Back to Journals » Cancer Management and Research » Volume 11
Long non-coding RNA NNT-AS1 functions as an oncogenic gene through modulating miR-485/BCL9 in cholangiocarcinoma
Authors Huang L , Jiang X, Kang P, Wang Z, Leng K, Ji D, Xu Y, Wang H, Cui Y
Received 12 March 2019
Accepted for publication 10 July 2019
Published 15 August 2019 Volume 2019:11 Pages 7739—7749
DOI https://doi.org/10.2147/CMAR.S207801
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Lining Huang,* Xingming Jiang,* Pengcheng Kang, Zhidong Wang, Kaiming Leng, Daolin Ji, Yi Xu, Hao Wang, Yunfu Cui
Department of HPB Surgery, The 2nd Affiliated Hospital of Harbin Medical University, Harbin 150086, People’s Republic of China
*These authors contributed equally to this work
Introduction: Growing evidence suggests that long non-coding RNAs (lncRNAs) could function as important regulators in carcinogenesis and cancer progression. Nicotinamide nucleotide transhydrogenase antisense RNA 1 (lncRNA NNT-AS1) is up-regulated in some human tumors and functions as a tumor promoter. This study aimed to detect the effect of NNT-AS1 on cholangiocarcinoma (CCA) prognosis.
Materials and methods: In this study, we detected NNT-AS1 expression in CCA tissue samples and cell lines, and analyzed the association between NNT-AS1 expression levels and clinical parameters of CCA patients. Moreover, we conducted loss-of-function studies in CCA cancer cells to explore the biological function and molecular mechanism of NNT-AS1. NNT-AS1 was downregulated by using RNAi technology. Cell proliferation was examined by CCK8 and clone formation assays. Cell migration and invasion were determined by wound healing and transwell assays. Western blot assays were used to explore protein expression.
Results: In this study, NNT-AS1 was expressed at high levels in CCA and closely associated with poor prognosis of patients with CCA. NNT-AS1 knockdown impaired cell proliferation, suppressed CCA cell migration and invasion, and restrained tumor growth in vitro. Moreover, NNT-AS1 directly bounded to miR-485 and further regulated BCL9. Finally, rescue assays verified that NNT-AS1 modulated the tumorigenesis of CCA by regulating miR-485.
Conclusion: Taken together, NNT-AS1 played a critical biological role in the development of CCA. Our results elucidated NNT-AS1/miR-485/BCL9 axis might lead to a further understanding of the molecular mechanism of CCA.
Keywords: lncRNA, NNT-AS1, cholangiocarcinoma, BCL9
Introduction
Cholangiocarcinoma (CCA) is the most common biliary tract cancer which represents 3% of all gastrointestinal malignancies, described as a malignancy originating from the ductal epithelial cells lining the intrahepatic and extrahepatic biliary ducts.1–3 Over the past few decades, the overall incidence and mortality rates of CCA have increased worldwide, especially in China and Thailand. Currently, there is no effective chemoprevention or radiotherapy for CCA. Surgical resection and liver transplantation are curative means for treatment, but more than 70% of patients missed surgery opportunities due to early diagnosis failures. Therefore, novel diagnosis and treatment approaches of CCA are urgently demanded.4,5
Long non-coding RNAs (lncRNAs), which are over 200 nucleotides (nt) in length, have no significant protein-coding potential.6,7 Owing to the recent developments in deep-sequencing technologies, lncRNAs have been demonstrated to be capable of serving as pivotal regulators in numerous biological processes, including cellular proliferation, invasion, and so on. RNA transcripts contain miRNA response elements that share miRNAs to communicate with and coregulate each other by titrating the availability of miRNAs, which function as competing endogenous RNAs (ceRNAs). Increasing evidences have shown that lncRNAs could act as endogenous miRNA sponges to modulate targets.8,9 It is worth noting that abnormal expression of lncRNAs have been confirmed in many malignant tumors,10 such as CCA.
Homo sapiens nicotinamide nucleotide transhydrogenase antisense RNA 1 (lncRNA NNT-AS1), locating in 5p12 with 3 exons, is a novel tumor-related lncRNA.11 It has been verified that NNT-AS1 is a cancer-promoting gene in gastric cancer,12 cervical cancer.13 However, the functions of NNT-AS1 in CCA are still largely uncertain. Thus, in the present study, we measured the expression of NNT-AS1 in human CCA tissues and adjacent normal bile duct tissues. Furthermore, the correlation between the expression and clinicopathological parameters of CCA patients was examined. Additionally, the effects of NNT-AS1 on CCA malignant biological behaviors were also detected in vitro and vivo, including cell proliferation, migration and invasion.
Materials and methods
Patient samples
A total of 48 CCA tissues and adjacent non-tumorous tissues were collected from CCA patients who was hospitalized in the Department of HPB Surgery, The 2nd Affiliated Hospital of Harbin Medical University, from August 2014 to July 2015. All the patients did not receive preoperative chemotherapy before enrollment, and their tissue specimens were immediately stored and kept in liquid nitrogen until analysis. Pathological examination was performed immediately after resection of samples. This study was approved by The 2nd Affiliated Hospital of Harbin Medical University. All of the written informed consents were obtained from each patient before recruitment, and the local medical ethics committee approved the study protocol.
Cell culture and subcellular fractionation address
HCCC-9810 and RBE cells used in the study were purchased from the Cell Bank of Type Culture of Chinese Academy of Sciences (Shanghai, People’s Republic of China). The other CCA cells including QBC939, Huh-28, HuCCT1, KMBC and CCLP-1 and human intrahepatic biliary epithelial cell (HIBEC) were preserved in our laboratory. Cells were maintained in RPMI-1640 (Gibco, Grand Island, NY, USA) or DMEM (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (Invitrogen Life Technologies, Carlsbad, CA, USA) in a humidified atmosphere at 37 °C and 5% CO2. All cell lines were passaged for less than 6 months. The division of nuclear as well as cytosolic fractions was constructed with the PARIS Kit (Life Technologies, Carlsbad, CA, USA) following the producer’s guides.
Transfection
CCA cells were planted in six-well plates till growth to half confluence, then transfected with particular siRNA (100 nM) or scramble negative control siRNA (100 nM) by using Lipofectamine 3000 (Thermo Fisher Scientific, USA) on the basis of the producer’s protocol. Human NNT-AS1-specific sequences were used, sh-NNT-AS1: 5ʹ-AATTCGAGGTGTCTGTGAGGGTGTTTCTGTATTCAAGAGATACAGAAACACCCTCACAGACACCTTTTTTTG-3ʹ. Small interfering RNA (siRNA) sequences: si-NNT-AS1-1, 5ʹ-CGACAGUGCUIGUGAACUUTT-3ʹ; si-NNT-AS1-2, 5ʹ-ACUGACGCUGACCAUGUGATT-3ʹ; si-NNT-AS1-3, 5ʹ-CCAAGGCUGGAACUGAUATT-3ʹ. And si-NC was obtained from GenePharma (Shanghai, China). MiR-485 mimic and miR-485 inhibitor were purchased from Ribobio (Guangzhou, China).
qRT-PCR analysis
Total RNA was extracted from CCA tissue specimens and cells by using Trizol (Thermo Fisher Scientific, Waltham, MA, USA). The Transcriptor First Strand cDNA Synthesis Kit (Roche, Germany) was used to reverse transcribe RNA into cDNA. FastStart Universal SYBR Green Master Kit (Roche, Germany) and a BIO-RAD C1000 Thermal Cycler were used for qRT-PCR experiments. GAPDH was used as the internal control. The primers for PCR were as follows: NNT-AS1, forward: 5ʹ-AGTTCCACCAAGTTTCTTCA-3ʹ, reverse: 5ʹ-AGGTTTTGCCAGCATAGAC-3ʹ; GAPDH, forward: 5′-GGGAGCCAAAAGGGTCAT-3′, reverse: 5′-GAGTCCTTCCACGATACCAA-3′. Specifically, the primers for miR-485 were purchased from RiboBio (Guangzhou, China) with the small nuclear RNA (snRNA) U6 as an endogenous control.
CCK-8 assays and colony formation assays
Cell viability was monitored by cell counting kit-8 (CCK8) kit (Houston TX, USA) following the producer’s suggestions. The CCLP1 and QBC-939 cells transfected with si-NNT-AS1 or si-NC (3000 cells/well) were cultivated in five 96-well plates with six replicate wells. In terms of the colony formation assay, altogether 200 transfected cells were addressed in a 6-well plate and kept in a medium with 10% FBS for 2 weeks with the replacement of the medium every 4 days. Then, colonies were confirmed with paraformaldehyde and dyed with 0.1% crystal violet (Beyotime, Beijing, China) for 30 min. The quantity of visibly stained colonies was counted in colony formation.
Wound-healing assay
4×105 cells were cultured into 12-well plates and grew until 80–90% confluence. The cell monolayer was scratched with a sterile pipette tip. At different times (0, 24 h), the images of cell morphology were captured under an Olympus microscope (10×10). The results were analyzed by software IPP Image-Pro Plus 6.0.
Transwell assay
The migration and invasion of CCA were examined by transwell assay. After 24 hrs of transfection, cells (5×104 cells/well) were detached with serum free RPMI-1640 and loaded in the upper section of a 24-well transwell unit with 8 μm polycarbonate nucleopore filters (Corning, NY, USA), while medium with 10% FBS was added to the lower chamber. The transwell unit was incubated for 24 hrs before the cells on the lower surface of the membrane were fixed and stained with crystal violet. The experiment was performed in triplicate. For the invasion assay, 40 μL Matrigel (BD Biosciences, San Jose, CA, USA) was coated in the top filter of the transwell unit and placed in an incubator at 37 °C for 4 h to form a reconstructed basement membrane. The methods used were identical to those applied to the migration assay.
Plasmid construction and luciferase reporter assay
The sequence of NNT-AS1 contained the predicted binding site with miR-485; then we cloned the putative sequences of the binding site into a psi-CHECK-2 vector to shape the reporter vector psi-CHECK-2-NNT-AS1-wild type (NNT-AS1-WT). NNT-AS1-mutation (NNT-AS1-MUT) psi-ChECK-2 plasmid was constructed by using the QuikChange® Site-Directed Mutagenesis kit (Stratagene, Wilmington, USA). BCL9-WT and BCL9-MUT reporter plasmid were constructed by the same method. HEK-293T cells were seeded into 6-well plates and co-transfected with the appropriate plasmids (NNT-AS1-WT, NNT-AS1-MUT; Bcl9-WT, Bcl9-MUT) and miR-485 mimics. After 24 h, cells were collected, firefly as well as Renilla luciferase activities were measured by using the Luciferase Reporter Gene Assay kit from Beyotime Institute of Biotechnology (Nantong, China). Renilla luciferase was regarded as the internal control and the RLU (relative light units) ratio of firefly luciferase relative to Renilla luciferase was calculated.
Western blot assay
Whole-cell lysate preparation and Western blot analysis were performed as previously described.14 Cells’ protein lysates were separated by 10% SDS-PAGE, then transferred to a 0.45 μm polyvinylidene fluoride (PVDF) membrane (GE, Piscataway, NJ, USA), and cultivated with specific antibodies. Densitometry (Quantity One software; Bio-Rad) was used to quantify the protein band density. The GAPDH antibody was employed as a control. Anti-BCL9 was obtained from Abcam (Cambridge, UK).
Xenograft mice model
To evaluate the in vivo tumorigenic effects, CCLP1 cells (5×106) transfected with sh-NNT-AS1 and sh-NC were inoculated subcutaneously in the left and right posterior flank of female BALB/c nude mice (6 weeks) separately. Xenograft were measured every 3 days and tumor volume was calculated. 21 days after CCLP1 inoculation, the mice were sacrificed and the samples were collected. All animal experiments were approved and performed in accordance with the guidelines by the Animal Care and Use Committee of The 2nd Affiliated Hospital of Harbin Medical University. All animal experiments were con-ducted according to the Principles of Laboratory Animal Care (National Society for Medical Research). All the experimental procedures were in accordance with the Declaration of Helsinki.
Statistical analysis
An independent t-test was performed to analyze the comparison of continuous data and chi-square test was applied into categorical data. Kaplan-Meier survival analysis and log-rank tests were used to evaluate the correlation betweenNNT-AS1 expression and prognosis of patients with CCA. All statistical analyses were performed by SPSS for Windows v.19.0 (Chicago, IL, USA) and p<0.05 was considered statistically significant for all results.
Results
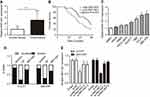
NNT-AS1 was up-regulated in CCA tissues and cells
To explore whether NNT-AS1 was related to CCA, we firstly tested the expression in tumor and non-tumor tissues. NNT-AS1 expression was relatively high in tumor tissues (n=48) ( Figure 1A ). In addition, the 48 patients were categorized into low (n=21) group and high (n=27) group according to the median expression level of NNT- AS1. Kaplan Meier analysis revealed that higher expression of NNT-AS1 causes lower overall survival rate. ( Figure 1B ). The clinicopathologic data of CCA patients were presented in Table 1 . The high expression level of NNT-AS1 was significantly associated with tumor size ( p =0.013), lymph node invasion ( p =0.017), and distant metastasis ( p =0.009). Meanwhile, NNT-AS1 was present at higher expression in CCA cells (CCLP-1, HCCC- 9810, HuCCT1, KMBC, Huh-28, QBC-939 and RBE) compared with HIBEC ( Figure 1C ). Since cellular location could dictate function of lncRNA, cellular fractionation was performed to identify the subcellular localization of NNT-AS1 in CCLP1 and QBC-939 cells. The results showed that NNT-AS1 was mainly expressed in the cytoplasm ( Figure 1D ). All these data confirmed that overexpressed NNT-AS1 was closely associated with poor prognosis in CCA.
 |
Table 1 Association between NNT-AS1 expression and clinicopathological characteristics of CCA |
NNT-AS1 inhibition suppressed CCA cell proliferation in vitro
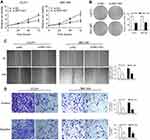
To explore the biological functions of NNT-AS1 in CCA progression, three siRNAs targeting the coding region of NNT-AS1 were tested for their knockdown efficiency ( Figure 1E ). Being the most efficient one, si-NNT-AS1-1 was selected for further experiments. CCK-8 assay showed that NNT-AS1 knockdown suppressed the proliferation ability of CCA cells (CCLP1, QBC-939) compared with control group ( Figure 2A ). In accordance with proliferation, the data of colony formation were also conspicuous. The clonogenic ability of si-NNT-AS1 transfected CCLP1 and QBC-939 cells was more subdued ( Figure 2B ).
Knockdown of NNT-AS1 in CCA cell lines inhibited cell invasion and migration
Further investigated about the potential impacts of NNT-AS1 on the migration and invasion of CCA cells were conducted with wound healing and transwell assays. Knockdown of NNT-AS1 with siRNA significantly reduced wound closure area (Figure 2C). In line with our predictions, as revealed by the transwell assays, knockdown of NNT-AS1 decreased the migration and invasion of CCLP1 and QBC-939 (Figure 2D).
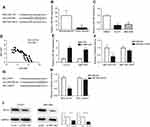
NNT-AS1 acted as a ceRNA by sponging miR-485 and indirectly regulated BCL9 expression
It is well known that lncRNA, as the molecular sponge mechanism of miRNA, is the most common molecular mechanism of lncRNA. NNT-AS1 is mainly located in cytoplasm. Therefore, we verified whether NNt-AS1 played a role as a “sponge” of miRNA in the process of CCA tumorigenesis. Bioinformatics online program (StarBase, http://starbase.sysu.edu.cn ) was used to predict the NNT-AS1 and miR-485 potential binding sites ( Figure 3A ). MiR-485 expression level was much lower than that in CCA tissues than in normal tissues ( Figure 3B ). MiR- 485 was also less expressed in CCLP1 and QBC- 939, compared with that in HEBIC ( Figure 3C ). Spearman’s correlation analysis suggested a significant negative correlation between NNT-AS1 and miR-485 in CCA patients’ tissues (R=−0.8717, p<0.0001, Figure 3D). In CCLP1 and QBC-939 transfected with si-NNT-AS1, the expression level of miR-485 was up-regulated (Figure 3E). To investigate the binding ability between miR-485 and NNT-AS1, a luciferase reporter assay was conducted using wild-type and mutant versions of NNT-AS1. The results of luciferase reporter assay showed that ectopic miR-485 expression evidently repressed the luciferase activity (Figure 3F). Moreover, we predicted a target by TargetScan (http://www.targetscan.org) and considered that miR-485 might regulate BCL9 (Figure 3G). By luciferase reporter assay, we confirmed miR-485 could bind to BCL9 mRNA (Figure 3H). Western blot analysis indicated that BCL9 expression was decreased after knockdown NNT-AS1 (Figure 3I).
MiR-485 reversed the carcinogenesis of NNT-AS1
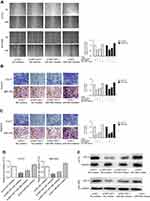
Rescue assays were carried out to validate the regulatory mechanism of NNT-AS1 in CCA. Wound-healing assay and transwell assay revealed that silencing of NNT-AS1 repressed the migration and invasion of CCA cells, which could be rescued by suppression of miR-485 ( Figure 4A –C). Moreover, CCK8 assays elucidated that the inhibitory impact of NNT-AS1 knockdown on proliferation of CCA cells could be abolished by miR-485 suppression (Figure 4D). In addition, the expression of BCL9 in transfected CCA cells were analyzed. The influence of si-NNT-AS1 on the expression was reversed by miR-485 inhibitor (Figure 4E). These data suggested that miR-485 reversed the promoting effect of NNT-AS1 on the proliferation and metastasis of CCA cells.
Ectopic expression of NNT-AS1 promoted CCA growth in vivo
In order to confirm whether NNT-AS1 caused CCA tumorigenesis in vivo, we conducted a subcutaneous tumor formation experiment in nude mice. CCLP1 cells treated with sh-NNT-AS1 or a control vector were injected into nude mice. After 21 days post-injection, the tumors established in the sh- NNT- AS1 group were dramatically smaller than those in sh-NC group ( Figure 5A and B). As shown in Figure 5C and D, sh-NNT-AS1 injected mice showed a reduction in tumor weight and volume at the end of the in vivo assay compared to the control group ( p<0.05). These findings indicated that silencing NNT-AS1 could repress CCA tumor growth in vivo.
Discussion
Cholangiocarcinoma (CCA) is one of the most aggressive malignancies of the digestive system. This disease is difficult to diagnose at its inchoate stage and has poor prognosis. Therefore, a clear understanding of pathogenesis and major influencing factors is the key to develop effective therapeutic methods for CCA. However, the mechanism of CCA progression, including cell proliferation, invasion, metastasis and anti-apoptosis, has not been elaborated. Increasing evidences have indicated that lncRNAs emerge as an important regulator of cancers,15 including CCA. NNT-AS1 has been reported to participate in the tumorigenesis of various cancer.16–18 In agreement with most of current studies of tumors on NNT-AS1, our study found that NNT-AS1 was highly expressed in CCA, and its expression level was correlated with clinical prognosis. We confirmed that NNT-AS1 played an oncogene role in CCA, and the specific molecular mechanism needed to be further explored.
A large number of lncRNAs have been shown to play an important role in the development of cholangiocarcinoma, such as NEAT119 and LINC01296.20 In the current study, our data showed that the NNT-AS1 expression level was significantly increased of in CCA tissues compared to adjacent non-tumor tissues. High NNT-AS1 expression was associated with advanced clinicopathological features and poor overall survival. Therefore, the evidence proved the oncogenic role of NNT-AS1 in CCA tumorigenesis. Functional experiments results revealed that NNT-AS1 knockdown inhibited the proliferation and invasion ability in vitro, and suppressed the CCA tumor growth in vivo.
Recently, the miRNA ‘sponge’ theory21 is well known. There was a significant negative correlation between NNT-AS1 and miR-485 in CCA. MiR-485 was reported to be abnormally expressed in many cancers. Kang have reported that miR-485 could suppress gastric cancer cell metastasis and sphere formation,22 miR-485 was also dysregulated in non-small-cell lung cancer (NSCLC) and markedly inhibited NSCLC cell growth and induced cell apoptosis.23 Moreover, miR-485 widely participated in cell proliferation and invasion, as well as autophagy and apoptosis.
Then we identified BCL9 as the potential biological target for miR-485 by using TargetScan Database. Luciferase reporter assay confirmed the molecular binding at 3ʹ-UTR. We hypothesized that there was a ‘ceRNA network’ among NNT-AS1, miR-485 and BCL9. The results of rescue assays showed that miR-485 reversed the promoting effect of NNT-AS1 on the proliferation and metastasis of CCA. NNT-AS1 was up-regulated and competed with BCL9 for binding to miR-485, thereby increasing the protein expression of BCL9 and promoting the proliferation, migration and invasion in CCA (Figure 5E). Published studies have shown that BCL9 was closely related to the development of cancers such as renal cell carcinoma,24 colorectal cancer,25 and gastric cancer.26 Moreover, BCL9 was involved in signal transduction through the Wnt pathway, promoting β-catenin’s transcriptional activity.27 As our knowledge, the Wnt/β-catenin signaling pathway was pathologically activated in CCA, and β-Catenin was a key mediator of the Wnt/β-catenin pathway. In this regard, NNT-AS1 might promote the CCA tumorigenesis by regulating the Wnt signaling pathway.
Conclusion
We have a conclusion that NNT-AS1 overexpression is associated with tumorigenesis and poor prognosis in CCA, NNT-AS1 promotes proliferation, migration and invasion of CCA via miR-485/BCL9 axis.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (No. 81602088), China Postdoctoral Science Foundation (No. 2017M621305), Heilongjiang Postdoctoral Science Foundation (No. LBH-Z16096), Health and Family Planning Commission Research Project of Heilongjiang Province (No. 2016-049), HMU College Students’ Innovation and Entrepreneurship Training Program (No. 201810226105) and Innovative Science Foundation of Harbin Medical University (No. 2016LCZX09).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang WT, Ye H, Wei PP, et al. LncRNAs H19 and HULC, activated by oxidative stress, promote cell migration and invasion in cholangiocarcinoma through a ceRNA manner. J Hematol Oncol. 2016;9:117. doi:10.1186/s13045-016-0348-0
2. Blechacz B. Cholangiocarcinoma: current knowledge and new developments. Gut and Liver. 2017;11:13–26. doi:10.5009/gnl15568
3. Afshar M, Khanom K, Ma YT, Punia P. Biliary stenting in advanced malignancy: an analysis of predictive factors for survival. Cancer Manag Res. 2014;6:475–479. doi:10.2147/CMAR.S71111
4. Doherty B, Nambudiri VE, Palmer WC. Update on the diagnosis and treatment of cholangiocarcinoma. Current Gastroenterology Reports. 2017;19:2. doi:10.1007/s11894-017-0542-4
5. Gatto M, Bragazzi MC, Semeraro R, et al. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis. 2010;42:253–260. doi:10.1016/j.dld.2009.12.008
6. Zhang Y, Tang L. The application of lncRNAs in cancer treatment and diagnosis. Recent Pat Anticancer Drug Discov. 2018;13:292–301. doi:10.2174/1574892813666180226121819
7. Chen X, Yang Y, Cao Y, et al. lncRNA PVT1 identified as an independent biomarker for prognosis surveillance of solid tumors based on transcriptome data and meta-analysis. Cancer Manag Res. 2018;10:2711–2727. doi:10.2147/CMAR.S166260
8. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi:10.1038/nm.3981
9. Li X, Du N, Zhang Q, et al. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014;5:e1479. doi:10.1038/cddis.2014.430
10. Li ZL, Li JL, Ji DL, et al. Overexpressed long noncoding RNA Sox2ot predicts poor prognosis for cholangiocarcinoma and promotes cell proliferation and invasion. Gene. 2018;645:131–136. doi:10.1016/j.gene.2017.12.017
11. Fagerberg L, Hallstrom BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi:10.1074/mcp.M113.035600
12. Wang X, Ren M, Li Y, et al. Long noncoding RNA NNT-AS1 promotes gastric cancer proliferation and invasion by regulating microRNA-363 expression. J Cell Biochem. 2018;120(4):5704–5712. doi:10.1002/jcb.27855
13. Hua F, Liu S, Zhu L, Ma N, Jiang S, Yang J. Highly expressed long non-coding RNA NNT-AS1 promotes cell proliferation and invasion through Wnt/beta-catenin signaling pathway in cervical cancer. Biomed Pharmacother. 2017;92:1128–1134. doi:10.1016/j.biopha.2017.03.057
14. Li X, Edwards M, Swaney KF, et al. Mutually inhibitory Ras-PI(3,4)P2 feedback loops mediate cell migration. Proc Natl Acad Sci U S A. 2018;115:E9125–E9134. doi:10.1073/pnas.1809039115
15. Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi:10.1038/onc.2017.184
16. Shen Q, Jiang Y. LncRNA NNT-AS1 promotes the proliferation, and invasion of lung cancer cells via regulating miR-129-5p expression. Biomed Pharmacother. 2018;105:176–181. doi:10.1016/j.biopha.2018.05.123
17. Li Y, Lv M, Song Z, Lou Z, Wang R, Zhuang M. Long non-coding RNA NNT-AS1 affects progression of breast cancer through miR-142-3p/ZEB1 axis. Biomed Pharmacother. 2018;103:939–946. doi:10.1016/j.biopha.2018.04.087
18. Cai Y, Dong ZY, Wang JY. LncRNA NNT-AS1 is a major mediator of cisplatin chemoresistance in non-small cell lung cancer through MAPK/slug pathway. Eur Rev Med Pharmacol Sci. 2018;22:4879–4887. doi:10.26355/eurrev_201808_15624
19. Parasramka M, Yan IK, Wang X, et al. BAP1 dependent expression of long non-coding RNA NEAT-1 contributes to sensitivity to gemcitabine in cholangiocarcinoma. Mol Cancer. 2017;16:22. doi:10.1186/s12943-017-0587-x
20. Zhang D, Li H, Xie J, et al. Long noncoding RNA LINC01296 promotes tumor growth and progression by sponging miR-5095 in human cholangiocarcinoma. Int J Oncol. 2018;52:1777–1786. doi:10.3892/ijo.2018.4362
21. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell. 2011;146:353–358. doi:10.1016/j.cell.2011.07.014
22. Kang M, Ren MP, Zhao L, Li CP, Deng MM. miR-485-5p acts as a negative regulator in gastric cancer progression by targeting flotillin-1. American Journal of Translational Research. 2015;7:2212–2222.
23. Jiang P, Xu C, Chen L, et al. Epigallocatechin-3-gallate inhibited cancer stem cell-like properties by targeting hsa-mir-485-5p/RXRalpha in lung cancer. J Cell Biochem. 2018;119:8623–8635. doi:10.1002/jcb.27117
24. Wang J, Ying Y, Bo S, Li G, Yuan F. Differentially expressed microRNA-218 modulates the viability of renal cell carcinoma by regulating BCL9. Mol Med Rep. 2016;14:1829–1834. doi:10.3892/mmr.2016.5403
25. Beaulieu JF. Tuning WNT-beta-catenin signaling via BCL9 proteins for targeting colorectal cancer cells. EBioMedicine. 2015;2:1846–1847. doi:10.1016/j.ebiom.2015.11.033
26. Han W, Mu Y, Zhang Z, Su X. Expression of miR-30c and BCL-9 in gastric carcinoma tissues and their function in the development of gastric cancer. Oncol Lett. 2018;16:2416–2426. doi:10.3892/ol.2018.8934
27. Takada K, Zhu D, Bird GH, et al. Targeted disruption of the BCL9/beta-catenin complex inhibits oncogenic Wnt signaling. Sci Transl Med. 2012;4:148ra117. doi:10.1126/scitranslmed.3003808
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.