Back to Journals » Cancer Management and Research » Volume 12
Long Non-Coding RNA AC118344.1 Promotes Gastric Cancer Cell Proliferation, Invasion, and Metastasis via AKT2 and Its Downstream Molecules HK2 and MMP2
Authors Cai L, Xue Y, Ding J, Zheng B
Received 12 September 2020
Accepted for publication 25 November 2020
Published 8 December 2020 Volume 2020:12 Pages 12613—12621
DOI https://doi.org/10.2147/CMAR.S281608
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Lixia Cai,1,* Yangji Xue,2,* Jianzu Ding,2,3 Bin Zheng2,3
1Department of Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Huanglong Science and Education Center, Hangzhou Medical College, Hangzhou, People’s Republic of China; 3Zhejiang Academy of Medical Sciences, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin Zheng
Huanglong Science and Education Center, Hangzhou Medical College, Hangzhou, 310013 Zhejiang, People’s Republic of China
Tel +86-571-88215606
Fax +86-591-88215602
Email [email protected]
Background: Gastric cancer (GC) is a highly occurring cancer with poor prognosis. Reports indicate that long non-coding RNA (LncRNA) potentially regulates tumor progression. Herein, we aim to explore the effect of LncRNA AC118344.1 on the progression of gastric cancer.
Methods: Overexpression and knockout experiments were used to clarify the potential molecular signaling mechanisms induced by AC118344.1. CCK-8, transwell and in vivo metastasis assay were used to detect the function of AC118344.1 in AGS and SGC-7901 cells. Additionally, shRNA silencing techniques, qRT-PCR and Western blot assay were used to explore the relationship between AC118344.1, AKT2, and its downstream molecules.
Results: Upregulating the expression of AC118344.1 induces cell proliferation, invasion in vitro, and lung metastasis in vivo whereas downregulating the expression of AC118344.1 inhibits these effects. Besides, silencing the expression of AC118344.1 downregulated the expression of AKT2 in both the two cells. On the other hand, silencing the expression of AKT2 by shRNA was unable to downregulate the expression of AC118344.1 in both the gastric cancer cells. Also, AC118344.1 regulated AKT2 via its downstream molecules including HK2 and MMP2.
Conclusion: AC118344.1 promotes gastric cancer cell proliferation and invasion and lung metastasis in nude mice by upregulating the expression of AKT2 and its downstream molecules (HK2 and MMP2). Therefore, our findings provide a novel mechanism of the AC118344.1-AKT2-HK2/MMP2 axis in regulating the development of gastric cancer cells.
Keywords: gastric cancer, lncRNA AC118344.1, AKT2, proliferation, invasion, metastasis
Introduction
Gastric cancer is the 4th leading incidence of malignant tumors across the globe. Besides, it is endemic in certain Asian countries, including China, Japan, Iran, and India.1 Specifically, China has a significantly high incidence of gastric cancer with its morbidity and mortality being approximately twice the global average.2 Existing evidence suggests that most patients with gastric cancer are already in an advanced stage at the time of diagnosis. Also, the treatment approach based on surgery or chemotherapy has not yielded satisfactory outcomes.3 As such, a continuous exploration of the molecular mechanism of gastric cancer is clinically essential for targeted therapy and improving the survival rate of patients diagnosed with gastric cancer.
Notably, protein kinase B (PKB or AKT) is one of the most important kinases linked to the regulation of cell growth, splitting, differentiation, invasion, and survival.4 So far, a total of 3 different subtypes of AKTs including AKT1 (PKBα), AKT2 (PKBβ), and AKT3 (PKBγ) have been identified. Despite their expression and distribution exhibiting differential organ or tissue inclination, these subtypes regulate the progression of human cancer and primarily exercise oncogenic roles.5 AKT2 is a well-documented oncogene, and several lines of evidence have shown that it is implicated in the development of a wide range of human cancers, particularly ovarian and breast cancers.6 Nonetheless, the molecular mechanism by which AKT2 promotes cancer progression remains elusive.
Accumulating studies have shown that non-coding RNAs also play essential roles in the regulation of cancer-related AKT1 and AKT2 signaling cascades, thereby promoting or attenuating the incidence and progression of cancer.7–9 Zhang J et al reported that AKT2 can act as a direct target of miR-625, hence, reversing the anti-cancer functions of miR-625 on glioma.10 Also, a study by Ma X revealed that microRNA-296-5p suppresses the progression of hepatocellular carcinoma cell (HCC) primarily including the inhibition of HCC proliferation, migration, and invasion by downregulated AKT2.11 The roles of long non-coding RNAs (lncRNAs) in human cancers have recently elicited more interest from researchers. Furthermore, emerging evidence has demonstrated that lncRNAs are closely related to the occurrence and progression of various cancers.12–14 Elsewhere, an investigation by Zhang Y displayed that silencing lncRNA LINC00152 inhibited the biological activity of lung cancer via inhibition of EGFR, PI3K, and AKT expression.15
Based on our previous investigation on gastric cancer, we used lncRNA expression profile microarray chip assays to identify the differential expression of lncRNAs in 6 paired gastric cancer and non-gastric cancer tissues. Also, qRT-PCR was used to further extensive sample verification. Consequently, lncRNA AC118344.1 was upregulated in gastric cancer tissues by both chip assay and qRT-PCR verification. Importantly, its expression levels in gastric cancer tissues were positively correlated with its potentially associated gene, ie, AKT2 (AKT2 is inferred as AC118344.1 associated gene, which was predicted by bioinformatics analysis). These data reveal that AKT2 might be a downstream molecule of AC118344.1 closely related to gastric cancer. Moreover, we used qRT-PCR to validate that the expression of AKT2 in primary gastric cancer specimens was positively correlated with AC118344.1 expression (Spearman R = 0.587, p <0.01).
Based on our previous study, this work investigated the function of AC118344.1 and the relationship between AC118344.1, AKT2, and its downstream molecules in gastric cancer development.
Materials and Methods
Cells, Reagents, and Mice
A total of 6 human gastric cancer cell lines including AGS, MGC-803, SGC-7901, BGC-823, MKN-45, and MKN-28 were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The human gastric epithelial cell line GES-1 was purchased from the Beijing Institute of Cancer Research (Beijing, China). Notably, the use of human gastric epithelial cell line GES-1 was approved by the Ethics Committee of Hangzhou Medical College. SGC-7901, BGC-823, MKN-45, MKN-28, and GES-1 cell lines were cultured in RPMI-1640 medium (Gibco), AGS cell line was cultured in DMEM culture medium (Gibco), and MGC-803 cell line was cultured in DF12 culture medium (Gibco). The concentration of fetal bovine serum (Gibco) in all culture media was 10%.
BALB/c nude mice aged 5 weeks were purchased from Shanghai Slack Laboratory Animal Co., Ltd., China, and raised under standard conditions. All the experiments performed on animals were approved by the Animal Care and Use Committee of Hangzhou Medical College and implemented following the Guide for the Care and Use of Laboratory Animals (GB/T 35892–2018).
Plasmid Construction and Cell Transfection
Plasmid pcDNA3.1+-AC118344.1 and empty vector pcDNA3.1+ were purchased from IBSBIO Biotechnology Co. Ltd. (Shanghai, China). Short hairpin RNA (shRNA) sequences were designed by Hanbio Biotechnology Co. Ltd. (Shanghai, China) to target human AC118344.1. Double strands of shRNA were inserted into lentiviral pHBLV-U6-Luc-T2A-Puro vector (Hanbio, Shanghai, China), named Lv-shAC118344.1, while negative control was named Lv-shNC. The sequences of primers and shRNA used for plasmid construction are shown in Table 1.
 |
Table 1 Primers and shRNA Sequences of AC11834.4 |
pLVX-Neo-IRES-ZsGreen-AKT2 and pSilencer-4.1-CMV-puro-AKT2 vectors that overexpressed and knocked-down AKT2, respectively, were constructed in our previous studies.16
Plasmid transfections were performed using Lipofectamine LTX (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Stable cell lines were established using corresponding drugs. Notably, cells were incubated for 24h before use in experiments.
Western Blot
Western blot analysis was used to evaluate the expression of AKT2, HK2, MMP2, STAT3, and p-STAT3 in AGS and SGC-7901 cells, respectively. A total of 5 primary antibodies including AKT2#2964, HK2#2867, MMP2#40994, STAT3#12640, and p-STAT3#9145 were purchased from Cell Signal Technology, Inc. (USA). GAPDH antibody was purchased from abMart Company (Shanghai, China). First, the band (expression intensity) was checked by Pierce™ ECL substrate then measured by ChemiDoc™ Touch Imaging System (Bio-Rad, USA). The GAPDH expression was detected in a similar sample as a control. The dilution of the primary antibody was performed based on the manufacturer’s instructions.
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
We previously described reagents and method for isolation of the total RNA in cultured cells were also used in this study.17 The extracted total RNA was used as the template for qRT-PCR for quantified the expression of AC118344.1 and AKT2 mRNA expression. The primers used were forward: 5′-GAGGCGACACAAGATGGGAG-3′ and reverse: 5′-GGTGAATACCCACAGCGAGA-3′ for AC118344.1; forward: 5′-CAAAGAAGGCTGGCTCCACA-3′ and reverse: 5′-ATCCACTCCTCCCTCTCGTC-3′ for AKT2 mRNA; forward: 5′-GTAACCCGTTGAACCCCATT-3′ and reverse: 5′-CCATCCAATCGGTAGTAGCG-3′ for 18sRNA (internal reference); forward: 5′-AGCCACATCGCTCAGACAC-3′, reverse: 5′-GCCCAATACGACCAAATCC-3′ for GAPDH (internal reference). The tests were repeated 3 times, and the results were obtained from these experiments which were expressed as the mean ± SD of ΔCt value.
Cell Proliferation Assay
In total, 5 different groups of AGS and SGC-7901 cells were included in the cell proliferation assays and checked by CCK-8 tests. The 5 groups included, untransfected cells (wild-type cells, NC), transduced with empty vector pcDNA3.1+, transduced with pcDNA3.1+-AC118344.1, transfected with scrambled shRNA (negative shRNA), and transfected with shAC118344.1. The cells were cultured in a density of 4000 cells/per well with 100 µL of culture medium in 96 well-plates. Each group was seeded repeatedly in 6 different wells. The cells were cultured continuously in the medium with lower fetal bovine serum (2% FBS) for 72h followed by culturing in the medium with 10% FBS in a 37°C incubator with 5% CO2 overnight. CCK-8 tests were conducted at time intervals of 6, 24, 48, and 12h, respectively, and were completed by placing CCK-8 (10 μL, 5 mg/mL) (Genview, Beijing, China) into each well after continual growth for another 1 h. The blank which contained CCK-8 and culture medium was set at each time interval. A microplate reader (Bio-Tek, VT, USA) was used to detect the OD450 (absorbance at 450nm) against that of the blank.
Transwell Assay
Corning® BioCoat™ Matrigel® Invasion Chambers (Corning, USA) was selected to evaluate the invasion ability of AGS and SGC-7901 cells in vitro. First, 20% FBS-containing medium was added to the lower chamber of the 24-well cell culture plate. Then, the AGS and SGC-7901 cell suspensions (density 1×105) in serum-free medium were, respectively, inoculated into the upper chamber coated with Matrigel (USA, BD). After culturing for 36 hours in a 5% CO2 humidified incubator at 37°C, cells that were not transferred via the upper cavity were removed. The cells on the underside of the filter were fixed with methanol for 30 minutes then stained with 1% crystal violet for 20 minutes. Subsequently, the filter membrane was removed and rinsed with double distilled water, and the cells were observed and counted in 5 fields of view under a 400x optical microscope.
Nude Mouse Lung Metastasis Experiment
A total of 75 female BALB/c mice were randomly divided into 5 groups (15 in each group), namely the negative control (NC), vector, AC118344.1, scramble shRNA, and shAC118344.1. After rearing for 1 week under SPF conditions, each mouse was injected with the corresponding cell suspension 1*106 (0.2 mL) through the tail vein. On the 45th day, the mice were euthanized, and then the lungs were removed to observe the tumor formation on the lung tissue. The tumor formation rate was calculated then the lung tissues were fixed with 10% paraformaldehyde and stained with hematoxylin-eosin (HE) for tissue disease scientific analysis.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7.0 (GraphPad, San Diego, California, USA). The single-factor analysis of variance (ANOVA) and independent t-test was used to compare cell proliferation, invasion, and nude mouse lung metastasis between groups. p<0.05 was considered as statistically significant.
Results
Upregulation of AC118344.1 in Human Gastric Cancer Cell Lines
The relative expression of AC11834 in 6 human gastric cancer cell lines (AGS, MGC-803, SGC-7901, BGC-823, MKN-45, and MKN-28) and a normal human gastric mucosal epithelial cell line Ges-1 were detected by qRT-PCR. As confirmed in Figure 1, the expression level of AC118344.1 in the 6 gastric cancer cell lines was significantly higher compared to that of the control Ges-1 cells (p <0.01). Additionally, AC118344.1 had the highest expression level in BGC-823 cells (p <0.01). Out of these, the expression of AC118344.1 in AGS and SGC-7901 cells was moderate and similar and considered as a suitable cell line for further research to construct stable overexpression or knockout cell lines.
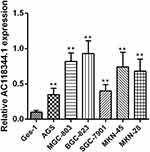 |
Figure 1 Upregulation of AC118344.1 expression in 6 human gastric cancer cells. **Indicates p <0.01. |
Overexpressed AC118344.1 Elevates AGS and SGC-7901 Cell Proliferation
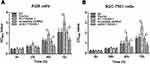
The cell proliferation was significantly elevated in AC118344.1 overexpressed AGS and SGC-7901 cells, and the statistically significant difference appeared at the 48h while the largest increased peak was at 72h compared to that in control cells (empty vector transduction) (p < 0.01) or in wild-type cells (p < 0.01). Whereas in terms of the differences between empty vector transduction and wild-type cells in cell proliferation, all p < 0.05 at the measured periods of this investigation, detecting by CCK-8 tests (Figure 2A and B). In AGS and SGC-7901 cells stably knocked down AC118344.1 expression by transfection with Lv-shAC118344.1, statistically significant differences were detected compared to that in Lv-scramble shRNA control groups (AGS and SGC-7901 cells transfected with scramble shRNA)(p < 0.01). These data assert that AC118344.1 promotes the regulation of cell proliferation in gastric cancer cells.
AC118344.1 Improves Gastric Cancer Cell Invasion
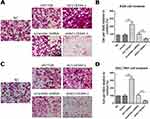
As shown in Figure 3A–D, transwell cell invasion analysis revealed that compared to empty vector transduction, overexpression of AC118344.1 significantly increased the invasion ability of AGS and SGC-7901 cells (p<0.01). Moreover, gastric cancer cells SGC-7901 overexpressing AC118344.1 harbored higher invasive ability compared to AGS cells. Notably, no statistically significant difference was detected in cell invasion ability between the 2 control groups (empty vector-transduced cells and wild-type cells, p<0.01). In contrast, using the shRNA method to knock down AC118344.1 significantly reduced the invasion ability of AGS and SGC-7901 cells (p<0.01). Similarly, there was no statistically significant difference in cell invasion ability between the 2 control groups (p<0.01).
AC118344.1 Promotes Lung Metastatic Nodules in Nude Mice
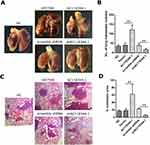
The mice were euthanized 45 days after injecting the corresponding AGS cells into their tail veins. In line with our transwell invasion in vitro results, it was found that unlike the blank control group and the empty vector transduction control group, overexpression of AC118344.1 in the AGS cell line significantly promoted the lung metastasis of AGS gastric cancer cells. Besides, and the difference was statistically significant (p <0.01; Figure 4A–D).
In contrast, unlike the Scramble shRNA control group, the use of shRNA in knocking down AC118344.1 significantly reduced the lung metastasis ability of AGS gastric cancer cells (p <0.01; Figure 4A–D). There was no statistically significant difference in lung metastasis between the blank control group and the Scramble shRNA control group (p>0.05; Figure 4A–D).
These findings further suggest that AC118344.1 improves the metastatic ability of gastric cancer cells and promotes the progress of gastric cancer.
AC118344.1 Potentially Act as the Upstream Molecule of AKT2
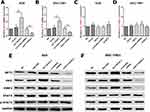
In our previous work on gastric cancer, AKT2 was predicted and verified to be potentially associated with mRNA of AC118344.1 (unreported data). Therefore, their relationship was investigated in this study. Using qRT-PCR and Western blot, overexpression of AC118344.1 in gastric cancer cells AGS and SGC-7901 significantly upregulated the expression of AKT2 mRNA (p < 0.01, Figure 5A and B) and protein (Figure 5E and F). On the contrary, knocking down AC118344.1 significantly downregulated the expression of AKT2 mRNA (p < 0.01, Figure 5A and B) and protein (Figure 5E and F). Of note, there was no significant difference in AKT2 expression between the 2 empty vector groups and wild-type AGS and SGC-7901 cells (all p < 0.05, Figure 5A and B).
Conversely, in gastric cancer cells AGS and SGC-7901, neither overexpression of AKT2 nor knockdown of AKT2 could change the expression of AC118344.1 (p < 0.01, Figure 5C and D). At the same time, there was no significant difference in the expression level of AC118344.1 between the 2 empty vector groups and wild-type AGS and SGC-7901 cells (all p < 0.05, Figure 5C and D).
These data imply that AC118344.1 might be the upstream molecule of AKT2 regulating AKT2 mediated gastric cancer cell progression.
AC118344.1 Regulates AKT2 to Promote the Progression of Gastric Cancer Cells Through Its Downstream Molecules HK2 and MMP2
Many findings have established that AKT2 regulates the occurrence and development of malignant tumors via its multiple downstream molecules (such as STAT3, PKM2, MMP2, HK2, etc.). Nonetheless, the AKT2 downstream molecules regulated by AC118344.1 to promote the progression of gastric cancer cells remain unreported. As shown in Figure 5E and F, it was found that overexpression of AC118344.1 in AGS and SGC7901 cells upregulated the expression of AKT2 and MMP2 and HK2. Nevertheless, the expression of STAT3 and p-STAT3 remained unchanged. In contrast, knocking down AC118344.1 downregulated the expression of AKT2 as well as MMP2 and HK2. Similarly, the expression of STAT3 and p-STAT3 remained unchanged. The above results indicate that AC118344.1 promotes the progression of gastric cancer cells via AKT2 and its downstream molecules MMP2 and HK2 except STAT3.
Discussion
AKT2 promotes the development of several human cancers. Specifically, its contribution to gastric cancer cell progression has been well established and widely documented.18,19 As such, AKT2 is arguably a true cancer therapy or treatment monitoring target. In recent years, accumulating evidence has identified the significance of AKT2 in cancer. For instance, data from the Miyoshi T group showed that large-cell neuroendocrine carcinoma of the lung (LCNEC) tissues existed in 4% AKT2 mutants, which might potentially exhibit value as targeted therapies.20 Ding Z et al demonstrated that using shRNA against AKT2 suppressed, elevated, as well as increased colorectal cancer cell proliferation, apoptosis, and paclitaxel chemosensitivity, respectively.21 Additionally, Gong JN et al reported that microRNA-29 family (miR-29a, −29b and −29c) regulated inhibited abnormal proliferation and induced cell apoptosis in acute myeloid leukemia (AML) bone marrow cells which might be related to their function in the inhibition of their targets, ie, AKT2 and CCND1 mRNA expression.22 Elsewhere, Zhou B et al reported that AKT2 acts as the direct target of microRNA let-7a in papillary thyroid carcinoma (PTC), and upregulating the expression of let-7a decreased cell proliferation, migration, and invasion in PTC cells via inhibiting AKT2 expression.23 Notably, numerous studies have demonstrated the essential roles of AKT2 in human cancers. Nonetheless, the precise molecular mechanisms on how AKT2 functions remain undetermined.
In recent years, increasing evidence has shown that lncRNA regulates the expression of numerous human genes, and is closely related to the occurrence and development of various human cancers.24,25 Besides, findings by Zhang Y reveal that lncRNA LINC00152 is linked to the expression AKT in lung cancer.26 Several sources of evidence also report additional lncRNAs associated with cancer via AKT2, including lncRNA H19 in gallbladder cancer cell proliferation and LncRNA Gm15290 in non-Small Cell Lung Cancer progression.27,28 However, the roles of lncRNA and AKT2 in gastric cancer remain unclear.
By in vitro cell function and in vivo nude mice lung metastasis assays, we first established that AC118344.1 potentially plays a vital role in increasing gastric cancer cell proliferation, invasion, and lung metastasis by regulating the expression of AKT2. Notably, AC118344.1 is an intron sense-overlapping lncRNA with a length of 1462nt, located in chromosome 19, whose associated gene was predicted to AKT2. Our data showed that suppressing the expression of AC118344.1 inhibited AGS and SGC-7901 cell proliferation, invasion, and lung metastasis as well as the expression of AKT2 mRNA and protein. In contrast, suppressing the expression of AKT2 showed no inhibition to the expression of AC118344.1. Furthermore, we demonstrated that AC118344.1 promotes the progression of gastric cancer cells by AKT2 and its downstream molecules MMP2 and HK2 except STAT3.
Therefore, AKT2 is potentially a downstream molecule of AC118344.1 regulating gastric cancer cell progression.
Conclusion
In conclusion, our findings suggest that both AC118344.1 and AKT2 promote gastric cancer cell development, and can potentially be used as therapeutic targets for gastric cancer.
Acknowledgments
This work was supported by the Zhejiang Medical and Health Science and Technology Plan (No. 2018KY347).
Disclosure
The authors declare no financial or nonfinancial conflicts of interest.
References
1. Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18:534–542. doi:10.1016/j.cgh.2019.07.045
2. Sano T. Gastric cancer: Asia and the world. Gastric Cancer. 2017;20:1–2. doi:10.1007/s10120-017-0694-9
3. Biagioni A, Skalamera I, Peri S, et al. Update on gastric cancer treatments and gene therapies. Cancer Metastasis Rev. 2019;38:537–548.
4. Shariati M, Meric-Bernstam F. Targeting AKT for cancer therapy. Expert Opin Investig Drugs. 2019;28:977–988. doi:10.1080/13543784.2019.1676726
5. Mirza M, Ekrami EM, Aghdas SAM, et al. Targeting PI3K/Akt/mTOR signaling pathway by polyphenols: implication for cancer therapy. Life Sci. 2020;255:117481. doi:10.1016/j.lfs.2020.117481
6. Noorolyai S, Shajari N, Baghbani E, et al. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–128. doi:10.1016/j.gene.2019.02.076
7. Yang Q, Zhang RW, Sui PC, et al. Dysregulation of non-coding RNAs in gastric cancer. World J Gastroenterol. 2015;21:10956–10981.
8. Wu Y, Qian Z. Long non-coding RNAs (lncRNAs) and microRNAs regulatory pathways in the tumorigenesis and pathogenesis of glioma. Discov Med. 2019;28:129–138.
9. Zong W, Ju S, Jing R, et al. Long non-coding RNA-mediated regulation of signaling pathways in gastric cancer. Clin Chem Lab Med. 2018;56:1828–1837. doi:10.1515/cclm-2017-1139
10. Zhang J, Zhang J, Qiu W, et al. MicroRNA-625 inhibits the proliferation and increases the chemosensitivity of glioma by directly targeting AKT2. Am J Cancer Res. 2017;7:1835–1849.
11. Ma X, Zhuang B, Li W. MicroRNA2965p downregulated AKT2 to inhibit hepatocellular carcinoma cell proliferation, migration and invasion. Mol Med Rep. 2017;16:1565–1572. doi:10.3892/mmr.2017.6701
12. Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi:10.1038/onc.2017.184
13. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi:10.1038/nm.3981
14. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–463. doi:10.1016/j.ccell.2016.03.010
15. Zhang Y, Xiang C, Wang Y, et al. lncRNA LINC00152 knockdown had effects to suppress biological activity of lung cancer via EGFR/PI3K/AKT pathway. Biomed Pharmacother. 2017;94:644–651. doi:10.1016/j.biopha.2017.07.120
16. Zheng B, Geng L, Zeng L, et al. AKT2 contributes to increase ovarian cancer cell migration and invasion through the AKT2-PKM2-STAT3/NF-kappaB axis. Cell Signal. 2018;45:122–131. doi:10.1016/j.cellsig.2018.01.021
17. Li H, Lu S, Chen Y, et al. AKT2 phosphorylation of hexokinase 2 at T473 promotes tumorigenesis and metastasis in colon cancer cells via NF-κB, HIF1α, MMP2, and MMP9 upregulation. Cell Signal. 2019;58:99–110. doi:10.1016/j.cellsig.2019.03.011
18. Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–1527. doi:10.1016/j.cellsig.2011.05.004
19. Hinz N, Jucker M. Distinct functions of AKT isoforms in breast cancer: a comprehensive review. Cell Commun Signal. 2019;7:154.
20. Miyoshi T, Umemura S, Matsumura Y, et al. Genomic profiling of large-cell neuroendocrine carcinoma of the lung. Clin Cancer Res. 2017;23:757–765. doi:10.1158/1078-0432.CCR-16-0355
21. Ding Z, Xu F, Li G, et al. Knockdown of Akt2 expression by shRNA inhibits proliferation, enhances apoptosis, and increases chemosensitivity to paclitaxel in human colorectal cancer cells. Cell Biochem Biophys. 2015;71:383–388. doi:10.1007/s12013-014-0209-9
22. Gong JN, Yu J, Lin HS, et al. The role, mechanism and potentially therapeutic application of microRNA-29 family in acute myeloid leukemia. Cell Death Differ. 2014;21:100–112. doi:10.1038/cdd.2013.133
23. Zhou B, Shan H, Su Y, et al. Let-7a inhibits migration, invasion and tumor growth by targeting AKT2 in papillary thyroid carcinoma. Oncotarget. 2017;8:69746–69755. doi:10.18632/oncotarget.19261
24. Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19(5):1310. doi:10.3390/ijms19051310
25. Sanchez A, Kawamura Y, Yamamoto Y, et al. Emerging roles of long non-coding RNA in cancer. Cancer Sci. 2018;109:2093–2100. doi:10.1111/cas.13642
26. Zhang L, Wang Y, Su H. Long non-coding RNA LINC00152/miR-613/CD164 axis regulates cell proliferation, apoptosis, migration and invasion in glioma via PI3K/AKT pathway. Neoplasma. 2020;67(04):762–772. doi:10.4149/neo_2020_190706N598
27. Wang SH, Wu XC, Zhang MD, et al. Long noncoding RNA H19 contributes to gallbladder cancer cell proliferation by modulated miR-194-5p targeting AKT2. Tumour Biol. 2016;37:9721–9730. doi:10.1007/s13277-016-4852-1
28. Dong Y, Huo X, Sun R, et al. lncRNA Gm15290 promotes cell proliferation and invasion in lung cancer through directly interacting with and suppressing the tumor suppressor miR-615-5p. Biosci Rep. 2018;38. doi:10.1042/BSR20181150
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.