Back to Journals » OncoTargets and Therapy » Volume 12
Long Non-Coding RNA HOTAIR Modulates KLF12 to Regulate Gastric Cancer Progression via PI3K/ATK Signaling Pathway by Sponging miR-618
Authors Xun J, Wang C, Yao J, Gao B, Zhang L
Received 20 July 2019
Accepted for publication 6 November 2019
Published 27 November 2019 Volume 2019:12 Pages 10323—10334
DOI https://doi.org/10.2147/OTT.S223957
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Jin Xun, Chunfeng Wang, Jianning Yao, Bing Gao, Lianfeng Zhang
Department of Gastroenterology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou City, Henan Province, People’s Republic of China
Correspondence: Lianfeng Zhang
Department of Gastroenterology, The First Affiliated Hospital of Zhengzhou University, No. 1 Jianshedong Road, Erqi District, Zhengzhou City 450052, Henan Province, People’s Republic of China
Tel/Fax +86-371-66862062
Email [email protected]
Purpose: Long non-coding RNA (lncRNA) HOX transcript antisense RNA (HOTAIR) has been reported to dysregulate in many tumors. However, the mechanism of HOTAIR was rarely reported in GC.
Methods: The levels of HOTAIR, microRNA-618 (miR-618) and Krueppel-like factor 12 (KLF12) in GC tissues and cells were detected by quantitative real-time polymerase chain reaction (qRT-PCR). The cell viability and apoptotic rate were assessed via cell counting kit-8 (CCK-8) assay and flow cytometry, respectively. The migrating and invading abilities were tested by Transwell assay. The protein levels of KLF12, p-PI3K, PI3K, p-ATK and ATK were measured by Western blot assay. These interactions between miR-618 and HOTAIR or KLF12 were predicted by DIANA tools, and then, dual-luciferase reporter assay and RNA immunoprecipitation (RIP) assay were conducted to validate these interactions. Besides, the xenograft tumor experiment was performed to further verify the roles of HOTAIR in GC.
Results: The levels of HOTAIR and KLF12 were significantly upregulated and the level of miR-618 was strikingly downregulated in GC tissues and cells. miR-618 was verified as a direct target of HOTAIR or KLF12. HOTAIR silencing blocked GC progression and PI3K/ATK signaling pathway by sponging miR-618 and also restrained xenograft tumor growth in vivo. miR-618 inhibited GC progression and PI3K/ATK signaling pathway by targeting KLF12. Mechanistically, HOTAIR modulated KLF12 expression by sponging miR-618 in GC cells.
Conclusion: These data unraveled that HOTAIR promoted GC progression through PI3K/ATK signaling pathway via miR-618/KLF12 axis.
Keywords: HOTAIR, miR-618, KLF12, PI3K/ATK signaling pathway, gastric cancer
Introduction
Gastric cancer (GC) is the second leading cause of cancer death worldwide, especially in some eastern Asia countries, including China, Japan and Korea.1–3 Despite some improvements in early detection and therapeutic in recent decades, the survival time of GC patients is still short and more serious in the advanced stage.4,5 Therefore, it is urgent to search for novel therapeutic targets for GC patients.
Long non-coding RNAs (lncRNAs), a group of non-coding RNAs with the length of more than 200 nucleotides (nt), may affect target gene expressions at the transcriptional and posttranscriptional stages.6 In GC, a number of reports showed that lncRNAs, including lncRNA GIHCG,7 ATB,8 FEZF1 antisense RNA 1 (FEZF1-AS1),9 SNHG1510 and CRNDE,11 were aberrantly expressed as well as related to the processes in cancer progression. HOX transcript antisense RNA (HOTAIR), located on human chromosome 12, has been documented to play an oncogenic role in cancer. Previous researches indicated that HOTAIR dysregulation was associated with cancer progressions, such as ovarian cancer12 and colon cancer.13 However, the biological mechanism of HOTAIR in GC was rarely reported.
MicroRNAs are a class of small RNAs with about 22 nt in length and suppress target gene expression by inhibiting the translation of message RNAs (mRNAs) or mediating the degradation mRNAs.14 Emerging evidence implicated that microRNA miR-618 was abnormally expressed in breast cancer,15 prostate cancer16 and anaplastic thyroid cancer,17 as well as in GC.18 Krueppel-like factor 12 (KLF12) is encoded by the KLF12 gene which is located on human chromosome 13. KLF12 was also reported to dysregulate in endometrial cancer19 and GC.20 Phosphoinositide 3-kinases (PI3K)/protein kinase B (AKT) signaling pathway, a signal transduction pathway and one of the most frequently deregulated pathways in cancer, is implicated to the pathogenesis of various human cancers.21 However, the mechanisms of miR-618 and KLF12 were barely defined in GC. In this study, we mainly explored the mechanism of HOTAIR in GC, thus in turn providing novel therapeutic target for GC patients.
Materials and Methods
Tissue Samples
The study was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University and performed according to the Declaration of Helsinki Principles. Thirty-five GC tissue samples were collected from The First Affiliated Hospital of Zhengzhou University as well as thirty-five corresponding adjacent normal tissue samples. The GC patients (n=35) were divided into two groups: patients with low HOTAIR expression (n=16) and patients with high HOTAIR expression (n=19). All tissue samples were immediately frozen in a −80°C refrigerator until further use. Written informed consent was provided by all GC patients or guardians.
Cell Culture and Transfection
Two human gastric carcinoma cell lines MGC-803 (CQ80145) and AGS (H007) and human stomach normal epithelial cell lines GES-1 (H054) were purchased from ChuanQiu Biotechnology (Shanghai, China). All cells were cultivated in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) in an incubator with the conditions of 37°C and 5% CO2.
Small interfering RNA (siRNA) targeting HOTAIR (si-HOTAIR#1, si-HOTAIR#2, si-HOTAIR#3) and negative control (si-NC), miR-618 mimics (miR-618) and negative control (miR-NC), miR-618 inhibitor (in-miR-618) and negative control (in-miR-NC), HOTAIT overexpression plasmid (HOTAIR) and KLF12 overexpression vector (KLF12) were all obtained from GenePharma (Shanghai, China). The transfection was performed using Lipofectamine 2000 (Invitrogen) according to the reference.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The RNA from MGC-803 and AGS cells was extracted using Trizol reagent (Thermo Fisher Scientific). After the detection of the concentration, the RNA samples were subjected to synthesize cDNA using miScript RT Kit (TaKaRa, Dalian, China). The quantitative PCR was performed using SYBR Premix Ex Taq II (TaKaRa) on a 96-well Real-Time system (Bio-Rad, Shanghai, China). The relative expression of HOTAIR, KLF12 and miR-618 was calculated by the method of 2−ΔΔCt and normalized by GAPDH or U6, respectively. The primers were synthesized in Beijing Genomics Institute (BGI, Shenzhen, China) and listed as follows: HOTAIR: (forward, 5ʹ-CAGTGGGGAACTCTGACTCG-3ʹ, reverse, 5ʹ-GTGCCTGGTGCTCTCTTACC-3ʹ); miR-618: (forward, 5ʹ-CGGCGGAAACTCTACTTGTCCTT-3ʹ, reverse, 5′-ATCCAGTGCAGGGTCCGAGG-3ʹ); KLF12: (forward, 5ʹ-CACCTGGAAATGTGAACAACA-3ʹ, reverse, 5ʹ-TTTTACTTTGTCTGGGAGATAGGC-3ʹ); GAPDH: (forward, 5ʹ-TGTTCGTCATGGGTGTGAAC-3ʹ, reverse, 5ʹ-ATGGCATGGACTGTGGTCAT-3ʹ) and U6: (forward, 5ʹ-CTCGCTTCGGCAGCACA-3ʹ, reverse, 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ).
Cell Counting Kit-8 (CCK-8) Assay
CCK-8 (Beyotime, Shanghai, China) was used to measure the cell viability. In brief, the MGC-803 and AGS cells (4×103 per well) were seeded into 96-well plate and incubated for 0 hr, 24 hrs, 48 hrs and 72 hrs. Then, 10 μL CCK-8 reagent was injected into each well and incubated for another 2 hrs. The absorbance at 450 nm was measured via a spectrophotometer (Thermo Fisher Scientific).
Transwell Assay
Transwell chambers (Corning, Tewksbury, MA, USA) were used to detect the migrating and invading abilities of transfected MGC-803 and AGS cells. For migration, the upper chamber was supplemented with serum-free DMEM, while DMEM with 10% FBS was added in the lower chamber. After 24-hr incubation, the cells in the lower chamber were fixed with 4% methanol and then stained with 0.1% crystal violet for 24 hrs. The cell counted for 5 randomly selected fields under a microscope (Olympus, Tokyo, Japan). For invasion, the protocols were similar to those in migration. The difference is that the Transwell chamber was coated with a Matrigel matrix (BD Biosciences, San Jose, CA, USA) in invasion experiments.
Cell Apoptosis Assay
Annexin V/PI cell apoptosis analysis kit (Servicebio, Wuhan, China) was used to evaluate the apoptotic rate of transfected MGC-803 and AGS cells. After digestion, the re-suspended samples were stained with Annexin V fluorescein isothiocyanate (FITC) and propidium iodide (PI) and further incubated for 15 mins. The cell apoptotic rate was analyzed by flow cytometry (BD Biosciences).
Western Blot Assay
The protein in MGC-803 and AGS cells was extracted by RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific). Following the measurement of concentration, the protein samples were separated via sodium dodecyl sulfonate-polyacrylamide gel electrophoresis (SDS-PAGE). Then, the protein samples were transferred onto a PVDF membrane (Millipore, Billerica, MA, USA). The membranes were blocked in skim milk for 2 hrs and then incubated with primary antibody overnight at 4°C as well as the incubation with secondary antibody for another 2 hrs. The chemiluminescence intensity was evaluated using eyoECL Plus Kit (Beyotime). All the antibodies were purchased from Bioss (Beijing, China).
Dual-Luciferase Reporter Assay
The interactions between miR-618 and HOTAIR or KLF12 were predicted by DIANA tools (http://diana.imis.athena-innovation.gr). The sequences of HOTAIR or its negative mutant were cloned and inserted into psiCHECK2 vector (Promega, Madison, WI, USA) to conduct the luciferase reporter, namely, HOTAIR WT or HOTAIR MUT. The co-transfection of luciferase reporter HOTAIR WT or HOTAIR MUT and miR-618 mimics or miR-NC were conducted using Lipofectamine 2000 Reagent (Invitrogen). The protocols were also applied to KLF12 3ʹUTR.
RNA Immunoprecipitation (RIP) Assay
RIP assay was carried out using Magna RNA immunoprecipitation kit (Millipore). After lysis of MGC-803 and AGS cells with RIP lysis buffer, the sample was incubated with magnetic beads conjugated with anti-Ago2 or anti-IgG antibodies. The enrichment of RNA was measured by qRT-PCR.
Mice Xenograft Models
The experiment in nude mice was performed according to the procedure and approved by the Animal Care Committee of The First Affiliated Hospital of Zhengzhou University. Six-week-old BALB/c nude mice were randomly divided into two groups (n = 6 per group) and implanted with AGS cells transfected with sh-HOTAIR or sh-NC. The tumor volume was measured every 7 d for 5 times and calculated with the (length × width2)/2 method. After injection for 35 d, the xenograft tumor was excised for the weight measurement or the further study.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7 (GraphPad, La Jolla, CA, USA). All values are presented as means ± standard deviation (SD). The comparison between two groups was processed by Student’s t-test, while among three or more than three groups, it was analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc. P< 0.05 was considered as statistical significance.
Results
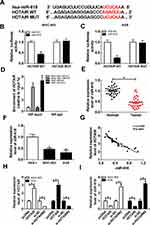
HOTAIR Was Strikingly Upregulated in GC Tissues and Cells
It is well known that lncRNA HOTAIR plays a role as an oncogenic molecule in different cancer cells. To explore the potential roles in GC, we firstly detected the level of HOTAIR in GC tissues and cells. The qRT-PCR results showed that the level of HOTAIR was conspicuously elevated in GC tissues related to that in corresponding adjacent normal tissues (Figure 1A) as well as elevated in human gastric carcinoma cell lines MGC-803 and AGS compared to that in human stomach epithelial cell lines GES-1 (Figure 1C). In addition, we demonstrated that a high level of HOTAIR had a low survival rate, while the low HOTAIR expression associated with a high survival rate (Figure 1B). These results implied that lncRNA HOTAIR was apparently enhanced in GC tissues and cells.
HOTAIR Knockdown Suppressed Cell Proliferation, Migration, Invasion and PI3K/ATK Signaling Pathway While Induced Cell Apoptosis in MGC-803 and AGS Cells
To investigate the functions of HOTAIR, si-HOTAIR was transfected into MGC-803 and AGS cells. The qRT-PCR results confirmed the knockdown efficiency, indicated by the notable downregulation of HOTAIR in MGC-803 and AGS cells (Figure 2A and B). Furthermore, the CCK-8 assay exhibited that si-HOTAIR significantly constrained cell viability in MGC-803 and AGS cells (Figure 2C and D). Also, the Transwell assay presented that the transfection of si-HOTAIR#2 remarkably reduced the cell migrating and cell invading abilities in MGC-803 and AGS cells (Figure 2E and F). The flow cytometry showed that the apoptotic rate was distinctly promoted in MGC-803 and AGS cells transfected with si-HOTAIR#2 (Figure 2G). Besides, the Western blot assay indicated that the ratios of p-PI3K/PI3K and p-ATK/ATK were both declined in MGC-803 and AGS cells transfected with si-HOTAIR#2 (Figure 2H). These data implicated that HOTAIR depletion repressed cell proliferation, migration, invasion and PI3K/ATK signaling pathway but promoted cell apoptosis in MGC-803 and AGS cells.
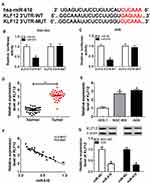
miR-618 Was Negatively Interacted with HOTAIR and Was Markedly Decreased in GC Tissues and Cells
To search the biological mechanism of HOTAIR in GC, DIANA tools online database was utilized to predict the putative target of HOTAIR. The results showed that miR-618 had complementary binding sites with HOTAIR (Figure 3A). Following dual-luciferase reporter assay indicated that the transfection with miR-618 mimics resulted in the dramatical decrease of luciferase activity of HOTAIR WT reporter in MGC-803 and AGS cells, while the luciferase activity of HOTAIR MUT had no significant fluctuation in any group (Figure 3B and C). Also, the RIP assay presented that the level of HOTAIR was distinctly more enriched by Ago2 antibody in MGC-803 and AGS cells transfected with miR-618 in contrast to in IgG group (Figure 3D). Moreover, the qRT-PCR results showed that the level of miR-618 was significantly downregulated in GC tissues and cells (Figure 3E and F). The scatter diagram indicated that the level of HOTAIR was negatively linear correlated with the level of miR-618 (Figure 3G). Additionally, the level of HOTAIR was strikingly increased and the level of miR-618 was evidently reduced in MGC-803 and AGS cells transfected with HOTAIR, while the transfection with si-HOTAIR#2 contributed to the downregulation of HOTAIR and the upregulation of miR-618 (Figure 3H and I). Taken together, these data demonstrated that miR-618 was a direct target of HOTAIR and was downregulated in GC tissues and cells.
miR-618 Inhibitor Counteracted the Inhibitory Impacts on Cell Proliferation, Migration, Invasion and PI3K/ATK Signaling Pathway, as Well as the Facilitated Effect on Cell Apoptosis in MGC-803 and AGS Cells Caused by HOTAIR Silencing
To explore the functions of HOTAIR and miR-618 in GC, the si-HOTAIR#2 and in-miR-618 were co-transfected into MGC-803 and AGS cells. The CCK-8 assay showed that the transfection with miR-168 inhibitor reversed the suppressive effect on the cell viability in MGC-803 and AGS cells caused by si-HOTAIR#2 (Figure 4A and B). Subsequently, the Transwell assay exhibited that miR-618 inhibitor mitigated the repressive impacts on the migrating and invading abilities in MGC-803 and AGS cells transfected with si-HOTAIR#2 (Figure 4C and D). Furthermore, the flow cytometry implied that the apoptotic rate was reverted in MGC-803 and AGS cells co-transfected si-HOTAIR and in-miR-618 compared to that in si-HOTAIR#2 group (Figure 4E). Besides, the Western blot assay demonstrated that the downregulation of miR-168 alleviated the suppressive effects on the ratios of p-PI3K/PI3K and p-ATK/ATK in MGC-803 and AGS cells transfected induced by si-HOTAIR#2 (Figure 4F). To sum up, these results revealed that miR-618 inhibitor relieved the inhibitory impacts on cell proliferation, migration, invasion and PI3K/ATK signaling pathway as well as the accelerated effect on cell apoptosis in MGC-803 and AGS cells induced via HOTAIR depletion.
KLF12 Was a Direct Target of miR-618 and Significantly Enhanced in GC Tissues and Cells
To explore the mechanism of miR-618 in GC, DIANA tools online database was used to search the putative target of miR-618. The results presented that KLF12 3ʹUTR had complementary sequences with miR-618 (Figure 5A). The dual-luciferase reporter assay implicated that the luciferase activity of KLF12 3ʹUTR-WT reporter was effectively declined in MGC-803 and AGS cells transfected with miR-618 related to that in miR-NC group; however, the luciferase activity of KLF12 3ʹUTR-MUT reporter had no apparent change in any treatment (Figure 5B and C). Moreover, the qRT-PCR assay indicated that the level of KLF12 was remarkably augmented in GC tissues and cells (Figure 5D and E). In addition, the scatter plot exhibited that the level of KLF12 was negatively linear correlated with the level of miR-618 (Figure 5F). The Western blot assay implied that the protein level of KLF12 was obviously retarded in MGC-803 and AGS cells transfected with miR-618 in comparison with that in miR-NC group (Figure 5G). These results manifested that KLF12 was negatively interacted with miR-618 and significantly upregulated in GC tissues and cells.
KLF12 Overexpression Alleviated the Restraint Effects on Cell Proliferation, Migration, Invasion and PI3K/ATK Signaling Pathway and the Facilitated Impact on Cell Apoptosis in MGC-803 and AGS Cells Induced by miR-618 Mimics
Based on the earlier results, we demonstrated that KLF12 was a direct target of miR-618. Subsequently, the functions of miR-618 and KLF12 were further studied. The CCK-8 assay exhibited that the cell viability was relieved in MGC-803 and AGS cells transfected with KLF12 caused via miR-618 (Figure 6A and B). Meanwhile, the Transwell assay implicated that the overexpression of KLF12 mitigated the restraint effects on cell migration and invasion ability in MGC-803 and AGS cells promoted by miR-618 (Figure 6C and D). Furthermore, the flow cytometry results showed that the apoptotic rate was apparently elevated in MGC-803 and AGS cells transfected miR-618, while the emergence of KLF12 weakened the promoted effect on cell apoptotic rate (Figure 6E). Besides, the Western blot assay uncovered that KLF12 overexpression regained the ratios of p-PI3K/PI3K and p-ATK/ATK in MGC-803 and AGS cells inhibited by miR-618 (Figure 6F). These data suggested that KLF12 overexpression receded the constraint effects on cell proliferation, migration, invasion and PI3K/ATK signaling pathway and the promoted impact on cell apoptosis in MGC-803 and AGS cells caused by miR-618 mimics.
HOTAIR Depletion Downregulated KLF12 Expression by Targeting miR-618
To explore the relationship among HOTAIR, miR-618 and KLF12, MGC-803 and AGS cells were co-transfected with si-HOTAIR#2 and in-miR-618. The qRT-PCR showed that the level of KLF12 was regained in MGC-803 and AGS cells transfected with in-miR-618 suppressed by si-HOTAIR#2 (Figure 7A). Also, Western blot assay indicated that miR-618 inhibitor rescued the protein level of KLF12 in MGC-803 and AGS cells transfected with si-HOTAIR#2 (Figure 7B). In addition, the qRT-PCR results exhibited that the level of HOTAIR was positively linear correlated with the level of KLF12 (Figure 7C). These data unraveled that HOTAIR silencing downregulated KLF12 expression by regulating miR-618.
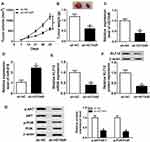
HOTAIR Depletion Constrained Xenograft Tumor Growth in vivo
To further investigate the role of HOTAIR in GC, sh-HOTAIR was transfected in AGS cells and then injected into mice. The measurement results implicated that the tumor volume and weight were both declined in mice injected with sh-HOTAIR related to that in sh-NC group (Figure 8A and B). The qRT-PCR results presented that the level of HOTAIR was notably decreased, and the level of miR-618 was obviously enhanced in sh-HOTAIR group compared to that in sh-NC group (Figure 8C and D). The qRT-PCR and Western blot assay showed that the mRNA and protein levels of KLF12 were both distinctly downregulated in sh-HOTAIR group (Figure 8E and F). Besides, the Western blot assay indicated that the ratios of p-PI3K/PI3K and p-ATK/ATK were also evidently declined in sh-HOTAIR group (Figure 8G). To sum, these results uncovered that HOTAIR silencing restrained xenograft tumor growth in vivo.
Discussion
Gastric cancer is the second leading cause of cancer-related death in the world.3 Accumulating evidence reported that lncRNAs play crucial roles in cancer. In this research, we explored the biological mechanism of lncRNA HOTAIR in GC. The results indicated that HOTAIR modulated KLF12 expression to promote cell proliferation, migration and invasion and repress cell apoptosis in GC through PI3K/ATK signaling pathway by sponging miR-618.
Recent researches indicated that HOTAIR was aberrantly expressed in diverse cancers. For example, a previous study in ovarian carcinoma indicated that the level of HOTAIR was apparently increased in ovarian carcinoma tissues and cell lines than that in the negative controls.12 Another study in colon cancer documented that the relative expression of HOTAIR was significantly upregulated in colon cancer tissues than that in matched adjacent normal tissues.13 In this study, we validated that the level of lncRNA HOTAIR was significantly increased in GC tissues and cells (MGC-803 and AGS). Following the transfection of si-HOTAIR resulted in the remarkable decrease of cell viability, migrating and invading abilities, as well as the distinct increase of apoptotic rate in MGC-803 and AGS cells. Besides, the patients with a high level of HOTAIR associated with a low survival rate. The mice xenograft models also indicated that HOTAIR silencing constrained the xenograft tumor growth and the ratios of p-PI3K/PI3K and p-ATK/ATK in vivo. These results demonstrated that HOTAIR knockdown inhibited GC progression.
Recently, a hypothesis proposed that lncRNAs may act as competing endogenous RNAs (ceRNAs) to recruit miRNAs, thus resulting in the depression of miRNA targets.22 For instance, a study in cervical cancer showed that HOTAIR functioned as a ceRNA to sponge miR-143-2p and then resulted in the promotion of cervical cancer cell growth.23 Another study demonstrated that HOTAIR augmented cell proliferation, migration and invasion and suppressed cell apoptosis in colorectal cancer by sponging miR-197.24 Also, Dong et al reported that HOTAIR promoted cell viability, migration, invasion and epithelial–mesenchymal transition (EMT) in GC by sponging miR-217.25 In the present study, the dual-luciferase reporter assay and RIP assay verified that the miR-618 was a direct target of HOTAIR. Besides, we found that the level of miR-618 was conspicuously declined in GC tissues and cells and was negatively linear correlated with HOTAIR. The further functional experiments indicated that miR-168 inhibitor relieved the restraint effects on cell viability, migrating and invading abilities as well as the facilitated effect on apoptotic rate induced by HOTAIR depletion. These data manifested that HOTAIR promoted GC progression by sponging miR-618.
Emerging evidence manifested that KLF12 was involved in the cancer progression of various cancers. For example, Ding et al reported that KLF12 facilitated cell proliferation, migration and inhibited cell apoptosis in vitro as well as promoted tumor size in vivo in endometrial cancer.19 Another study in GC indicated that lncRNA TTN-AS1 modulated the expression of KLF12 to induce cell proliferation, cell migration and invasion and impaired cell apoptosis by targeting miR-376b-3p.20 In this study, we demonstrated that KLF12 negatively interacted with miR-618 by the results of dual-luciferase reporter assay. Furthermore, the level of KLF12 was apparently enhanced in GC tissues and cells and negatively linear correlated with the level of KLF12. The following functional study implied that KLF12 overexpression alleviated the suppressive impacts on cell viability, migration and invasion ability, as well as the promoted impact on the apoptotic rate in MGC-803 and AGS cells caused by miR-618 overexpression. The restoration experiments implicated that the transfection of miR-618 inhibitor regained the mRNA and protein levels of KLF12 in MGC-803 and AGS cells transfected with si-HOTAIR. These results unraveled that HOTAIR contributed to GC progression via miR-618/KLF12 axis.
Recent studies demonstrated that PI3K/AKT signaling pathway was implicated in many processes in tumor progression, including cell proliferation, metastasis and apoptosis.21,26 For example, a study in endometrial cancer indicated that KLF12 facilitated tumor progression in endometrial cancer by activating PI3K/ATK signaling pathway.19 Another study in human thyroid carcinomas demonstrated that miR-618 blocked tumor progression in thyroid carcinomas by confining PI3K/ATK signaling pathway.27 In the current research, HOTAIR depletion inhibited the protein levels of p-PI3K and p-ATK in GC cells by regulating miR-618. miR-618 confined p-PI3K and p-ATK expression by targeting KLF12 in GC cells. Furthermore, p-PI3K and p-ATK were also declined in sh-HOTAIR group. These data disclosed that HOTAIR/miR-618/KLF12-induced GC progression was mediated by PI3K/ATK signaling pathway.
In conclusion, we confirmed that the levels of HOTAIR and KLF12 were strikingly augmented and the level of miR-618 drastically decreased in GC tissues and cells. Combined with the functional and mechanistical experiment results, we concluded that HOTAIR positively regulated KLF12 expression to promote GC progression via PI3K/ATK signaling pathway by sponging miR-618.
Ethical Approval
The study was approved from the Animal Care Committee of The First Affiliated Hospital of Zhengzhou University and followed for the welfare of the animals in the guidelines of the National Institutes of Health.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Catalano V, Labianca R, Beretta GD, et al. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127–164. doi:10.1016/j.critrevonc.2009.01.004
2. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.21262
3. Rubayat R, Akwi WA, Jamal AI. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483–4490. doi:10.3748/wjg.v20.i16.4483
4. Pasechnikov V, Chukov S, Fedorov E, et al. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20:13842–13862. doi:10.3748/wjg.v20.i38.13842
5. Tinoco A, Gottardi LF, Boechat ED. Gastric cancer in the excluded stomach 10 years after gastric bypass. Case Rep Surg. 2015;2015:468293.
6. Wang J, Sun J, Wang J, et al. Long noncoding RNAs in gastric cancer: functions and clinical applications. Onco Targets Ther. 2016;9:681–697. doi:10.2147/OTT.S95412
7. Liu G, Jiang Z, Qiao M, et al. Lnc-GIHCG promotes cell proliferation and migration in gastric cancer through miR- 1281 adsorption. Mol Genet Genomic Med. 2019;7:e711. doi:10.1002/mgg3.711
8. Lei K, Liang X, Gao Y, et al. Lnc-ATB contributes to gastric cancer growth through a MiR-141-3p/TGFβ2 feedback loop. Biochem Biophys Res Commun. 2017;484:514–521. doi:10.1016/j.bbrc.2017.01.094
9. Wu X, Zhang P, Zhu H, et al. Long noncoding RNA FEZF1-AS1 indicates a poor prognosis of gastric cancer and promotes tumorigenesis via activation of Wnt signaling pathway. Biomed Pharmacother. 2017;96:1103–1108. doi:10.1016/j.biopha.2017.11.113
10. Chen SX, Yin JF, Lin BC, et al. Upregulated expression of long noncoding RNA SNHG15 promotes cell proliferation and invasion through regulates MMP2/MMP9 in patients with GC. Tumour Biol. 2016;37:6801–6812. doi:10.1007/s13277-015-4404-0
11. Hu CE, Du PZ, Zhang HD, et al. Long noncoding RNA CRNDE promotes proliferation of gastric cancer cells by targeting miR-145. Cell Physiol Biochem. 2017;42:13–21. doi:10.1159/000477107
12. Chang L, Guo R, Yuan Z, et al. LncRNA HOTAIR regulates CCND1 and CCND2 expression by sponging miR-206 in ovarian cancer. Cell Physiol Biochem. 2018;49:1289–1303. doi:10.1159/000493408
13. Luo ZF, Zhao D, Li XQ, et al. Clinical significance of HOTAIR expression in colon cancer. World J Gastroenterol. 2016;22:5254–5259. doi:10.3748/wjg.v22.i22.5254
14. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi:10.1038/nrg3074
15. Xu Y, Yao Y, Leng K, et al. Increased expression of circular RNA circ_0005230 indicates dismal prognosis in breast cancer and regulates cell proliferation and invasion via miR-618/CBX8 signal pathway. Cell Physiol Biochem. 2018;51:1710–1722. doi:10.1159/000495675
16. Song XL, Tang Y, Lei XH, et al. miR-618 inhibits prostate cancer migration and invasion by targeting FOXP2. J Cancer. 2017;8:2501–2510. doi:10.7150/jca.17407
17. Cheng Q, Zhang X, Xu X, et al. MiR-618 inhibits anaplastic thyroid cancer by repressing XIAP in one ATC cell line. Ann Endocrinol (Paris). 2014;75:187–193. doi:10.1016/j.ando.2014.01.002
18. Shi J, Gong L, Chen L, et al. miR-618 suppresses metastasis in gastric cancer by downregulating the expression of TGF-β2. Anat Rec (Hoboken). 2019;302:931–940. doi:10.1002/ar.24083
19. Ding L, Ding Y, Kong X, et al. Dysregulation of krüppel-like factor 12 in the development of endometrial cancer. Gynecol Oncol. 2019;152:177–184. doi:10.1016/j.ygyno.2018.10.028
20. Dong MM, Peng SJ, Yuan YN, et al. LncRNA TTN-AS1 contributes to gastric cancer progression by acting as a competing endogenous RNA of miR-376b-3p. Neoplasma. 2019;66:564–575. doi:10.4149/neo_2018_180927N721
21. Martini M, De Santis MC, Braccini L, et al. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46:372–383. doi:10.3109/07853890.2014.912836
22. Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi:10.1038/nature12986
23. Liu M, Jia J, Wang X, et al. Long non-coding RNA HOTAIR promotes cervical cancer progression through regulating BCL2 via targeting miR-143-3p. Cancer Biol Ther. 2018;19:391–399. doi:10.1080/15384047.2018.1423921
24. Lu X, Liu Z, Ning X, et al. The long noncoding RNA HOTAIR promotes colorectal cancer progression by sponging miR-197. Oncol Res. 2018;26:473–481. doi:10.3727/096504017X15105708598531
25. Dong X, He X, Guan A, et al. Long non-coding RNA hotair promotes gastric cancer progression via miR-217-GPC5 axis. Life Sci. 2019;217:271–282. doi:10.1016/j.lfs.2018.12.024
26. Vara JÁF, Casado E, de Castro J, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi:10.1016/j.ctrv.2003.07.007
27. Yi L, Yuan Y. MicroRNA-618 modulates cell growth via targeting PI3K/Akt pathway in human thyroid carcinomas. Indian J Cancer. 2015;52:186. doi:10.4103/0019-509X.186577
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.