CRC patients. Finally, we investigated the functional effects of STARD13-AS on CRC cell proliferation, metastasis and EMT.
Materials And Methods
Clinical Samples
CRC tissue samples and the corresponding adjacent normal tissue samples (at least 5 cm away from the tumor) were collected by surgical resection from 40 CRC patients who underwent colectomy at the Sun Yat-sen Memorial Hospital. The clinicopathological data of the 40 patients was shown in Table 1. We ensured that none of the patients have received preoperative chemotherapy or radiotherapy and obtained signed informed consents from all patients. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Sun Yat-sen Memorial Hospital, Sun Yat-sen University. The diagnosis and clinicopathological characteristics, which include tumor size, lymphatic metastasis and distant metastasis, were blindly confirmed by two experienced pathologists. All the collected surgical specimens were stored in liquid nitrogen till RNA isolation.
 |
Table 1 The Clinicopathological Data Of The 40 Patients Enrolled In This Study |
Bioinformatics Analysis
Bioinformatics tool “GEPIA” (http://gepia.cancer-pku.cn/detail.php), a web-based tool to deliver fast and customizable functionalities based on TCGA and GTEx data, was used to analyze the potential expression of STARD13-AS in colon adenocarcinoma (COAD) and rectum adenocarcinoma (READ).14
qRT-PCR
The total RNA from the CRC tissues or CRC cell line was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA). The reverse transcription reaction was performed using the PrimeScript RT Master Mix (Takara, Dalian, China) to obtain the first strand cDNA. All PCR reactions were carried out using the ABI 7500 RT-PCR system (Applied Biosystems, Foster City, CA, USA) with a SYBR® Premix Ex Taq™ Kit (TaKaRa). The primers were obtained from GenePharma (Shanghai, China), the primers sequences were as follows: STARD13-AS: forward, 5ʹ-CCACAGAGAGGATTCCAGAA-3ʹ reverse, 5ʹ-TCCAGGCTCTGTATAGAAGCT −3ʹ. 18srRNA: forward, 5ʹ-CCTGGATACCGCAGCTAGGA-3ʹ reverse, 5ʹ-GCGGCGCAATACGAATGCCCC-3ʹ. The expression of STARD13-AS was standardized by using 18srRNA. Relative STARD13-AS expression was calculated by the 2−ΔΔCT method.15 Each experiment was repeated three times.
Cell Culture
Normal human colon mucosal epithelial cell line NCM460 and the human CRC cell lines LoVo, RKO, HCT-116, HT-29, SW620, and SW480 were obtained from the Shanghai Institute of Biological Sciences (Shanghai, China). All of the cell lines were cultured in complete culture medium, containing 90% Dulbecco’s modified Eagle’s medium (Hyclone, Logan, UT, USA), and 10% fetal bovine serum (FBS; Hyclone). And 1% penicillin-streptomycin (penicillin final concentration: 100µ/mL; streptomycin final concentration: 100µg/mL; Thermo Fisher Scientific, Waltham, MA, USA). All cells were cultured at 37°C with a humidity of 5% CO2.
Plasmid Construction And Cell Transfection
The complete sequence of STARD13-AS (NR_046693) was cloned from the human genome obtained from the adjacent normal tissue samples of CRC patients. It was then cloned into the pcDNA 3.1 vector (Invitrogen) using the restriction enzymes HindIII and XhoI. The correct sequence was confirmed by sequencing done by Shenggong Biology (Shanghai, China). The empty pcDNA3.1 vector was considered as a negative control (NC). The transfections were performed with Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA). Transfected cells were used for further assays after 24 h.
MTT Assay
CRC cell proliferation was evaluated by MTT Cell Proliferation Assay Kit (Beyotime, Shanghai, China). In brief, 2×103 cells/100μl/well were added to the wells of 96-well plates in triplicate. After transfection, 10 μl MTT (5 mg/mL) was added into the wells at each point in time. After the cells were cultured for 4 h at 37°C, 100 μl of Formazan dissolved solution was added into the wells. A microplate reader (Bio-Rad, Hercules, CA, USA) was used to detect the optical density at 570 nm (OD570 nm). MTT assay was repeated three times.
Cell Cycle Analysis
Cell Cycle Detection Kit (Keygentec, Nanjing, China) was used to assess the cell cycle. Cells were collected after transfection for 48 h and fixed in 70% ethanol at 25°C for 2 h. After washed with PBS thrice, cells werestained with propidium iodide (PI, 25 μg/mL) with 1 μg/mL RNase. After incubation at 37°C for 0.5 h in the dark, the percentages of the cell population in different phases were assessed using flow cytometry (BD Biosciences, San Jose, CA, USA). Cell cycle analysis was repeated three times.
Cell Apoptosis Assay
Cell apoptosis assay was carried our using the Annexin V-FITC Apoptosis Detection Kit (Keygentec, Nanjing, China). Briefly, after transfection for 48 h, cells were washed with cold PBS thrice and re-suspended in 500 µl of binding buffer. Next, 5 μl of Annexin V-FITC and 5 μl propidium iodide were added to cells, and they were incubated for 15 min at 25°C in the dark. The percentages of the cell apoptosis was assessed by flow cytometry (BD Biosciences). Cell apoptosis assay was repeated three times.
Transwell Migration And Invasion Assay
BD 24-well Transwell chamber (BD Biosciences) was used to assess the cell migration and invasion. In brief, the top chamber of the wells were supplemented with serum-free medium containing 1×105 cells/100 μl. Then, a mixture of serum-free medium and Matrigel (BD Biosciences) was added to the upper chamber for the invasion assay and Matrigel-free for the migration assay. The lower chamber was supplemented with 10% FBS medium for the Transwell assay. This system was then cultured at 37°C with 5% CO2 for 48 h. The cells present in the Transwell membrane were stained with 0.5% crystal violet for 15 min. The stained cells were counted using a microscope (Olympus, Tokyo, Japan). Transwell assay was repeated three times.
Western Blotting
RIPA lysis buffer with protease inhibitor (Beyotime, Shanghai, China) was used to extract the total protein samples from the cells. The denatured proteins (approximately 20 μg) were separated by SDS-PAGE and after electrophoresis, were transferred onto polyvinylidene fluoride membranes. Tris-buffered saline (TBS) with 5% skim milk was used to block the membrane for 2 h. Next, the membranes were incubated at 4°C with diluted primary antibodies overnight: anti-Cyclin E and anti-Cyclin D antibody (dilution 1:1000); anti-E-cadherin antibody (dilution 1:1000); anti-vimentin antibody (dilution 1:1000); anti-N-cadherin (dilution 1:1500) and anti-GAPDH antibody (dilution 1:2000). All the antibodies were purchased from Kangchen (Shanghai, China). After incubation, the membranes were washed with TBS containing Tween 20 (TBS-T) thrice, incubated with the HRP-labeled secondary antibody (dilution 1:5000) for 2 h at 25°C and then washed again with TBS-T thrice. Finally, the bands corresponding to the proteins were quantified using enhanced chemiluminescence (Keygentec) and ChemiDoc™ XRS systems (Bio-Rad).
Statistical Analysis
SPSS 19.0 statistical software (IBM Inc., Chicago, IL, USA) was used to perform statistical analyses. The experimental data were reported as means ± standard deviation (SD). The comparison between the groups was performed using student t-test or nonparametric t-test. P values less than 0.05 were regarded as statistically significant.
Results
STARD13-AS Expression Was Decreased In CRC
The results of the prediction using the bioinformatics tool “GEPIA” showed that STARD13-AS expression was remarkably downregulated in COAD and READ tissues compared to that in the non-cancerous normal tissues (Figure 1A). In addition, the qRT-PCR results showed that STARD13-AS expression was markedly downregulated in 40 CRC tissues compared to their adjacent normal tissues (Figure 1B and C). These results suggested that downregulation of STARD13-AS is important for the development of CRC.
STARD13-AS Expression Is Associated With The Clinicopathologic Characteristics Of CRC Patients
To study the clinical significance of STARD13-AS, the relationship between STARD13-AS expression and clinicopathologic characteristics of CRC patients were further explored. The results revealed that while the expression of STARD13-AS was not associated with age and gender, it was significantly associated with distant metastasis, lymphatic metastasis, and tumor size in CRC patients (Figure 2).
 |
Figure 2 The relationship between STARD13-AS expression and clinicopathological characteristics of CRC patients. |
STARD13-AS Expression In CRC Cell Lines
To investigate the biological function of STARD13-AS in CRC, we first evaluated the relative expression of STARD13-AS in different types of CRC cell lines and normal human colon mucosal epithelial cell line NCM460. The results showed that STARD13-AS expression was downregulated in all CRC cell lines compared to NCM460 and STARD13-AS expression in SW620 and LoVo cell lines was lowest (Figure 3). Therefore, SW620 and LoVo cell lines were selected for further functional experiments.
 |
Figure 3 The relative expression of STARD13-AS in the CRC cell lines and normal human colon mucosal epithelial cell line NCM460 were measured using qRT-PCR. |
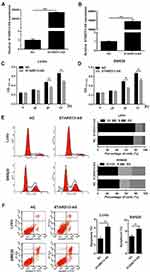
The Effect Of STARD13-AS Overexpression On Cell Proliferation, Cell Cycle, And Apoptosis In CRC Cells
To overexpress STARD13-AS, LoVo and SW620 cells were transfected with pcDNA3.1-STARD13-AS (STARD13-AS) plasmids and pcDNA3.1-negative control (NC) plasmids were transfected as the control. The qRT-PCR results indicated that the STARD13-AS expression was markedly increased both in LoVo and SW620 cells compared to the control group cells (Figure 4A and B). Next, we further analyzed the effect of STARD13-AS overexpression on cell proliferation in LoVo and SW620 cells. MTT assay showed that STARD13-AS overexpression significantly suppressed cell proliferation compared to the control group (Figure 4C and D). In addition, we found that percentage of LoVo cells in G1 phase was obviously increased in STARD13-AS overexpression group compared to that in NC group, and percentage of SW620 cells in S phase was obviously increased in STARD13-AS overexpression group compared to that in NC group S(Figure 4E). Moreover, STARD13-AS overexpression could increase the rate of apoptotic cells in LoVo and SW620 cells (Figure 4F).
STARD13-AS Overexpression Suppressed Cell Migration And Invasion In CRC Cells
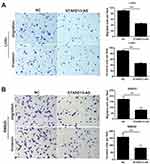
Transwell assays were performed to evaluate the effects of STARD13-AS overexpression on cell migration and invasion. We found that the migratory and invasion ability of LoVo (Figure 5A) and SW620 cells (Figure 5B) was inhibited by STARD13-AS overexpression when compared with their control group cells, respectively.
The Effect Of STARD13-AS Overexpression On Cell Cycle Regulator And EMT-Related Marker Expression In CRC Cells
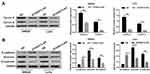
The Western blot results indicated that STARD13-AS overexpression markedly inhibited the Cyclin E and Cyclin D expression both in LoVo and SW620 cells compared to the control cells (Figure 6A). Moreover, compared to the control group, STARD13-AS overexpression markedly upregulated E-cadherin expression, while downregulating vimentin and N-cadherin expression (Figure 6B).
Discussion
There is significant evidence confirming that lncRNAs play a key role in various human tumors including CRC.16,17 Hence, it is crucial to identify the key cancer-related lncRNAs and elucidate their functional mechanism. However, the function of lncRNAs in CRC remains largely unclear and need be further explored. Here, we have demonstrated that STARD13-AS expression was markedly decreased in CRC tissues and the low STARD13-AS expression was significantly associated with tumor size, lymphatic metastasis, and distant metastasis in CRC patients. STARD13-AS overexpression significantly suppressed cell proliferation, migration, and invasion both in LoVo and SW620 cells via modulation of cell cycle regulator and EMT-related marker expression.
Recently, many studies have reported that aberrant expression or dysfunctional activities of lncRNAs play a key role in the tumorigenesis and progression of CRC.18,19 For instance, lncRNA BX357664 was markedly decreased in CRC tissues and cells overexpressing BX357664 showed suppressed proliferation and metastasis as well as enhanced apoptosis.20 LncRNA CPS1-IT1 was markedly reduced in CRC tissues and cell lines and this low CPS1-IT1 expression was associated with poor survival of CRC patients. Moreover, CPS1-IT1 overexpression inhibited CRC cell proliferation, invasion and metastasis.21 However, the detailed function and molecular mechanisms of STARD13-AS in CRC remain to be elucidated. Here, we confirmed that STARD13-AS expression was remarkably decreased in human CRC tissues compared to that in the adjacent normal tissues, Additionally, our data indicated that the low STARD13-AS expression in CRC tissues was closely associated with tumor size, lymphatic metastasis and distant metastasis in CRC patients. This finding suggested that STARD13-AS might be involved in the progression of human CRC metastasis. Similar to the expression in CRC tissues, STARD13-AS was also decreased in all CRC cell lines compared to NCM460, a normal human colon mucosal epithelial cell line. Then, STARD13-AS was overexpressed in LoVo and SW620 cells and the effects on cell proliferation, cycle, apoptosis, migration, and invasion were investigated. The results showed that while the STARD13-AS overexpression markedly suppressed cell proliferation, migration, and invasion as well as promoted apoptosis. These findings indicated that STARD13-AS might act as a tumor suppressor in CRC.
The cell cycle is considered as a key factor regulating cell proliferation. Cyclin D and Cyclin E are the main regulators of the cell cycle and aberrant expression of these proteins is closely related to the tumor growth.22 Our data showed that STARD13-AS overexpression markedly inhibited the expression levels of Cyclin D and Cyclin E proteins in both LoVo and SW620 cells, which is consistent with the proliferation inhibition mediated by STARD13-AS overexpression. Therefore, the results of this study strongly suggested that STARD13-AS overexpression suppresses CRC cell proliferation by affecting the cell cycle. Tumor metastasis is a multi-step process and EMT is considered to enhance the activity and invasiveness of cancer cells.23,24 The first step in the EMT process is the inhibition of E-cadherin, thereby reducing cell adhesion.25 Another important aspect of EMT is the upregulated expression of non-epithelial cadherins, including N-cadherin and vimentin.26 Our results showed that STARD13-AS overexpression enhanced E-cadherin expression and reduced vimentin and N-cadherin expression both in LoVo and SW620 cells, which causes suppression of metastasis. These data showed that STARD13-AS might influence metastasis in CRC patients by affecting the EMT processes.
Nevertheless, there are some limitations in our study. First, although STARD13-AS overexpression could drastically inhibit proliferation and metastasis of CRC cells in vitro, the underlying mechanism needs to be explored. The target gene of STARD13-AS should be identified to further illuminate the underlying mechanism. STARD13-AS is an antisense RNA of coding RNA STARD13, so we predicted that the target gene of STARD13-AS may be STARD13. Secondly, animal models need to be adopted to verify the effect of STARD13-AS overexpression on CRC development. We will perform further experiments to remedy the above-mentioned gaps.
In summary, this study confirmed that STARD13-AS expression was markedly decreased in CRC cell lines as well as tissues and STARD13-AS overexpression suppresses CRC cell proliferation and metastasis. Hence, STARD13-AS might act as a potential target for CRC treatment.
Abbreviations
lncRNAs, long non-coding RNAs; CRC, colorectal cancer; COAD, colon adenocarcinoma; READ, rectum adenocarcinoma; NC, negative control.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) (81572925)
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi:10.3322/caac.21395
2. Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27(1):2–12.
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Stein A, Atanackovic D, Bokemeyer C. Current standards and new trends in the primary treatment of colorectal cancer. Eur J Cancer. 2011;47(Suppl 3):S312–S314. doi:10.1016/S0959-8049(11)70183-6
5. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi:10.1016/S0140-6736(13)61649-9
6. Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157(1):77–94. doi:10.1016/j.cell.2014.03.008
7. Kita Y, Yonemori K, Osako Y, et al. Noncoding RNA and colorectal cancer: its epigenetic role. J Hum Genet. 2017;62(1):41–47. doi:10.1038/jhg.2016.66
8. Weng M, Wu D, Yang C, et al. Noncoding RNAs in the development, diagnosis, and prognosis of colorectal cancer. Transl Res. 2017;181:108–120. doi:10.1016/j.trsl.2016.10.001
9. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. doi:10.1016/j.cell.2009.02.006
10. Kondo Y, Shinjo K, Katsushima K. Long non-coding RNAs as an epigenetic regulator in human cancers. Cancer Sci. 2017;108(10):1927–1933. doi:10.1111/cas.2017.108.issue-10
11. Han D, Wang M, Ma N, Xu Y, Jiang Y, Gao X. Long noncoding RNAs: novel players in colorectal cancer. Cancer Lett. 2015;361(1):13–21. doi:10.1016/j.canlet.2015.03.002
12. Sun J, Ding C, Yang Z, et al. The long non-coding RNA TUG1 indicates a poor prognosis for colorectal cancer and promotes metastasis by affecting epithelial-mesenchymal transition. J Transl Med. 2016;14:42. doi:10.1186/s12967-016-0786-z
13. Huang G, Wu X, Li S, Xu X, Zhu H, Chen X. The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci Rep. 2016;6:26524. doi:10.1038/srep26524
14. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Research. 2017;45(W1):W98–w102. doi:10.1093/nar/gkx247
15. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408.
16. Liu T, Han Z, Li H, Zhu Y, Sun Z, Zhu A. LncRNA DLEU1 contributes to colorectal cancer progression via activation of KPNA3. Mol Cancer. 2018;17(1):118. doi:10.1186/s12943-018-0873-2
17. Bhan A, Soleimani M, Mandal SS. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017;77(15):3965–3981. doi:10.1158/0008-5472.CAN-16-2634
18. Tsai KW, Lo YH, Liu H, et al. Linc00659, a long noncoding RNA, acts as novel oncogene in regulating cancer cell growth in colorectal cancer. Mol Cancer. 2018;17(1):72. doi:10.1186/s12943-018-0821-1
19. Sun Z, Liu J, Chen C, et al. The biological effect and clinical application of long noncoding RNAs in colorectal cancer. Cell Physiol Biochem. 2018;46(2):431–441. doi:10.1159/000488610
20. Liu F, Wang X, Liu H, et al. LncRNA BX357664 inhibits cell proliferation and invasion and promotes cell apoptosis in human colorectal cancer cells. Oncol Lett. 2018;15(6):8237–8244. doi:10.3892/ol.2018.8435
21. Zhang W, Yuan W, Song J, Wang S, Gu X. LncRna CPS1-IT1 suppresses cell proliferation, invasion and metastasis in colorectal cancer. Cell Physiol Biochem. 2017;44(2):567–580. doi:10.1159/000485091
22. De Falco M, De Luca A. Cell cycle as a target of antineoplastic drugs. Curr Pharm Des. 2010;16(12):1417–1426. doi:10.2174/138161210791033914
23. Rafael D, Doktorovova S, Florindo HF, et al. EMT blockage strategies: targeting Akt dependent mechanisms for breast cancer metastatic behaviour modulation. Curr Gene Ther. 2015;15(3):300–312. doi:10.2174/1566523215666150126123642
24. Jung HY, Fattet L, Yang J. Molecular pathways: linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clin Cancer Res. 2015;21(5):962–968. doi:10.1158/1078-0432.CCR-13-3173
25. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi:10.1016/j.cell.2009.11.007
26. Broders-Bondon F, Paul-Gilloteaux P, Gazquez E, et al. Control of the collective migration of enteric neural crest cells by the Complement anaphylatoxin C3a and N-cadherin. Dev Biol. 2016;414(1):85–99. doi:10.1016/j.ydbio.2016.03.022
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.