Back to Journals » Cancer Management and Research » Volume 12
Long Non-coding RNA BLACAT1 Induces Tamoxifen Resistance in Human Breast Cancer by Regulating miR-503/Bcl-2 Axis
Authors Qu R, Hu C, Tang Y, Yu Q, Shi G
Received 25 November 2019
Accepted for publication 31 January 2020
Published 10 March 2020 Volume 2020:12 Pages 1771—1777
DOI https://doi.org/10.2147/CMAR.S239981
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
This paper has been Retracted.
Rongfeng Qu,* Chunmei Hu,* Yan Tang, Qiong Yu, Guang Shi
Department of Hematology and Oncology, The Second Hospital of Jilin University, Changchun 130041, Jilin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guang Shi
Department of Hematology and Oncology, The Second Hospital of Jilin University, No. 218 Ziqiang Street, Changchun 130041, Jilin, People’s Republic of China
Email [email protected]
Introduction: At present, drug resistance remains a major obstacle for breast cancer (BCa) patients who receive tamoxifen (TAM) chemotherapy. In this study, we aimed to investigate the functional role of long non-coding RNA BLACAT1 in the acquisition of TAM resistance in BCa.
Methods: TAM-resistant BCa cells were derived by exposure to 1 μM of TAM for 6 months. The expression levels of BLACAT1 and miR-503 were detected by RT-qPCR analysis. Chemosensitivity of BCa cells to TAM was measured by MTT assay. Apoptosis of BCa cells was detected by flow cytometric analysis, and the expression levels of apoptosis-related proteins were detected by Western blot analysis. The direct binding relation between BLACAT1 and miR-503 was predicted by bioinformatics analysis and verified by dual-luciferase reporter assay.
Results: Our findings showed that BLACAT1 was significantly upregulated in TAM-resistant BCa cells (MCF-7/TR and T47D/TR), and BLACAT1 knockdown markedly reduced the TAM resistance in these cells. Importantly, we observed that BLACAT1 might function as a competing endogenous RNA of miR-503 in MCF-7/TR and T47D/TR cells, thereby increasing the expression of oncogenic Bcl-2 protein. Rescue experiments showed that miR-503 inhibition partly blocked the inhibitory effect of BLACAT1 knockdown on TAM resistance of MCF-7/TR and T47D/TR cells.
Conclusion: To conclude, this study revealed that overexpressed BLACAT1 induces TAM resistance in human BCa partly by regulating miR-503/Bcl-2 axis, potentially benefiting BCa treatment in the future.
Keywords: breast cancer, tamoxifen resistance, long non-coding RNA BLACAT1, miR-503, Bcl-2
Introduction
Breast cancer (BCa) is the second leading cause of cancer-related death among women worldwide, and around two-thirds of all BCa cases are estrogen receptor positive (ER+).1,2 Tamoxifen (TAM) is an estrogen antagonist in the breast, and TAM-based chemotherapy is one of the most widely used treatments for patients with ER+ BCa.3 However, approximately half of the patients have poor response to TAM treatment, and TAM resistance still remains a major challenge for BCa treatment.4 Therefore, elucidation of molecular mechanisms underlying TAM resistance may provide feasible ways to effectively reduce drug resistance and improve the therapeutic outcomes of BCa patients.
Long non-coding RNAs (lncRNAs) are defined as a group of non-protein coding RNA molecules that are longer than 200 nucleotides length.5 Over the past several years, lncRNAs have gained widespread attention for their regulatory functions in many biological and cellular processes, including cancer progression.6 Among many lncRNAs, bladder cancer-associated transcript 1 (BLACAT1), located on human chromosome 1q32.1, has been recently recognized as an oncogene in a wide variety of cancers, such as colorectal cancer, cervical cancer and osteosarcoma.7–9 In addition, knockdown of BLACAT1 reversed the resistance of afatinib in non-small cell lung cancer.10 In the present study, we aimed to investigate the functional role of BLACAT1 in TAM resistance of BCa, as well as its underlying molecular mechanisms.
Materials and Methods
Cell Culture and Treatments
The ER+ and TAM-sensitive human BCa cell lines (MCF-7 and T47D), obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA), were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
TAM-resistant BCa cells (MCF-7/TR and T47D/TR) were derived by exposure to 1 μM of TAM (Sigma-Aldrich, St. Louis, MO, USA) for 6 months.
The small interfering RNA (siRNA) targeting BLACAT1 (si-BLACAT1), miR-503 mimics, miR-503 inhibitor and the scrambled oligonucleotides (NC) were designed and synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). Cells were plated into six-well plates at a density of 2×105 cells/well. Upon reaching 80% confluence, the cells were transfected with the oligonucleotides using Lipofectamine 2000 reagent (Invitrogen) at a final concentration of 100 nM. After 48 h, cells were collected and used for further analysis.
RNA Extraction and RT-qPCR Analysis
Total RNA was extracted using the TRIzol reagent (Invitrogen). Cytoplasmic and nuclear fractions were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Inc., Waltham, MA, USA). cDNA was synthesized using the PrimeScript™ RT reagent kit (TaKaRa, Dalian, China) with 1 μg RNA as template. The subsequent PCR amplification was carried out on an ABI PRISM 7500 fast Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using the SYBR Premix Ex Taq II kit (TaKaRa). The PCR thermocycling was initiated by polymerase activation step for 10 min at 95 °C followed by 40 cycles of denaturation (95 °C for 30 s) and annealing/extension (60 °C for 1 min). The 2−ΔΔCt method was used for data quantification,11 with GAPDH and U6 as an internal reference. The primer sequences were: BLACAT1 forward, 5ʹ-CCTGCTTGGAAACTAATGACC-3ʹ; BLACAT1 reverse, 5ʹ-AGGCTCAACTTCCCAGACTCA-3ʹ; miR-503 forward, 5′-CCTATTTCCCATGATTCCTTCATA-3′; miR-503 reverse, 5′-GTAATACGGTTATCCACGCG-3′; GAPDH forward, 5ʹ-ATGGGTGTGAACCATGAGAA-3ʹ; GAPDH reverse, 5ʹ-GTGCTAAGCAGTTGGTGGTG-3ʹ; U6 forward, 5′-TCGCTTCGGCAGCACATATACT-3′; and U6 reverse, 5′-ACGCTTCACGAATTTGCGTGTC-3′. Each sample was tested in triplicate.
MTT Assay
Chemosensitivity was measured using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay. Cells were seeded in 96-well plates and cultured with medium containing different doses of TAM. After 24 h, 20 μL of MTT dye (5 mg/mL; Sigma-Aldrich) was added to the culture medium in each well. After incubation for additional 4 h, the medium was discarded, and 150 μL of DMSO (Sigma-Aldrich) was added to dissolve the formazan crystals. Optical density (OD) values were measured at a wavelength of 570 nm using a microplate reader (MultiskanEX, Lab systems, Helsinki, Finland). Each sample was tested in triplicate.
Cell Apoptosis Analysis
Cell apoptosis was measured using an Annexin V-FITC apoptosis detection kit (BD Biosciences, Franklin Lake, NJ, USA). Cells were seeded in 6-well plates at a density of 1×105 cells/well, treated with 1 μM TAM for 24 h, resuspended in binding buffer and stained with 5 μL Annexin V-FITC and 10 μL PI in the dark for 10 min at room temperature. Then, the stained cells were analyzed by flow cytometer (BD Biosciences) equipped with CellQuest software. Each sample was tested in triplicate.
Western Blot Analysis
Total protein was extracted using radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Shanghai, China), and qualified using a BCA protein assay kit (Beyotime). Protein sample (20 µg) mixed with 2× SDS loading buffer was loaded per lane. The proteins were separated by SDS-polyacrylamide gels, and transferred to PVDF membranes (Millipore, Bedford, MA, USA). The membranes were then blocked with 5% skimmed milk, followed by incubation with specific primary antibodies against Bcl-2 (1;1000; cat. no. ab32124; Abcam, Cambridge, UK), Bax (1;1000; cat. no. ab32503; Abcam) and GAPDH (1;1000; cat. no. ab9485; Abcam) at 4 °C overnight. The membranes were washed and incubated for 2 h with horseradish peroxidase-conjugated secondary antibodies (1:5000; cat. no. sc-2004; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The signals were visualized by an enhanced chemiluminescent detection kit (Beyotime), and GAPDH was used as the internal loading control. Each sample was tested in triplicate.
Dual-Luciferase Reporter Assay
The wild or mutant BLACAT1 and Bcl-2 mRNA containing predicted miR-503 binding sites were amplified by PCR and inserted into the psiCHECK-2 luciferase reporter vector (Promega, Madison, WI, USA). Cells were seeded in six-well plates (2×105 cells/well) and co-transfected with the luciferase reporter and miR-503 mimics or NC using Lipofectamine 2000 reagent. At 48 h post-transfection, the luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega). Each sample was tested in triplicate.
Statistical Analysis
Each experimental value was expressed as mean ± standard deviation (SD). All statistical analyses were performed using GraphPad Prism 6.0 software (GraphPad Software Inc., San Diego, CA, USA). All data points represented the mean of triplicates, and the significance of differences between groups was evaluated by Student’s t-test or one-way ANOVA. A value of P<0.05 was considered to indicate a statistically significant result.
Results
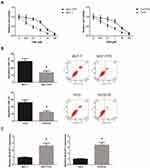
BLACAT1 Is Overexpressed in TAM-Resistant BCa Cells
We first generated TAM-resistant BCa cells (MCF-7/TR and T47D/TR), and we observed that, after 24 h of TAM exposure, these cells showed an acquired resistance to TAM compared with the parental cells (Figure 1A). Flow cytometric analysis disclosed that upon TAM exposure (1 μM), the apoptosis rates of MCF-7/TR and T47D/TR cells were also remarkably decreased (Figure 1B). RT-qPCR analysis confirmed that MCF-7/TR and T47D/TR cells expressed higher level of BLACAT1 than the parental cells (Figure 1C).
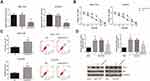
BLACAT1 Knockdown Reduces TAM Resistance in TAM-Resistant BCa Cells
Next, we analyzed the functional role of BLACAT1 in the acquisition of TAM resistance in BCa cells. By introducing with si-BLACAT1, the expression levels of BLACAT1 in MCF-7/TR and T47D/TR cells were strikingly decreased (Figure 2A). As shown in Figure 2B, the acquired resistance to TAM in MCF-7/TR and T47D/TR cells was obviously blocked by BLACAT1 knockdown. In addition, upon TAM exposure (1 μM), BLACAT1 knockdown also notably increased the apoptosis rate and decreased the Bcl-2/Bax expression ratio in MCF-7/TR and T47D/TR cells (Figure 2C and D).
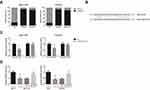
BLACAT1 Directly Binds to miR-503 in TAM-Resistant BCa Cells
We found that BLACAT1 is mostly located in the cytoplasm of MCF-7/TR and T47D/TR cells (Figure 3A), indicating the ceRNA potential for BLACAT1. Through the Starbase database (http://starbase.sysu.edu.cn/index.php), we observed that BLACAT1 sequence contains the complementary binding sites of miR-503, as shown in Figure 3B. To validate the predicted binding interaction, dual-luciferase reporter assay was subsequently carried out, and the results indicated that the luciferase activity of BLACAT1-WT was markedly decreased after transfection with miR-503 mimics in MCF-7/TR and T47D/TR cells (Figure 3C). We further observed that, compared with the parental cells, the expression levels of miR-503 were remarkably reduced in MCF-7/TR and T47D/TR cells, and this reduction could be blocked by BLACAT1 knockdown (Figure 3D).
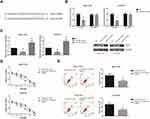
miR-503 Inhibition Blocks the Inhibitory Role of BLACAT1 Knockdown on TAM Resistance
By the TargetScan database (http://www.targetscan.org), Bcl-2 was predicted to be a potential target of miR-503 (Figure 4A). Dual-luciferase reporter assay demonstrated that miR-503 mimics markedly reduced the luciferase activity of Bcl-2-WT in MCF-7/TR and T47D/TR cells (Figure 4B). We also found that miR-503 mimics decreased, while miR-503 inhibitor increased the Bcl-2 expression levels in MCF-7/TR and T47D/TR cells (Figure 4C). We then performed rescue experiments, and the results showed that miR-503 inhibition notably rescued the impaired TAM resistance in MCF-7/TR and T47D/TR cells with BLACAT1 knockdown (Figure 4D and E).
Discussion
At present, intrinsic or acquired resistance to TAM remains a particular clinical concern for effective cancer therapy. Several studies have demonstrated the involvement of lncRNAs in the TAM resistance of BCa. For example, knockdown of CCAT2 improves the sensitivity to TAM of TAM-resistant BCa cells.12 Besides, exosomes mediated transfer of UCA1 leads to increased TAM resistance in BCa cells.13 In a previous study, the oncogenic role of BLACAT1 in BCa was reported.14 Here, we observed that BLACAT1 was significantly upregulated in TAM-resistant BCa cells, and BLACAT1 knockdown in TAM-resistant BCa cells increased their sensitivity to TAM.
LncRNAs exert their regulatory functions through various mechanisms. The ceRNA hypothesis, first proposed by Salmena et al, suggests that lncRNAs can serve as competing endogenous RNAs (ceRNAs) to bind specific miRNAs, thereby reducing the depression of their target mRNAs.15,16 The tumor suppressive role of miR-503 has been previously identified in BCa,17 and in this study, miR-503 was confirmed to be directly bound by BLACAT1 in BCa. Rescue experiments further showed that miR-503 inhibition blocked the inhibitory role of BLACAT1 knockdown on TAM resistance. MiRNAs regulate gene expression by binding to the 3′-UTR of target mRNAs,18 and thus we speculated that BLACAT1 might act as a ceRNA for miR-503 to increase the expression of its target, Bcl-2, thus inducing TAM resistance in BCa. Further investigations regarding the association between BLACAT1 and TAM resistance using clinical human samples and animal models are required.
In conclusion, our study, for the first time, revealed that overexpressed BLACAT1 induces TAM resistance in human BCa, at least in part by regulating miR-503/Bcl-2 axis, potentially benefiting BCa treatment in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. (). doi:10.3322/caac.21208
2. Ignatiadis M, Sotiriou C. Luminal breast cancer: from biology to treatment. Nat Rev Clin Oncol. 2013;10(9):494–506. (). doi:10.1038/nrclinonc.2013.124
3. Ahmad R, Kaus NHM, Hamid S. Synthesis and characterization of PLGA-PEG thymoquinone nanoparticles and its cytotoxicity effects in tamoxifen-resistant breast cancer cells. Adv Exp Med Biol. 2018;2:431–439. Epub 2018/ 12/19.
4. Clarke R, Tyson JJ, Dixon JM. Endocrine resistance in breast cancer–An overview and update. Mol Cell Endocrinol. 2015;418:220–234. doi:10.1016/j.mce.2015.09.035
5. Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193(3):651–669. doi:10.1534/genetics.112.146704
6. Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26(2):155–165. doi:10.1038/modpathol.2012.160
7. Su J, Zhang E, Han L, et al. Long noncoding RNA BLACAT1 indicates a poor prognosis of colorectal cancer and affects cell proliferation by epigenetically silencing of p15. Cell Death Dis. 2017;8(3):e2665. doi:10.1038/cddis.2017.83
8. Shan D, Shang Y, Hu T. Long noncoding RNA BLACAT1 promotes cell proliferation and invasion in human cervical cancer. Oncol Lett. 2018;15(3):3490–3495. doi:10.3892/ol.2018.7773
9. Dong Z, Wang Y. LncRNA BLACAT1 accelerates the proliferation and migration of osteosarcoma cells through regulating STAT3. Pathol Res Pract. 2019;215(3):571–579. doi:10.1016/j.prp.2019.01.017
10. Shu D, Xu Y, Chen W. Knockdown of lncRNA BLACAT1 reverses the resistance of afatinib to non-small cell lung cancer via modulating STAT3 signalling. J Drug Target. 2019;.28(3):1–7. Epub 2019/ 07/31.
11. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
12. Cai Y, He J, Zhang D. [Suppression of long non-coding RNA CCAT2 improves tamoxifen-resistant breast cancer cells’ response to tamoxifen]. Mol Biol (Mosk). 2016;50(5):821–827. Russian. doi:10.7868/S0026898416030046
13. Xu CG, Yang MF, Ren YQ, Wu CH, Wang LQ. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells. Eur Rev Med Pharmacol Sci. 2016;20(20):4362–4368.
14. Hu X, Liu Y, Du Y, Cheng T, Xia W. Long non-coding RNA BLACAT1 promotes breast cancer cell proliferation and metastasis by miR-150-5p/CCR2. Cell Biosci. 2019;9:14. doi:10.1186/s13578-019-0274-2
15. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi:10.1016/j.cell.2011.07.014
16. Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505(7483):344–352. doi:10.1038/nature12986
17. Long J, Ou C, Xia H, Zhu Y, Liu D. MiR-503 inhibited cell proliferation of human breast cancer cells by suppressing CCND1 expression. Tumor Biology. 2015;36(11):8697–8702. doi:10.1007/s13277-015-3623-8
18. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi:10.1016/j.cell.2009.01.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.