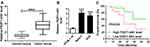Back to Journals » OncoTargets and Therapy » Volume 13
Long Non-Coding RNA FEZF1-AS1 Modulates CXCR4 to Promote Cell Proliferation, Warburg Effect and Suppress Cell Apoptosis in Osteosarcoma by Sponging miR-144
Authors Liu J, Feng G, Li Z, Li R, Xia P
Received 24 October 2019
Accepted for publication 10 January 2020
Published 5 April 2020 Volume 2020:13 Pages 2899—2910
DOI https://doi.org/10.2147/OTT.S235970
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Jun Liu,1 Guang Feng,2 Zhengwei Li,3 Rui Li,1 Peng Xia1
1Department of Hand Surgery, The Second Hospital of Jilin University, Changchun, People’s Republic of China; 2The Fourth Medical Center of PLA General Hospital, Beijing, People’s Republic of China; 3Department of Orthopaedics, The Second Hospital of Jilin University, Changchun, People’s Republic of China
Correspondence: Peng Xia
Department of Orthopaedics, The Second Hospital of Jilin University, No. 218 ZiQiang Street, NanGuan District, Changchun 130000, Jilin Province, People’s Republic of China
Tel +86 13514497595
Email [email protected]
Background: Osteosarcoma (OS) is a common bone tumor among children, adolescents, and young adults. Long non-coding RNA (lncRNA) FEZF1 antisense RNA 1 (FEZF1-AS1) has been reported as an oncogene in diverse tumors including colorectal cancer, pancreatic cancer and hepatocellular carcinoma, as well as in osteosarcoma. This study focused on the functions and mechanism of lncRNA FEZF1-AS1 in osteosarcoma.
Methods: The levels of FEZF1-AS1, microRNA miR-144 and CXC motif chemokine receptor 4 (CXCR4) in OS tissues and cells (Saos-2 and HOS) were measured by quantitative real-time polymerase chain reaction (qRT-PCR) or Western blot assay. The interactions between miR-144 and FEZF1-AS1 or CXCR4 were predicted by DIANA tools online database. Then, the dual-luciferase reporter assay and RNA immunoprecipitation (RIP) assay were conducted to validate the interactions. Moreover, the cell viability and apoptotic rate in transferred Saos-2 and HOS cells were assessed via 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay and flow cytometry, respectively. The levels of glucose and lactate productions were measured by glucose uptake and lactate production assay. In addition, the protein levels of Warburg-effect-related protein hexokinase 2 (HK2) and apoptosis-related proteins Bcl-2 or Bax in transferred Saos-2 and HOS cells were detected via Western blot assay.
Results: The levels of FEZF1-AS1 and CXCR4 were strikingly up-regulated, and miR-144 was notably down-regulated in OS tissues and cells. DIANA tools online database exhibited that miR-144 was a direct target of FEZF1-AS1 and CXCR4 was a direct target of miR-144. Then the interactions were validated by dual-luciferase reporter assay and RIP assay. Functionally, FEZF1-AS1 silencing or miR-144 overexpression inhibited cell viability, the glucose and lactate productions and promoted cell apoptosis in Saos-2 and HOS cells. Furthermore, miR-144 inhibitor mitigated the inhibitory effects on cell viability, the glucose and lactate productions and the promoted effect on cell apoptosis rate in Saos-2 and HOS cells induced by FEZF1-AS1 depletion. Mechanistically, FEZF1-AS1 regulated CXCR4 in Saos-2 and HOS cells by sponging miR-144.
Conclusion: We verified that FEZF1-AS1, CXCR4 were up-regulated, and miR-144 was downregulated in OS tissues and cells. Furthermore, FEZF1-AS1 promoted cell proliferation, Warburg effect and suppressed cell apoptosis in osteosarcoma via miR-144/CXCR4 axis, this novel pathway may provide a basis for the further study of osteosarcoma.
Keywords: lncRNA FEZF1-AS1, miR-144, CXCR4, Warburg effect, osteosarcoma
Introduction
Osteosarcoma (OS), which mainly involves long tubular bone, is a common primary bone tumor among children, adolescents, and young adults.1 Though there are many improvements in the treatment of OS patients, such as surgery, radiotherapy or chemotherapy, the 5-year survival rate of patients with advanced OS was only 30–40%, and OS patients still have the risk of relapse and cancer metastasis.2–5 However, the mechanism of OS progression remains unclear.
Warburg effect is a phenomenon that cancer cells mainly relied on aerobic glycolysis to generate the energy needed for cellular processes, while the normal cells depended on mitochondrial oxidative phosphorylation. Synchronously, relevant research has showed that cancer cells change their metabolic way to meet the high growth rate for energy, which may provide new insight into the process of adaptation of cancer cells.6 Accelerated glucose transport, aerobic glycolysis and lactate production were the main characteristics of Warburg effect.7 Warburg effect was reported in many cancers, such as breast cancer,8 ovarian cancer,9 lung cancer,10 and OS.11
Long non-coding RNAs (lncRNAs), a class of non-coding RNAs with >200 nucleotides (nts) in length, have been reported to function as competing endogenous RNAs (ceRNAs) to regulate the expression of miRNAs, and further affect the deposition of target protein.12 Furthermore, dysregulation of lncRNAs has been reported in diverse cancers including OS. For instance, previous studies indicated that lncRNA MALAT1,13 SNHG1,14 and HOST215 were significantly up-regulated in OS tissues and cells. Notably, lncRNA FEZF1-AS1 was also reported to regulate tumor progression in various cancers, such as ovarian cancer,16 pancreatic cancer,17 and OS.18 In addition, lncRNA FEZF1-AS1 was documented to participate in Warburg effect in colorectal cancer19 and pancreatic ductal adenocarcinoma.20 However, the biological mechanism of FEZF1-AS1 was rarely reported in OS.
MicroRNAs (miRNAs), a class of non-coding RNAs with about 18–23 nts in length, can suppress target gene expression by inhibiting message RNAs (mRNAs) translation or by mediating the degradation of mRNAs.21 Moreover, some studies confirmed that the aberrant expression of miRNAs was closely associated with OS progression. For example, miR-211-5p,22 miR-885-5p,23 and miR-142-5p24 were markedly decreased in OS tissues and cells and acted as tumor suppressors by repressing cell proliferation, migration, invasion, as well as promoting cell apoptosis in OS development. Intriguingly, prior reports showed that miR-144 could hinder OS growth and metastasis by the target genes, such as ROCK125 TAGLN26 and EZH2,27 suggesting the vital role of miR-144 in OS development.
CXC motif chemokine receptor 4 (CXCR4), a 352-amino acid rhodopsin-like transmembrane G protein-coupled cell surface receptors, is a crucial mediator in tumor progression in many cancers.28 Also, accumulating evidence validated that CXCR4 overexpression promoted tumor progression in OS.29,30 However, the biological mechanism of miR-144 and CXCR4 in OS is still unknown.
In this study, we found that FEZF1-AS1 was increased in OS tissues and cells, and the knockdown of FEZF1-AS1 suppressed growth and Warburg effect. Moreover, bioinformatics analysis discovered that FEZF1-AS1 had some complementary pairing with miR-144, and CXCR4 was an underlying target gene of miR-144 in OS cells. Therefore, we aimed to explore whether FEZF1-AS1 could regulate OS progression partly through the miR-144/CXCR4 axis.
Materials and Methods
Tissue Sample
The study was permitted by the Ethics Committee of The Second Hospital of Jilin University, and performed according to the Declaration of Helsinki Principles. Forty-two OS tissues and these corresponding adjacent normal tissues were obtained from The Second Hospital of Jilin University. All tissues were frozen at −80°C for the following experiments. Written informed consent was provided by all OS patients or their guardians.
Cell Culture
Two human osteosarcoma cell lines Saos-2 (HTB-85) and HOS (CRL-1543), as well as human osteoblast cell line hFOB 1.19 (CRL-11372) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were cultivated in RPMI‑1640 medium (Thermo Fisher Scientific, Rockville, MD, USA) containing 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) in an incubator with the parameters of 37°C and 5% CO2.
Cell Transfection
Small interference RNA (siRNA) targeting FEZF1-AS1 (si-FEZF1-AS1) and its control si-NC, FEZF1-AS1 overexpression vector (FEZF1-AS1) and empty vector (pcDNA), miR-144 mimics (miR-144) and its control (miR-NC), miR-144 inhibitor (anti-144) and its control (anti-NC) were bought from GenePharma (Shanghai, China). Transfection was performed by using Lipofectamine 2000 Reagent (Invitrogen) according to the manual.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA from Saos-2, HOS and hFOB 1.19 cells were extracted using miRNeasy Mini Kit (Qiagen, Valencia, CA, USA). Then, the concentration of RNA samples was detected using NanoDrop 2000c (Thermo Fisher Scientific), and cDNA was synthesized using miScript RT Kit (TaKaRa, Dalian, China). QPCR was conducted using SYBR Premix Ex Taq II (TaKaRa) on ABI Prism 7700 Sequence Detection System (Thermo Fisher Scientific). The relative expression of FEZF1-AS1, miR-144 and CXCR4 was calculated by the 2−ΔΔCt method. The primers were synthesized in RiBoBio (Guangzhou, China), and list as follows: FEZF1-AS1: (Forward, 5ʹ-TTAGGAGGCTTGTTCTGTGT-3ʹ, Reverse, 5ʹ-GCGCAGGTACTTAAGAAAGA-3ʹ); miR-144: (Forward, 5ʹ -TACAGTATAGATGAT-3ʹ, Reverse, 5ʹ-GTGCAGGGTCCGAGGT-3ʹ); CXCR4: (Forward, 5ʹ-TCAGTGGCTGACCTCCTCTT-3ʹ, Reverse, 5ʹ-CTTGGCCTTTGACTGTTGGT-3ʹ); GAPDH: (Forward, 5ʹ-TGTTCGTCATGGGTGTGAAC-3ʹ, Reverse, 5ʹ-ATGGCATGGACTGTGGTCAT-3ʹ) and U6: (Forward, 5ʹ-CTCGCTTCGGCAGCACA-3ʹ, Reverse, 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ).
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium Bromide (MTT) assay
For cell proliferation assay, MTT (Sigma, St. Louis, MO, USA) was used to detect the proliferative capacity. In brief, cells (2×104 per well) were injected into 96-well plates and cultivated until the cell confluency rate reached 80%. After transfection for 48 h, the cells were incubated for 0 h, 24 h, 48 h, and 72 hrs. Then, 20 μL of 5 mg/mL MTT solution (Sigma) was added into each well and incubated for another 4 hrs. Subsequently, dimethyl sulfoxide (DMSO, Sigma) was added into each well to dissolve the formazan. The colorimetric analysis at 490 nm was detected using a spectrophotometer (Thermo Fisher Scientific).
Glucose Uptake and Lactate Production Assay
The levels of glucose and lactate were measured using Glucose Uptake Colorimetric Assay Kit (Sigma) and Lactic Acid Assay Kit (Jiancheng, China) in accordance with the manual, respectively.
Western Blot Assay
Total protein from cells was extracted using a protein extraction kit (Beyotime, Shanghai, China), and the concentration of protein was measured using BCA Protein Assay Kit (Beyotime). The protein sample was first separated by SDS-PAGE and then transferred onto a PVDF membrane (Millipore, Billerica, MA, USA). After being blocked in non-fat milk for 2 hrs, then the membrane was incubated in primary antibody HK2 (ab104836), Bcl-2 (ab194583), Bax (ab32503), CXCR4 (ab74012) or β-actin (ab8226) at 4°C overnight, followed by incubation with secondary antibodies for another 2hrs. The chemiluminescence intensity analysis was detected using eyoECL Plus Kit (Beyotime). Above antibodies were purchased from Abcam (Cambridge, MA, USA).
Cell Apoptosis Assay
Fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit (BD Biosciences, USA) was used to assess the cell apoptosis rate. In brief, the cultivated cells were suspended in binding buffer and then incubated with Annexin V-FITC and propidium iodide (PI) for 10mins in the dark. Then, the treated cells were analyzed by flow cytometry (BD Biosciences).
Dual-Luciferase Reporter Assay
The putative binding sites between miR-144 and FEZF1-AS1 or CXCR4 were predicted by DIANA tools (http://diana.imis.athena-innovation.gr). The sequences of FEZF1-AS1, CXCR4 3ʹUTR and their negative mutants were cloned and then inserted into psiCHECK2 vector (Promega, Madison, WI, USA) to synthesize luciferase reporter plasmids, namely FEZF1-AS1-WT, FEZF1-AS1-MUT, CXCR4-WT or CXCR4-MUT. In Saos-2 and HOS cells, miR-144 mimics (miR-NC) and the luciferase reporter were co-transfected using Lipofectamine 2000. Following incubation for 48 hrs, Dual-Luciferase® Reporter Assay system (Promega) was used to detect the luciferase activity.
RNA Immunoprecipitation (RIP) Assay
For RIP assay, EZ-Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore) was used to enrich the fragment of FEZF1-AS1 or CXCR4. The lyase sample of cells transfected with miR-144 or miR-NC was incubated with magnetic beads conjugated with anti-Ago2 or IgG antibody. Then, the enrichment of FEZF1-AS1 or CXCR4 was analyzed by qRT-PCR assay.
Statistical Analysis
All data were presented as the means ± standard deviation (SD) from at least three independent experiments using GraphPad Prism 7 (GraphPad Inc., San Diego, CA, USA). The comparisons between the two groups were analyzed by Student’s t-test and differences among three or more groups were analyzed by one-way analysis of variance (ANOVA). P< 0.05 was considered as statistically significant.
Results
FEZF1-AS1 Was Up-Regulated in OS Tissues and Cells and Correlated with the Pathological Characteristics of OS Patients
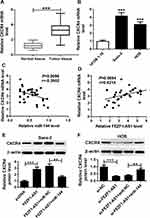
To explore the role of FEZF1-AS1 on OS, the qRT-PCR assay was used to detect the relative expression of FEZF1-AS1 in OS tissues and cells. The results exhibited that FEZF1-AS1 was apparently up-regulated in OS tissues and cells (Saos-2 and HOS) compared with that in corresponding adjacent normal tissues or human osteoblasts cells (hFOB 1.19) (Figure 1A and B). Moreover, the OS patients were divided into low group (n=18) and high group (n=24) by the median expression of FEZF1-AS1. The patients with high level of FEZF1-AS1 had lower survival rates, while low level of FEZF1-AS1 was associated with higher survival rates (Figure 1C). In addition, the chi-squared test presented that highly expressed FEZF1-AS1 was closely associated with clinical stage (p=0.0069), tumor size (p=0.0369) but uncorrelated with ages, gender and anatomic location (Table 1). These data implicated that the level of FEZF1-AS1 was involved in the progression of OS.
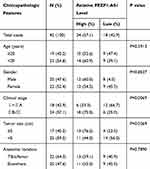 |
Table 1 Association of FEZF1-AS1 Expression with Clinicopathological Features of Osteosarcoma |
FEZF1-AS1 Knockdown Inhibited Cell Proliferation, Warburg Effect and Promoted Cell Apoptosis in Saos-2 and HOS Cells
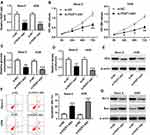
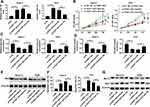
To investigate the function of FEZF1-AS1 in OS, FEZF1-AS1 was knocked down in this study. The qRT-PCR results confirmed the knockdown efficiency, demonstrated by the down-regulation of FEZF1-AS1 in Saos-2 and HOS cells (Figure 2A). Moreover, MTT assay, glucose uptake and lactate production assay results showed that FEZF1-AS1 silencing suppressed cell viability and the levels of glucose, lactate in Saos-2 and HOS cells (Figure 2B–D). As HK2 was the Warburg-effect-related protein,31 the Western blot assay exhibited that the protein level of HK2 was decreased in Saos-2 and HOS cells transfected with si-FEZF1-AS1 (Figure 2E). In addition, the flow cytometry and Western blot assay showed that the apoptotic rate and the protein level of pro-apoptosis factor Bax were apparently up-regulated and the protein level of apoptosis-inhibited protein Bcl-2 was dramatically down-regulated in Saos-2 and HOS cells transfected with si-FEZF1-AS1 (Figure 2F and G). Taken together, the depletion of FEZF1-AS1 restrained cell proliferation, Warburg effect, and induced cell apoptosis in Saos-2 and HOS cells.
miR-144 Was Negatively Interacted with FEZF1-AS1
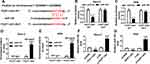
To explore the biological mechanism of FEZF1-AS1 in OS, DIANA tools was used to search the putative target of FEZF1-AS1. The result showed that miR-144 had complementary sequences with FEZF1-AS1 (Figure 3A). Following luciferase reporter assay implicated that the transfection of miR-144 contributed to the significant decrease of luciferase activity of FEZF1-AS1-WT reporter in Saos-2 and HOS cells, while the luciferase activity had no apparent change in miR-NC group; however, the luciferase activity of FEZF1-AS1-MUT reporter had no significant fluctuation in any group (Figure 3B and C). Also, the RIP assay indicated that FEZF1-AS1 was strikingly enriched by Ago2 antibody in Saos-2 and HOS cells transfected with miR-144 mimics in comparison with IgG control group (Figure 3D and E). In addition, the level of miR-144 was remarkably decreased in Saos-2 and HOS cells transfected with pcDNA-FEZF1-AS1, while the level of miR-144 showed the opposite trend in cells transfected with si-FEZF1-AS1 (Figure 3F and G). These data revealed that miR-144 was negatively interacted with FEZF1-AS1.
miR-144 Was Distinctly Reduced in OS Tissues and Cells, and miR-144 Overexpression Suppresses Cell Proliferation, Warburg Effect and Promotes Cell Apoptosis in Saos-2 and HOS Cells
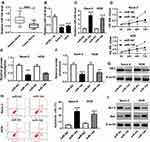
To investigate the functions of miR-144 in OS, miR-144 mimics were transfected into Saos-2 and HOS cells. First, the level of miR-144 was measured in OS. The qRT-PCR results showed that the level of miR-144 was markedly reduced in OS tissues and cells (Figure 4A and B). Moreover, qRT-PCR results exhibited the transfection efficiency, presented as the dramatical up-regulation of miR-144 in Saos-2 and HOS cells (Figure 4C). Furthermore, MTT assay exhibited that miR-144 overexpression suppressed cell viability of Saos-2 and HOS cells (Figure 4D). Also, the glucose uptake and lactate production assay results showed that miR-144 upregulation constrained cell viability and the levels of glucose, lactate in Saos-2 and HOS cells (Figure 4E and F). Meanwhile, the Western blot assay results indicated that the protein level of HK2 was decreased in Saos-2 and HOS cells transfected with miR-144 mimics (Figure 4G). In addition, the flow cytometry and Western blot assay implied that the transfection of miR-144 mimics resulted in the notable increase of apoptotic rate, the protein level of Bax and the decrease of the protein level of Bcl-2 in Saos-2 and HOS cells (Figure 4H and I). These results demonstrated that the level of miR-144 was conspicuously down-regulated in OS tissues, cells, and miR-144 overexpression restrained cell proliferation, Warburg effect and promoted cell apoptosis in Saos-2 and HOS cells.
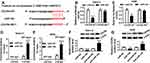
miR-144 Inhibitor Attenuated the Suppressive Effects on Cell Proliferation, Warburg Effect and the Promoted Effect on Cell Apoptosis in Saos-2 and HOS Cells Induced by FEZF1-AS1 Silencing
To investigate the biological mechanism and functions of FEZF1-AS1 and miR-144 in OS, si-FEZF1-AS1 and anti-144 were co-transfected into Saos-2 and HOS cells. The qRT-PCR results showed that the level of miR-144 was dramatically up-regulated in si-FEZF1-AS1-transfected Saos-2 and HOS cells, while anti-144 reversed the promotion effect (Figure 5A). Subsequently, the MTT assay indicated that miR-144 inhibitor mitigated the inhibitory effects on cell viability in Saos-2 and HOS cells transfected with si-FEZF1-AS1 (Figure 5B). Also, the glucose uptake and lactate production assay implicated that anti-144 receded the inhibitory effects on the levels of glucose and lactate in si-FEZF1-AS1-transfected Saos-2 and HOS cells (Figure 5C and D). Furthermore, Western blot assay exhibited that the protein level of HK2 was reverted in Saos-2 and HOS cells co-transfected with si-FEZF1-AS1 and anti-144 (Figure 5E). In addition, the Western blot and flow cytometry presented that anti-144 alleviated the promotion effects on cell apoptosis and the level of Bax, as well as the inhibitory effect on Bcl-2 expression level in Saos-2 and HOS cells caused by si-FEZF1-AS1 (Figure 5F and G). These results unraveled that miR-144 inhibitor blocked the suppressive effects on cell proliferation, Warburg effect and the promotion effect on cell apoptosis in Saos-2 and HOS cells induced by FEZF1-AS1 silencing.
CXCR4 Was a Direct Target of miR-144
To illustrate the biological mechanism of miR-144 in OS, DIANA tools was used to predict the putative target of miR-144. The results exhibited that CXCR4 3ʹUTR had complementary binding site with miR-144 (Figure 6A). Dual-luciferase reporter assay indicated that the luciferase activity of CXCR4-WT reporter was strikingly decreased in Saos-2 and HOS cells transfected with miR-144 mimics compared to that in miR-NC group, while the luciferase activity of CXCR4-MUT reporter had no distinct change (Figure 6B and C). Following RIP assay also indicated that the transfection of CXCR4 drastically enriched miR-144 by Ago2 antibody than IgG antibody in Saos-2 and HOS cells (Figure 6D and E). In addition, Western blot assay results showed that the protein level of CXCR4 was apparently decreased in Saos-2 and HOS cells transfected with miR-144 mimics, while elevated in cells transfected with anti-miR-144 (Figure 6F and G). To sum, CXCR4 was negatively interacted with miR-144.
FEZF1-AS1 Silencing Down-Regulated CXCR4 in Saos-2 and HOS Cells by Sponging miR-144
Firstly, the qRT-PCR results showed that the level of CXCR4 was notably upregulated in OS tissues and cells (Figure 7A and B). The scatter diagram presented that the level of CXVR4 was negatively linear correlated with miR-144, and positively linear correlated with FEZF1-AS1 (Figure 7C and D). To explore the interactions among FEZF1-AS1, miR-144 and CACR4 in OS, CXCR4 levels were explored in Saos-2, and HOS cells co-transfected FEZF1-AS1 and miR-144. Western blot assay implicated that the protein level of CXCR4 was obviously elevated in Saos-2 cells transfected with FEZF1-AS1, while the promoted impact was mitigated by miR-144 mimics in Saos-2 cells (Figure 7E). Also, the protein level of CXCR4 was conspicuously reduced in si-FEZF1-AS1 transfected HOS cells, while the transfection of anti-144 reversed the promotion effect (Figure 7F). Taken together, these data disclosed that FEZF1-AS1 modulated CXCR4 in Saos-2 and HOS cells by sponging miR-144.
Discussion
Osteosarcoma (OS) is a common bone malignant tumor among children, adolescents, and young adults. Convincing evidence indicated that lncRNAs play crucial roles in the progression of various cancers. In this program, we focused on the mechanism and functions of lncRNA FEZF1-AS1 in OS. The results unraveled that lncRNA FEZF1-AS1 promoted OS progression partially through up-regulating CXCR4 expression by sponging miR-144.
Accumulating evidence documented that dysregulation of lncRNAFEZF1-AS1 associated with many processes in tumor progression, including OS. For instance, previous studies indicated that FEZF1-AS1 was notably up-regulated, and FEZF1-AS1 knockdown inhibited proliferation, invasion, epithelial-mesenchymal transition (EMT), and induced apoptosis in multiple cancers, such as colorectal cancer,19 ovarian cancer,16 pancreatic cancer,17 gastric cancer,32 and hepatocellular carcinoma.33 Also, lncRNA FEZF1-AS1 has been found to be associated with Warburg effect in many cancers including colorectal cancer,19 pancreatic ductal adenocarcinoma.20 In this study, we verified that lncRNA FEZF1-AS1 was highly expressed in OS tissues and cells (Saos-2 and HOS), compared with previous studies.18 Meanwhile, the chi-squared test indicated that highly expressed FEZF1-AS1 was closely correlated with the pathological characteristics of OS patients, such as clinical stage, tumor size. Furthermore, functional experiment implicated that FEZF1-AS1 silencing repressed cell proliferation, Warburg effect and induced cell apoptosis in Saos-2 and HOS cells. In addition, the patients with high level of FEZF1-AS1 had low overall survival rate compared to that in low level of FEZF1-AS1 group. To sum, lncRNA FEZF1-AS1 plays vital roles in OS progression.
It is well documented that the aberrant expression of miR-144 takes part in the tumor progression in various cancers, including OS. Yin et al demonstrated that miR-144 expression was apparently reduced in breast cancer, and miR-144 overexpression blocked cell proliferation, migration and invasion, as well as promoted cell cycle arrest and cell apoptosis.34 Another study reported that miR-144 could repress the malignancy of cervical cancer by inhibiting cell proliferation and invasion.35 In the present study, miR-144 was predicted as the putative target of FEZF1-AS1, and following dual-luciferase reporter assay and RIP assay validated this interaction. Subsequently, we validated that miR-144 was strikingly down-regulated in OS tissues and cells.26,27,36,37 Functionally, miR-144 overexpression restrained cell proliferation, Warburg effect and induced cell apoptosis in Saos-2 and HOS cells. Furthermore, FEZF1-AS1 depletion inhibited cell proliferation, Warburg effect and induced cell apoptosis by sponging miR-144. These data revealed that FEZF1-AS1 regulated OS progression by sponging miR-144.
Emerging evidence reported that CXCR4 played an important role in tumor progression, including OS. Actually, previous study showed that CXCR4 overexpression promoted tumor progression in OS.29,30 In this study, the interaction between miR-144 and CXCR4 was first predicted by DIANA tools and then verified by dual-luciferase reporter assay and RIP assay. CXCR4 was found to be apparently increased in OS tissues and cells. In addition, CXCR4 was negatively correlated with miR-144 and positively correlated with FEZF1-AS1. Furthermore, the restoration experiment implied that FEZF1-AS1 overexpression up-regulated the level of CXCR4 in Saos-2 cells by targeting miR-144. Rescue assay proved that FEZF1-AS1 silencing decreased the expression of CXCR4 via sponging miR-144 in HOS cells. To sum, lncRNA FEZF1-AS1 regulated OS progression via miR-144/CXCR4 axis.
In summary, lncRNA FEZF1-AS1, CXCR4 was dramatically up-regulated, and miR-144 was significantly down-regulated in OS tissues and cells. FEZF1-AS1 silencing or miR-144 overexpression impeded cell proliferation, Warburg effect and induced cell apoptosis in Saos-2 and HOS cells. Moreover, lncRNA FEZF1-AS1 served as a sponge of miR-144 to upregulate CXCR4 expression, thereby promoting OS progression, providing an underlying therapeutic target for OS treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Anderson ME. Update on survival in osteosarcoma. Orthop Clin. 2016;47(1):283–292. doi:10.1016/j.ocl.2015.08.022
2. Allison DC, Carney SC, Ahlmann ER, et al. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. 2012;2012.
3. Link MP, Goorin AM, Horowitz M, et al. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the multi-institutional osteosarcoma study. Clin Orthop Relat Res. 1991;270:8–14.
4. Ferrari S, Serra M. An update on chemotherapy for osteosarcoma. Expert Opin Pharmacother. 2015;16(18):2727–2736. doi:10.1517/14656566.2015.1102226
5. Briccoli A, Rocca M, Salone M, et al. High grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985–2005. Surg Oncol. 2010;19(4):193–199. doi:10.1016/j.suronc.2009.05.002
6. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. science. 2009;324(5930):1029–1033. doi:10.1126/science.1160809
7. Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015;34(1):111. doi:10.1186/s13046-015-0221-y
8. Yao L, Wang L, Cao Z-G, et al. High expression of metabolic enzyme PFKFB4 is associated with poor prognosis of operable breast cancer. Cancer Cell Int. 2019;19(1):165. doi:10.1186/s12935-019-0882-2
9. Zhang S, Pei M, Li Z, et al. Double‐negative feedback interaction between DNA methyltransferase 3A and microRNA‐145 in the Warburg effect of ovarian cancer cells. Cancer Sci. 2018;109(9):2734. doi:10.1111/cas.13734
10. Li J, Zhang J, Xie F, et al. Macrophage migration inhibitory factor promotes Warburg effect via activation of the NF-κB/HIF-1α pathway in lung cancer. Int J Mol Med. 2018;41(2):1062–1068. doi:10.3892/ijmm.2017.3277
11. Song J, Wu X, Liu F, et al. Long non-coding RNA PVT1 promotes glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Biochem Biophys Res Commun. 2017;490(2):217–224. doi:10.1016/j.bbrc.2017.06.024
12. Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–1109. doi:10.1016/j.bbagrm.2014.08.012
13. Duan G, Zhang C, Xu C, et al. Knockdown of MALAT1 inhibits osteosarcoma progression via regulating the miR‑34a/cyclin D1 axis. Int J Oncol. 2019;54(1):17–28. doi:10.3892/ijo.2018.4600
14. Jiang Z, Jiang C, Fang J. Up-regulated lnc-SNHG1 contributes to osteosarcoma progression through sequestration of miR-577 and activation of WNT2B/Wnt/β-catenin pathway. Biochem Biophys Res Commun. 2018;495(1):238–245. doi:10.1016/j.bbrc.2017.11.012
15. Wang W, Li X, Meng F-B, et al. Effects of the long non-coding RNA HOST2 on the proliferation, migration, invasion and apoptosis of human osteosarcoma cells. Cell Physiol Biochem. 2017;43(1):320–330. doi:10.1159/000480412
16. Zhao X, Cheng Z, Wang J. Long noncoding RNA FEZF1-AS1 promotes proliferation and inhibits apoptosis in ovarian cancer by activation of JAK-STAT3 pathway. Med Sci Monit. 2018;24:8088. doi:10.12659/MSM.911194
17. Ou ZL, Zhang M, Ji LD, et al. Long noncoding RNA FEZF1‐AS1 predicts poor prognosis and modulates pancreatic cancer cell proliferation and invasion through miR‐142/HIF‐1α and miR‐133a/EGFR upon hypoxia/normoxia. J Cell Physiol. 2019. doi:10.1002/jcp.28188
18. Zhou C, Xu J, Lin J, et al. Long noncoding RNA FEZF1-AS1 promotes osteosarcoma progression by regulating the miR-4443/NUPR1 axis. Oncol Res Featuring Preclinical Clin Cancer Ther. 2018;26(9):1335–1343.
19. Bian Z, Zhang J, Li M, et al. LncRNA–FEZF1-AS1 promotes tumor proliferation and metastasis in colorectal cancer by regulating PKM2 signaling. Clin Cancer Res. 2018;24(19):4808–4819. doi:10.1158/1078-0432.CCR-17-2967
20. Ye H, Zhou Q, Zheng S, et al. FEZF1-AS1/miR-107/ZNF312B axis facilitates progression and Warburg effect in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9(2):34. doi:10.1038/s41419-017-0052-1
21. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–874. doi:10.1038/nrg3074
22. Song D, Yang K, Wang W, et al. MicroRNA-211-5p promotes the apoptosis and inhibits the migration of osteosarcoma cells by targeting proline-rich protein PRR11. Biochem Cell Biol. 2019. doi:10.1139/bcb-2018-0380
23. Liu Y, Bao Z, Tian W, et al. miR‑885‑5p suppresses osteosarcoma proliferation, migration and invasion through regulation of β‑catenin. Oncol Lett. 2019;17(2):1996–2004. doi:10.3892/ol.2018.9768
24. Cheng D, Li J, Zhang L, et al. miR-142-5p suppresses proliferation and promotes apoptosis of human osteosarcoma cell line, HOS, by targeting PLA2G16 through the ERK1/2 signaling pathway. Oncol Lett. 2019;17(1):1363–1371. doi:10.3892/ol.2018.9712
25. Huang J, Shi Y, Li H, Yang M, Liu G. MicroRNA-144 acts as a tumor suppressor by targeting Rho-associated coiled-coil containing protein kinase 1 in osteosarcoma cells. Mol Med Rep. 2015;12(3):4554–4559. doi:10.3892/mmr.2015.3937
26. Zhao M, Huang J, Gui K, et al. The downregulation of miR-144 is associated with the growth and invasion of osteosarcoma cells through the regulation of TAGLN expression. Int J Mol Med. 2014;34(6):1565–1572. doi:10.3892/ijmm.2014.1963
27. Cao J, Han X, Qi X, Jin X, Li X. TUG1 promotes osteosarcoma tumorigenesis by upregulating EZH2 expression via miR-144-3p. Int J Oncol. 2017;51(4):1115–1123. doi:10.3892/ijo.2017.4110
28. Fredriksson R, Lagerström MC, Lundin L-G, et al. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63(6):1256–1272. doi:10.1124/mol.63.6.1256
29. Zhu Y, Tang L, Zhao S, et al. CXCR 4‐mediated osteosarcoma growth and pulmonary metastasis is suppressed by Micro RNA‐613. Cancer Sci. 2018;109(8):2412–2422. doi:10.1111/cas.2018.109.issue-8
30. Zhu G, Liu X, Su Y, et al. Knockdown of urothelial carcinoma-associated 1 suppressed cell growth and migration through regulating miR-301a and CXCR4 in osteosarcoma MHCC97 cells. Oncol Res Featuring Preclinical Clin Cancer Ther. 2018;27(1):55–64. doi:10.3727/096504018X15201143705855
31. Wolf A, Agnihotri S, Micallef J, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208(2):313–326. doi:10.1084/jem.20101470
32. Liu Y-W, Xia R, Lu K, et al. LincRNA FEZF1-AS1 represses p21 expression to promote gastric cancer proliferation through LSD1-mediated H3K4me2 demethylation. Mol Cancer. 2017;16(1):39. doi:10.1186/s12943-017-0588-9
33. Wang Y-D, Sun X-J, Yin -J-J, et al. Long non-coding RNA FEZF1-AS1 promotes cell invasion and epithelial-mesenchymal transition through JAK2/STAT3 signaling pathway in human hepatocellular carcinoma. Biomed Pharmacother. 2018;106:134–141. doi:10.1016/j.biopha.2018.05.116
34. Yin Y, Cai J, Meng F, et al. MiR-144 suppresses proliferation, invasion, and migration of breast cancer cells through inhibiting CEP55. Cancer Biol Ther. 2018;19(4):306–315. doi:10.1080/15384047.2017.1416934
35. Zhu Y, Wu Y, Yang L, et al. Long non‐coding RNA activated by transforming growth factor‐β promotes proliferation and invasion of cervical cancer cells by regulating the miR‐144/ITGA6 axis. Exp Physiol. 2019. doi:10.1113/EP087656
36. Liu JL, Li J, Xu JJ, et al. MiR-144 inhibits tumor growth and metastasis in osteosarcoma via dual-suppressing RhoA/ROCK1 signaling pathway. Mol Pharmacol. 2019;95(4):451–461. doi:10.1124/mol.118.114207
37. Ren YF, Zhang TH, Zhong S, et al. miR-144 suppresses proliferation and induces apoptosis of osteosarcoma cells via direct regulation of mTOR expression. Oncol Lett. 2018;15(1):1163–1169. doi:10.3892/ol.2017.7364
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.