Back to Journals » OncoTargets and Therapy » Volume 13
Long Non-Coding RNA BLACAT1 in Human Cancers
Authors Ye T , Yang X , Liu H, Lv P, Ye Z
Received 6 May 2020
Accepted for publication 7 August 2020
Published 20 August 2020 Volume 2020:13 Pages 8263—8272
DOI https://doi.org/10.2147/OTT.S261461
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Tao Ye,1 Xiaoqi Yang,1 Haoran Liu,1,2 Peng Lv,1 Zhangqun Ye1
1Department of Urology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, People’s Republic of China; 2Department of Urology, The Second Affiliated Hospital of Kunming Medical University, Kunming 650000, People’s Republic of China
Correspondence: Zhangqun Ye Department of Urology
Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, People’s Republic of China
Tel/Fax +86-2783663454
Email [email protected]
Abstract: Long non-coding RNAs (lncRNAs) are a cluster of RNAs with more than 200 nucleotides in length, which lack protein-coding capacity. They are important regulators of numerous cellular processes, including gene transcription, translation, and posttranslational modification, especially in tumor initiation and progression. Aberrant expression of lncRNA bladder cancer–associated transcript 1 (BLACAT1) has been reported in various human cancers and was usually associated with unfavorable prognosis. Previous studies have revealed that dysregulation of BLACAT1 could promote the proliferation and metastasis of cancer cells. In this review, we summarize the present understanding of the functions and underlying mechanisms of BLACAT1 in the occurrence and development of various human cancers and discuss the roles of this lncRNA in cancers, including its promising application as a prognostic biomarker or a novel therapeutic target for malignancies.
Keywords: lncRNA, BLACAT1, cancer, review
Introduction
Despite great achievements in modern medicine, malignant cancer remains an intractable threat to human lives and health. In 2018, the International Agency for Research on Cancer reported increase in the number of new cancer cases and cancer-related deaths up to 18.1 million and 9.6 million worldwide, respectively.1 Moreover, the morbidity and mortality associated with cancer is increasing especially in undeveloped regions of the world.2 Genome sequencing analyses have revealed that less than 2% human genome can be transcribed into mature protein-encoding RNAs, while the remaining into non-coding RNAs (ncRNAs).3,4
Long non-coding RNAs (lncRNAs) have emerged as a cluster of RNAs that have over 200 nucleotides in length with no detectable open reading frames (ORFs).5,6 However, lncRNAs are reportedly involved in many cellular functions such as proliferation, differentiation, migration, invasion, and apoptosis. They also play an important part as regulators of gene transcription, translation, and posttranslational modification.7–9 The role of lncRNAs in the occurrence and development of various human cancers have been widely investigated.10−12 Some lncRNAs were reported to act as promising biomarkers for prognosis prediction or diagnosis supplementation for malignant tumors.13–15
Bladder cancer–associated transcript 1 (BLACAT1), also named linc-UBC1, is located at human chromosome 1q32.1 with a transcript of 2616 kb and only 1 exon, between LEM domain containing 1 (LEMD1) and cyclin-dependent kinase 18 (CDK18) genes.16,17 It was first identified by He et al in 2013 and described as non-coding based on sequence analysis.17 In addition, nuclear fractionation of bladder cancer cells demonstrated that BLACAT1 preferentially localized in the nucleus. Further analysis by reverse transcription-polymerase chain reaction (RT-PCR) analysis showed it had no effect on the expression of its neighboring genes, kelch domain containing 8A (KLHDC8A), LEMD1, and CDK18, indicating that BLACAT1 might function in trans. Following these findings, BLACAT1 attracted a large amount of interest from cancer researchers. Many emerging studies demonstrated that BLACAT1 was aberrantly expressed in different cancers, including bladder cancer, breast cancer, cervical cancer (CC), colorectal cancer (CRC), esophageal squamous cell carcinoma (ESCC), gastric cancer (GC), glioma, hepatocellular carcinoma (HCC), lung cancer (LC), osteosarcoma (OS), and thyroid cancer (TC). Additionally, a meta-analysis conducted by Lu et al summarized that high BLACAT1 expression could predict shorter overall survival, advanced TNM stage, poor tumor grade, and more lymph node metastasis in solid tumors.18 Chen et al also observed significant upregulation of BLACAT1 in both serum and tissues of 12 types of cancer compared to those of matched healthy participants. Further, serum BLACAT1 expression was reported to function as a non-specific diagnostic biomarker in various cancers, particularly in endometrial cancer (EMC).16 These evidences displayed the clinical significance of BLACAT1 in the research and treatment of carcinomas. In this review, latest studies are summarized to strengthen our understanding of the function, mechanism, and clinical value of BLACAT1 in carcinogenesis and progression of malignancies (Tables 1 and 2).
 |
Table 1 Functional Characterization of BLACAT1 in Various Cancers |
 |
Table 2 Clinical Significance of BLACAT1 in Various Cancers |
BLACAT1 in Human Cancers
BLACAT1 in Glioma
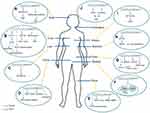
BLACAT1 in 23 glioma tissues and 6 cell lines was remarkably overexpressed than non-tumor controls, and its expression in grade I/II patients was significantly lower than that in grade III/IV patients, suggesting that BLACAT1 might reflect the grade of glioma. Additionally, Kaplan-Meier analysis showed that patients with high BLACAT1 expression had a reduced survival rate. Moreover, findings from mechanical experiments indicated that BLACAT1 could competitively bind to miR-605-3p as a competing endogenous RNA (ceRNA) to modulate the expression of vasodilator-simulated phosphoprotein (VASP), thereby promoting glioma growth, migration, and invasion. The BLACAT1/miR-605-3p/VASP axis is suggested to play a critical role in the progression of glioma.19 Li et al also discovered elevated BLACAT1 expression in 35 glioma tissues and 5 cell lines and introduced a different mechanism. They described that BLACAT1 could activate WNT/β-catenin pathway to promote proliferation and invasion of glioma cells.20 These results showed the potential function of BLACAT1 as a novel oncogenic lncRNA and a prospective therapeutic target in glioma (Figure 1A).
BLACAT1 in Lung Cancer
Lung cancer is the most aggressive human malignancy, accounting for 11.6% of new cancer cases and 18.4% of total cancer death worldwide in 2018, based on the report of International Agency for Research on Cancer.1 There are two subtypes of lung cancer: non-small cell lung cancer (NSCLC, 80–85%, including adenocarcinoma and squamous carcinoma) and small cell lung cancer (SCLC, 10–15%).21 Wang et al found 1364 differentially expressed mRNAs (DEmRNAs) and 260 DElncRNAs by analyzing the expression profile data of lung adenocarcinoma (LUAD) patients from The Cancer Genome Atlas (TCGA) database, and finally selected 8 DElncRNAs as optimal diagnostic biomarkers for LUAD patients, including BLACAT1.22 Ding et al also verified BLACAT1 as a DElncRNA using the high-throughput sequencing data from a Gene Expression Omnibus (GEO) database (GSE104854) of LUAD.23 Adenocarcinomas in situ (AIS) is regarded as the early stage of LUAD, and can develop into an invasive entity. In the research of Dan Li and his colleagues, twenty AIS-specific genes (including two lncRNAs, CTD-2527I21.15 and BLACAT1) were identified as an early-stage LC signature using the random forest feature selection, this signature had approximately 98% accuracy to differentiate early-stage LC from normal cases.24
Ye et al using qRT-PCR confirmed notably increased expression of BLACAT1 in 48 NSCLC tissues and 4 NSCLC cell lines compared to adjacent normal tissues and normal human bronchial epithelial cells (NHBE), respectively. Statistical analysis of clinicopathological characteristics and prognosis indicated that elevated BLACAT1 expression was closely related to smoking status, more lymph node metastasis, advanced TNM stage, and shorter overall survival. Functional assays showed that BLACAT1 knockdown could suppress proliferation, migration, invasion, and induce G0/G1 cycle arrest in NSCLC cells, and inhibit tumor growth and metastasis in vivo. In addition, further experiments illustrated that miR-144 downregulation could reverse the effect of BLACAT1 silencing on NSCLC cell. These results suggested BLACAT1 and miR-144 might be potential therapy targets for NSCLC.25 Xu et al similarly revealed that high expression of BLACAT1 was dramatically related to reduced overall survival and progression-free survival, further reporting a significant correlation of BLACAT1 with TNM stage and response to chemotherapy. Besides, silencing BLACAT1 depressed NSCLC cell proliferation, migration, and invasion via regulating epithelial–mesenchymal transition (EMT) and WNT/β-catenin signaling pathway.26
Huang et al observed remarkably upregulated BLACAT1 and downregulated miR-17 in cisplatin (DDP)-resistant NSCLC cells, and both were identified to affect the viability of the cancer cells. Bioinformatic analyses and dual-luciferase reporter assays confirmed the potential for miR-17 to bind to the 3ʹ-untranslated region (3ʹ-UTR) of BLACAT1. The interaction of BLACAT1 with miR-17 was also reported to be involved in cell autophagy and chemoresistance by regulating autophagy-related gene 7 (ATG7) expression.27 Further, elevated BLACAT1 expression was found in afatinib-resistant NSCLC cells, and BLACAT1 depletion could reverse the cancer cells’ resistance to afatinib. Mechanically, BLACAT1 was identified to promote the phosphorylation of signal transducer and activator of transcription 3 (STAT3) to facilitate drug-resistance.28 These results supported a promising strategy for chemotherapy-resistant NSCLC by targeting BLACAT1.
Chen et al detected upregulated BLACAT1 expression in SCLC tissues and 4 SCLC cell lines. Increased BLACAT1 expression was closely related to clinical stage, tumor size, lymph node metastasis, distant metastasis, and unfavorable overall survival. Moreover, results from univariate and multivariate Cox analyses all proved BLACAT1 could be used as an independent prognostic factor for SCLC. Functionally, knockdown of BLACAT1 was found to attenuate cell proliferation, migration, invasion, and arrest G0/G1 cell cycle in SCLC cells.29 Taken together, BLACAT1 could potentially serve as a novel prognostic predictor and therapeutic target for lung cancer patients (Figure 1B).
BLACAT1 in Hepatocellular Carcinoma
Peng et al reported that the BLACAT1 expression was dramatically higher in 37 HCC tissues and 8 HCC cell lines than in the normal controls. Biologically, BLACAT1 downregulation notably suppressed HCC proliferation and invasion in vitro and in vivo. Mechanically, they revealed that BLACAT1 promoted HCC progression by directly targeting miR-485-5p and negatively modulating its expression.30 This study described the functional characterization of BLACAT1 and its potential value as a therapeutic target for HCC (Figure 1C).
BLACAT1 in Cervical Cancer
A previous study found that BLACAT1 was upregulated in 133 CC tissues and 4 CC cell lines using qRT-PCR, and significantly correlated with Federation International of Gynecology and Obstetrics (FIGO) stage, histological grade, and distant metastasis in CC patients. Survival analyses revealed that high BLACAT1 expression was dramatically associated with poor overall survival and progression-free survival, and multivariate Cox regression analyses identified BLACAT1 as an independent prognostic indicator for overall survival in CC patients. Functional experiments showed the proliferation and metastasis ability of CC cells with BLACAT1 silencing was lower than that of cells in the control group. In addition, the expression of β-catenin and matrix metallopeptidase 7 (MMP-7) was decreased after BLACAT1 knockdown, demonstrating that BLACAT1 might contribute to the progression of CC by regulating the WNT/β-catenin signal pathway.31 Shan et al also reported that BLACAT1 was observably overexpressed in 99 CC tissues and 5 CC cell lines than adjacent non-tumor tissues and normal cervical epithelial cells (NCECs), respectively. Furthermore, mechanical experiments showed that BLACAT1 depletion inhibited cell proliferation and metastasis of CC cells and induced G0/G1 cell cycle arrest by suppressing the cell cycle regulators cyclin B1 (CCNB1) and cell division cycle 25C (CDC25C) and modulating EMT markers (N-cadherin and E-cadherin) (Figure 1D).32
BLACAT1 in Osteosarcoma
Osteosarcoma is the most frequent and aggressive malignant bone tumor which occurs predominantly in the long bones.33 According to the study of Dong et al, the expression of BLACAT1 was higher in 82 OS tissues and 4 cell lines than corresponding normal controls. Increased BLACAT1 expression was notably correlated with lower overall survival rate in OS by Kaplan-Meier analysis. Additionally, a significant correlation was identified between BLACAT1 and TNM stage, tumor size and lymph node metastasis. Functionally, downregulation of BLACAT1 caused G0/G1 cell cycle arrest, promoted cell apoptosis, and suppressed cell proliferation, migration, and invasion in OS, while its upregulation could seemingly reverse these effects. RNA pull-down assay revealed that BLACAT1 exhibited its function in OS via regulating the phosphorylation level of STAT3 (Figure 1E).34
BLACAT1 in Bladder Cancer
Globally, bladder cancer is one of the most prevalent cancer, with an estimated 386 000 new cases and 150 000 deaths annually.35 BLACAT1 was found to be overexpressed in the tissues of 60 bladder cancer cases compared to adjacent non-tumor tissues. The high BLACAT1 expression was identified in three bladder cancer cell lines (UMUC-3, TCCSUP, and 5637). Moreover, elevated BLACAT1 expression was positively correlated with lymph node metastasis. Patients in high BLACAT1 expression group tended to have a shorter overall survival and metastasis-free survival by Kaplan-Meier analysis. Functionally, BLACAT1 knockdown assays indicated that its depletion inhibited cell proliferation, motility, and invasion in vitro, as well as tumorigenicity and metastatic capability in vivo. Mechanically, BLACAT1 was found to interact with enhancer of zeste 2 (EZH2) and suppressor of zeste 12 (SUZ12), which were critical components of polycomb repressive complex 2 (PRC2) complex, regulating the expression of some target genes, such as cyclin D2 (CCND2), homeobox A5 (HOXA5), kruppel like factor 4 (KLF4), serpin family B member 2 (SERPINB2), and bone morphogenetic protein 2 (BMP2). Furthermore, chromatin immunoprecipitation (ChIP) assay indicated that BLACAT1 could modulate histone H3-lysine 27 (H3K27) trimethylation level in the promoter regions of the above-mentioned genes, exerting its oncogenic role in bladder cancer.17 Similarly, Droop et al also reported stronger BLACAT1 expression in cancer tissues than in adjacent benign tissues, and high BLACAT1 expression was significantly associated with poor survival in the cohort of TCGA bladder cancer. However, in a different cohort of 106 tumor cases, no obvious differences were observed in the BLACAT1 expression between tumor and benign tissues, and no significant association was found with survival or lymph node metastasis. The inconsistency might be due to heterogeneity in patient populations, or variation in the detection assays used.36 In summary, these results showed that BLACAT1 was likely to serve as a prognostic predictor and a promising therapeutic target for bladder cancer (Figure 1F).
BLACAT1 in Colorectal Cancer
Colorectal cancer (CRC) is the second most common malignancy in females and the third most common in males.37 Dai et al conducted a comprehensive analysis of 8 GEO datasets (GSE77199, GSE76855, GSE62321, GSE8671, GSE32323, GSE21815, GSE39582, and GSE20842), and identified 9 significantly differentially expressed lncRNAs, including upregulated BLACAT1. Moreover, they reported a significant elevation of plasma BLACAT1 in CRC patients compared to healthy controls, demonstrating its potential as a supplementary diagnostic biomarker for CRC.38 Through analyzing three other independent CRC datasets (GSE9348, GSE23878, and GSE22598), Bu et al identified obvious upregulation of BLACAT1 expression in all three datasets. Higher BLACAT1 expression was also observed in five CRC cell lines compared to a normal colon cell line (NCM460). In addition, clinical data of CRC patients in the GEPIA database revealed that BLACAT1 expression was significantly increased in colorectal adenocarcinoma tissues, especially in advanced-stage cancer tissues. A similar analysis of the GEO dataset (GSE18105) found a positive relationship between high BLACAT1 level and distant metastasis in CRC. Functional experiments demonstrated that proliferation and invasiveness of CRC cells were reduced after downregulating BLACAT1. The downregulation was found to significantly suppress expression of proliferation markers (CCND1 and CDK6) and mesenchymal marker (Vimentin), while increasing the expression of epithelial marker (E-cadherin).39 Furthermore, Gao et al also reported increased expression of BLACAT1 in 96 CRC tissues and 3 cell lines (HT29, SW620 and HCT116) using qRT-PCR compared with paired adjacent normal mucosa and human intestinal epithelial cells (NCM460 and SW480). BLACAT1 expression was more markedly expressed in CRC patients with larger tumor size, deeper tumor invasion, advanced TNM stage, and more lymph node metastasis. Kaplan-Meier survival analysis revealed high BLACAT1 expression was obviously related to reduced overall survival time, and multivariate analysis by Cox regression confirmed BLACAT1, TNM stage, and distant metastasis as independent prognostic factors affecting overall survival of CRC patients. After silencing BLACAT1, CRC cell cycle was blocked in M phase, inhibiting cell proliferation and facilitating apoptosis; meanwhile, the migration and invasion abilities of CRC cells were decreased.40 Su et al also observed BLACAT1 expression was remarkably amplified in 48 CRC samples, and significantly related to advanced TNM stage. Moreover, univariate and multivariate analyses confirmed high BLACAT1 expression as an independent indicator for unfavorable overall survival. Functional experiments demonstrated that BLACAT1 silencing caused stalling of CRC cells at G0/G1 phase and inhibited cell proliferation. Tumorigenicity assay in nude mice also revealed that BLACAT1 silencing postponed the growth of CRC in vivo. Mechanically, BLACAT1 silencing was found to reduce EZH2 chromatin binding and H3K27me3 level on p15 promoter, thus suppressing the cell proliferation in CRC (Figure 1G).41
BLACAT1 in Gastric Cancer
Gastric cancer is the second leading cause of mortality in cancer patients globally.42 A previous study reported upregulated expression of BLACAT1 in 85 GC tissues compared with adjacent normal gastric tissues, and the same was identified in GC cell lines. Upregulation of BLACAT1 was positively correlated with tumor size, lymph node metastasis, and TNM stage. Kaplan-Meier analysis detected GC patients in high BLACAT1 group had unfavorable overall survival. Additionally, BLACAT1 depletion was found to significantly decrease proliferation and invasion abilities of GC cell lines (HGC-27 and SGC-7901).43 Wu et al also observed that BLACAT1 was dramatically overexpressed in oxaliplatin (OXA)-resistant GC tissue and cells than in OXA-sensitive GC tissue and cells. Further research revealed BLACAT1 silencing facilitated apoptosis and suppressed metastasis and tumor growth of OXA-resistant GC cells in vitro and in vivo. Mechanical experiments verified that BLACAT1 modulated the expression of ATP binding cassette subfamily B member 1 (ABCB1) protein via targeting miR-361, and provided the pathway of BLACAT1/miR-361/ABCB1 contributing to the development of GC chemoresistance (Figure 1H).44
BLACAT1 in Breast Cancer
Among females worldwide, breast cancer is the most common malignancy.45 Hu et al observed remarkable overexpression of BLACAT1 in 72 breast cancer tissues and 7 cancer cell lines. The upregulated expression was positively correlated with TNM stage and lymph node metastasis. Kaplan-Meier analysis showed shorter survival time among breast cancer patients with high BLACAT1 expression compared to those with lower BLACAT1 expression. Moreover, knockdown of BLACAT1 significantly suppressed cell growth and metastasis of breast cancer in vitro. Further mechanical research demonstrated the potential of BLACAT1 to serve as a ceRNA binding to miR-150-5p, which has a target in the 3ʹ-UTR of C-C motif chemokine receptor 2 (CCR2) mRNA. Then, they validated the critical role of BLACAT1/miR-150-5p/CCR2 axis in the proliferation and metastasis of breast cancer cells.46 Owing to its oncogenic role in breast cancer progression, BLACAT1 was recommended as a specific therapeutic target (Figure 1I).
BLACAT1 in Esophageal Squamous Cell Carcinoma
A study by Niu et al reported signally higher BLACAT1 expression in ESCC tissues and cell lines, which correlated with TNM stage and lymph node metastasis. In addition, patients with low BLACAT1 expression tended to have better overall survival. Then, they further confirmed BLACAT1 knockdown could inhibit migration and invasion in ESCC cells. Moreover, E-cadherin protein was increased and EZH2 was decreased after the knockdown of BLACAT1 in ESCC cells. These results disclosed that BLACAT1 might influence ESCC metastasis through the regulation of EZH2 and E-cadherin (Figure 1J).47
BLACAT1 in Thyroid Cancer
Thyroid cancer is the most frequent endocrine carcinoma globally, with papillary thyroid carcinoma (PTC) accounting for the majority of thyroid cancer, nearly 80%.1 Inconsistent with the results of previous studies in other cancer types, Liao et al observed lower expression of BLACAT1 in the plasma of PTC cases and discovered obvious relationships between BLACAT1 expression and gender as well as lymph node metastasis. Additionally, the ROC curve was 0.825 for using plasma BLACAT1 to predict the occurrence of lymph node metastasis. Multivariable analysis showed that plasma BLACAT1 downregulation could be an independent indicator for better prognosis in PTC, suggesting BLACAT1 might act as a tumor suppressor in PTC.48 More studies are warranted to explore the mechanism of the effect of BLACAT1 on the progression of PTC.
Conclusion and Future Perspectives
In the recent years, the dysregulation of lncRNAs has been gradually identified to be involved in carcinoma progression through high-throughput sequencing technologies. Accumulating evidence has demonstrated that lncRNAs exert various functions in the pathophysiological processes of malignant tumors by regulating cell proliferation, apoptosis, differentiation, immune response, and invasion.49,50 The molecular mechanisms of lncRNAs in cellular biological behavior are extremely complex, as they serve as guides, scaffolds, decoys, or tethers for other biomolecules.51,52 In addition, many lncRNAs have been shown to significantly correlate with the prognosis of human cancers and likely to be regarded as promising tumor biomarkers or specific therapeutic targets.53–55
BLACAT1 was first discovered to be upregulated in bladder cancer tissues, with similar findings now reported in the tissues of breast cancer, CC, CRC, ESCC, GC, glioma, HCC, LC, and OS, while downregulation of BLACAT1 was found in the plasma of PTC (Table 1). According to the current evidence, increased BLACAT1 expression is commonly associated with advanced tumor stage, larger tumor size, metastasis, and reduced survival in human cancers, except for PTC (Table 2). Moreover, BLACAT1 has also been reported to contribute to chemotherapy resistance in GC and NSCLC.
Functional experiments have indicated that BLACAT1 depletion inhibits the progression of carcinogenesis by suppressing cell proliferation, migration, invasion, and promoting apoptosis. A previous study revealed that BLACAT1 might act mainly in trans, and partially modulate downstream targets by physically associating with the PRC2 complex in bladder cancer.17 Su et al reported that BLACAT1 was involved in the epigenetic repression of p15 by targeting PRC2, thus promoting the cell proliferation of CRC.41 Additionally, BLACAT1 was found to exert its oncogenic role by interacting with some miRNAs or modulating several classical signaling pathways in tumorigenesis (Figure 1 and Table 1). Liu et al showed BLACAT1 could bind to miR-605-3p and hereby upregulate miR-605-3p’ target gene VASP, promoting the development of glioma.19 Hu et al reported that BLACAT1 regulated oncogene CCR2 by competitively binding to miR-150-5p in breast cancer.46 The interactions between BLACAT1 and several cancer-related signaling pathways further emphasize its role in tumorigenesis. It was reported to regulate EMT-associated proteins and promote the progression of glioma, cervical cancer, and lung cancer by activating WNT/β-catenin pathway.20,26,31 Besides, present evidence pointed out that BLACAT1 could interact with STAT3 and enhance the phosphorylation of STAT3, accelerating the proliferation and migration of lung cancer and osteosarcoma.28,34
However, our understanding of the role of BLACAT1 in tumor biology remains in a preliminary stage, and detailed molecular mechanisms of BLACAT1 are still needed to be systematically explored. Accordingly, further studies are essential to determine the accurate roles of BLACAT1 in human cancers; moreover, studies with a larger sample size should be conducted to improve our understanding of the clinical application of BLACAT1 as a diagnosis/prognosis biomarker for malignancies.
Disclosure
The authors report no funding and no conflicts of interest for this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi:10.1016/S0140-6736(17)33326-3
3. Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–108. doi:10.1038/nature11233
4. ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi:10.1038/nature11247.
5. Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genomics Proteomics Bioinformatics. 2016;14(1):42–54. doi:10.1016/j.gpb.2015.09.006
6. Wu T, LncRNAs: DY. From basic research to medical application. Int J Biol Sci. 2017;13(3):295–307. doi:10.7150/ijbs.16968
7. Iyer MK, Niknafs YS, Malik R, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199–208. doi:10.1038/ng.3192
8. Sherstyuk VV, Medvedev SP, Zakian SM. Noncoding RNAs in the regulation of pluripotency and reprogramming. Stem Cell Rev Rep. 2018;14(1):58–70. doi:10.1007/s12015-017-9782-9
9. Liu H, Luo J, Luan S, He C, Li Z. Long non-coding RNAs involved in cancer metabolic reprogramming. Cell Mol Life Sci. 2019;76(3):495–504. doi:10.1007/s00018-018-2946-1
10. Shi C, Sun L, Song Y. FEZF1-AS1: a novel vital oncogenic lncRNA in multiple human malignancies. Biosci Rep. 2019;39(6). doi:10.1042/BSR20191202
11. Ghafouri-Fard S, Taheri M. Nuclear Enriched Abundant Transcript 1 (NEAT1): a long non-coding RNA with diverse functions in tumorigenesis. Biomed Pharmacother. 2019;111:51–59. doi:10.1016/j.biopha.2018.12.070
12. Thin KZ, Tu JC, Raveendran S. Long non-coding SNHG1 in cancer. Clin Chim Acta. 2019;494:38–47. doi:10.1016/j.cca.2019.03.002
13. Qiu JJ, Lin XJ, Tang XY, Zheng TT, Lin YY, Hua KQ. Exosomal metastasis‑associated lung adenocarcinoma Transcript 1 promotes angiogenesis and predicts poor prognosis in epithelial ovarian cancer. Int J Biol Sci. 2018;14(14):1960–1973. doi:10.7150/ijbs.28048
14. Chen J, Yu Y, Li H, et al. Long non-coding RNA PVT1 promotes tumor progression by regulating the miR-143/HK2 axis in gallbladder cancer. Mol Cancer. 2019;18(1):33. doi:10.1186/s12943-019-0947-9
15. Ou ZL, Zhang M, Ji LD, et al. Long noncoding RNA FEZF1-AS1 predicts poor prognosis and modulates pancreatic cancer cell proliferation and invasion through miR-142/HIF-1α and miR-133a/EGFR upon hypoxia/normoxia. J Cell Physiol. 2019. doi:10.1002/jcp.28188
16. Chen X, Dai M, Zhu H, et al. Evaluation on the diagnostic and prognostic values of long non-coding RNA BLACAT1 in common types of human cancer. Mol Cancer. 2017;16(1):160. doi:10.1186/s12943-017-0728-2
17. He W, Cai Q, Sun F, et al. linc-UBC1 physically associates with polycomb repressive complex 2 (PRC2) and acts as a negative prognostic factor for lymph node metastasis and survival in bladder cancer. Biochim Biophys Acta. 2013;1832(10):1528–1537. doi:10.1016/j.bbadis.2013.05.010
18. Lu H, Liu H, Yang X, et al. LncRNA BLACAT1 may serve as a prognostic predictor in cancer: evidence from a meta-analysis. Biomed Res Int. 2019:1275491. doi:10.1155/2019/1275491.
19. Liu N, Hu G, Wang H, Wang Y, Guo Z. LncRNA BLACAT1 regulates VASP expression via binding to miR-605-3p and promotes giloma development. J Cell Physiol. 2019;234(12):22144–22152. doi:10.1002/jcp.28778
20. Li X, Qi S, Ma D, Fan J, Wang J. Long non-coding RNA BLACAT1 promotes the proliferation and invasion of glioma cells via Wnt/β-catenin signaling. Exp Ther Med. 2019;17(6):4703–4708. doi:10.3892/etm.2019.7468
21. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–1380. doi:10.1056/NEJMra0802714
22. Wang Y, Fu J, Wang Z, Lv Z, Fan Z, Lei T. Screening key lncRNAs for human lung adenocarcinoma based on machine learning and weighted gene co-expression network analysis. Cancer Biomark. 2019;25(4):313–324. doi:10.3233/CBM-190225
23. Ding X, Zhang S, Li X, et al. Profiling expression of coding genes, long noncoding RNA, and circular RNA in lung adenocarcinoma by ribosomal RNA-depleted RNA sequencing. FEBS Open Bio. 2018;8(4):544–555. doi:10.1002/2211-5463.12397
24. Li D, Yang W, Zhang Y, et al. Genomic analyses based on pulmonary adenocarcinoma in situ reveal early lung cancer signature. BMC Med Genomics. 2018;11(Suppl 5):106. doi:10.1186/s12920-018-0413-3
25. Ye JR, Liu L, Zheng F. Long noncoding RNA bladder cancer associated Transcript 1 promotes the proliferation, migration, and invasion of nonsmall cell lung cancer through sponging miR-144. DNA Cell Biol. 2017;36(10):845–852. doi:10.1089/dna.2017.3854
26. Xu R, Cao XR, Zhang BQ, Wang JL, Wang L, Sun WQ. BLACAT1 is negatively associated with prognosis in patients with NSCLC and inhibits cell progression, metastasis and epithelial-mesenchymal transition through down-regulating Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(14):6217–6225. doi:10.26355/eurrev_201907_18439
27. Huang FX, Chen HJ, Zheng FX, et al. LncRNA BLACAT1 is involved in chemoresistance of non‑small cell lung cancer cells by regulating autophagy. Int J Oncol. 2019;54(1):339–347. doi:10.3892/ijo.2018.4614
28. Shu D, Xu Y, Chen W. Knockdown of lncRNA BLACAT1 reverses the resistance of afatinib to non-small cell lung cancer via modulating STAT3 signalling. J Drug Target. 2019;1–7. doi:10.1080/1061186X.2019.1650368
29. Chen W, Hang Y, Xu W, et al. BLACAT1 predicts poor prognosis and serves as oncogenic lncRNA in small-cell lung cancer. J Cell Biochem. 2018. doi:10.1002/jcb.27548
30. Peng Y, Leng W, Duan S, Hong M. Long noncoding RNA BLACAT1 is overexpressed in hepatocellular carcinoma and its downregulation suppressed cancer cell development through endogenously competing against hsa-miR-485-5p. Biomed Pharmacother. 2019;116:109027. doi:10.1016/j.biopha.2019.109027
31. Wang CH, Li YH, Tian HL, Bao XX, Wang ZM. Long non-coding RNA BLACAT1 promotes cell proliferation, migration and invasion in cervical cancer through activation of Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(10):3002–3009. doi:10.26355/eurrev_201805_15057
32. Shan D, Shang Y, Hu T. Long noncoding RNA BLACAT1 promotes cell proliferation and invasion in human cervical cancer. Oncol Lett. 2018;15(3):3490–3495. doi:10.3892/ol.2018.7773
33. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33(27):3029–3035. doi:10.1200/JCO.2014.59.4895
34. Dong Z, Wang Y. LncRNA BLACAT1 accelerates the proliferation and migration of osteosarcoma cells through regulating STAT3. Pathol Res Pract. 2019;215(3):571–579. doi:10.1016/j.prp.2019.01.017
35. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi:10.1002/ijc.25516
36. Droop J, Szarvas T, Schulz WA, et al. Diagnostic and prognostic value of long noncoding RNAs as biomarkers in urothelial carcinoma. PLoS One. 2017;12(4):e0176287. doi:10.1371/journal.pone.0176287
37. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.21254
38. Dai M, Chen X, Mo S, et al. Meta-signature LncRNAs serve as novel biomarkers for colorectal cancer: integrated bioinformatics analysis, experimental validation and diagnostic evaluation. Sci Rep. 2017;7:46572. doi:10.1038/srep46572
39. Bu CW, Wang B, Zhang D, et al. Overexpression of the long non-coding RNA BLACAT1 promotes cell proliferation and invasion in colorectal cancer. Transl Cancer Res. 2019;8(1):35–43. doi:10.21037/tcr.2018.12.26
40. Gao X, Wen J, Gao P, Zhang G, Zhang G. Overexpression of the long non-coding RNA, linc-UBC1, is associated with poor prognosis and facilitates cell proliferation, migration, and invasion in colorectal cancer. Onco Targets Ther. 2017;10:1017–1026. doi:10.2147/OTT.S129343
41. Su J, Zhang E, Han L, et al. Long noncoding RNA BLACAT1 indicates a poor prognosis of colorectal cancer and affects cell proliferation by epigenetically silencing of p15. Cell Death Dis. 2017;8(3):e2665. doi:10.1038/cddis.2017.83
42. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
43. Hu Y, Pan J, Wang Y, Li L, Huang Y. Long noncoding RNA linc-UBC1 is negative prognostic factor and exhibits tumor pro-oncogenic activity in gastric cancer. Int J Clin Exp Pathol. 2015;8(1):594–600.
44. Wu X, Zheng Y, Han B, Dong X. Long noncoding RNA BLACAT1 modulates ABCB1 to promote oxaliplatin resistance of gastric cancer via sponging miR-361. Biomed Pharmacother. 2018;99:832–838. doi:10.1016/j.biopha.2018.01.130
45. DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. doi:10.3322/caac.21203
46. Hu X, Liu Y, Du Y, Cheng T, Xia W. Long non-coding RNA BLACAT1 promotes breast cancer cell proliferation and metastasis by miR-150-5p/CCR2. Cell Biosci. 2019;9:14. doi:10.1186/s13578-019-0274-2
47. Niu G, Zhuang H, Li B, Cao G. Long noncoding RNA linc-UBC1 promotes tumor invasion and metastasis by regulating EZH2 and repressing E-cadherin in esophageal squamous cell carcinoma. J BUON. 2018;23(1):157–162.
48. Liao D, Lv G, Wang T, Min J, Wang Y, Liu S. Prognostic value of long non-coding RNA BLACAT1 in patients with papillary thyroid carcinoma. Cancer Cell Int. 2018;18:47. doi:10.1186/s12935-018-0544-9
49. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–1261. doi:10.1038/nm.3981
50. Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1(5):391–407. doi:10.1158/2159-8290.CD-11-0209
51. Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21. doi:10.1038/nrg3606
52. Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–914. doi:10.1016/j.molcel.2011.08.018
53. Leyten GH, Hessels D, Jannink SA, et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol. 2014;65(3):534–542. doi:10.1016/j.eururo.2012.11.014
54. Zhan Y, Du L, Wang L, et al. Expression signatures of exosomal long non-coding RNAs in urine serve as novel non-invasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Mol Cancer. 2018;17(1):142. doi:10.1186/s12943-018-0893-y
55. Lin LY, Yang L, Zeng Q, et al. Tumor-originated exosomal lncUEGC1 as a circulating biomarker for early-stage gastric cancer. Mol Cancer. 2018;17(1):84. doi:10.1186/s12943-018-0834-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.