Back to Journals » Cancer Management and Research » Volume 12
Long Intergenic Non-Coding RNA LINC00922 Aggravates the Malignant Phenotype of Breast Cancer by Regulating the microRNA-424-5p/BDNF Axis
Received 13 June 2020
Accepted for publication 24 July 2020
Published 21 August 2020 Volume 2020:12 Pages 7539—7552
DOI https://doi.org/10.2147/CMAR.S267665
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
This paper has been retracted.
Xin Yue, Zhuo Wang
Department of Breast Surgery, The First People’s Hospital of Jingzhou, Jingzhou, Hubei, 434000, People’s Republic of China
Correspondence: Zhuo Wang Department of Breast Surgery
The First People’s Hospital of Jingzhou, No. 8 Hangkong Road, Shashi District, Jingzhou, Hubei 434000, People’s Republic of China
Email [email protected]
Purpose: Long intergenic non-coding RNA 922 (LINC00922) plays a critical role in the progression of lung cancer. In this study, we aimed to quantify LINC00922 expression in breast cancer and determine its influence on the malignant behavior of breast cancer cells in vitro and in vivo. We also investigated the mechanism by which LINC00922 affects the progression of breast cancer.
Materials and Methods: Reverse transcription-quantitative polymerase chain reaction was performed to quantify LINC00922 expression in breast cancer tissues and cell lines. The cell counting kit-8 assay, flow cytometry, Transwell migration and invasion assays, and tumor model assays were performed to determine the effects of LINC00922 deficiency on breast cancer cell proliferation, apoptosis, migration and invasion in vitro, and tumor growth in vivo, respectively. Furthermore, bioinformatics analysis was performed to predict the potential target microRNA of LINC00922. The prediction was further evaluated using luciferase reporter and RNA immunoprecipitation assays.
Results: LINC00922 was clearly overexpressed in breast cancer tissues and cell lines. LINC00922 depletion restricted breast cancer cell proliferation, migration, and invasion but induced cell apoptosis in vitro. Additionally, its knockdown evidently repressed tumor growth of breast cancer cells in vivo. Mechanistically, LINC00922 was demonstrated to serve as a molecular sponge for miR-424-5p in breast cancer cells. Furthermore, brain-derived neurotrophic factor (BDNF) was verified as a direct target of miR-424-5p in breast cancer cells, and BDNF expression was found to be positively regulated by LINC00922 through sponging miR-425-5p. Rescue experiments further revealed that the influences on breast cancer cell proliferation, apoptosis, migration, and invasion induced by LINC00922 silencing were abrogated by increasing the output of the miR-424-5p/BDNF axis.
Conclusion: The LINC00922/miR-424-5p/BDNF pathway is implicated in the acceleration of the malignant behavior of breast cancer cells. These findings suggest that this pathway is a promising novel molecular target in breast cancer therapy.
Keywords: RNA immunoprecipitation, quantitative reverse transcription PCR, brain-derived neurotrophic factor, apoptosis, molecular sponge
Introduction
Breast cancer is the most prevalent malignant tumor and the leading cause of tumor-related mortality among women.1 Globally, approximately 1.7 million women are predicted to be diagnosed with breast cancer annually, leading to 522,000 mortalities.2 Owing to advances that allow early detection of cancer and curative therapeutic methods, the therapeutic effects and long-term prognosis of breast cancer have improved in the past decade, with a 5-year survival of approximately 90%.3 However, the clinical outcomes of patients diagnosed with advanced-stage breast cancer remain unfavorable, with a 5-year survival of <6%.4 Advanced-stage breast cancer is difficult to treat because of its aggressive nature and may result in tumor recurrence, metastasis, or death.5 Thus, a better understanding of the molecular mechanisms underlying breast carcinogenesis and progression may contribute to the identification of attractive anticancer therapies.
In recent years, non-coding RNAs have drawn more and more attention.6 Long non-coding RNAs (lncRNAs) are RNA transcripts that comprise >200 nucleotides, lack open reading frames, and have no protein-coding capacity.7 Accumulating evidence suggests that lncRNAs execute critical roles in regulating pathological and physiological behaviors of cancer cells.8–11 During cancer genesis and progression, cancer-inhibiting and cancer-promoting cellular pathways are regulated by lncRNAs via direct or indirect effects, including gene expression regulation, chromatin remodeling, post-transcriptional modulation, and translational regulation.12 Recent evidence has revealed the dysregulation of lncRNAs in breast cancer and has shown that aberrantly expressed lncRNAs are implicated in breast cancer pathogenesis.13–15
MicroRNAs (miRNAs) are a category of highly stable, single-stranded, endogenous RNA molecules comprising 18–25 nucleotides.16 miRNAs can negatively regulate gene expression via complete or incomplete binding to the 3′-untranslated regions (3′-UTRs) of their target genes, thereby decreasing translation and/or promoting the degradation of mRNAs.17,18 Many studies have revealed that miRNAs serve as important regulators of breast cancer oncogenesis and are engaged in a wide spectrum of malignant processes.19 In recent years, increasing evidence has identified the cross-modulation between lncRNAs and miRNAs in human cancer.20–22 lncRNAs can function as competing endogenous RNAs (ceRNAs) to regulate mRNA expression by competitively binding to shared miRNAs.23,24 Consequently, elucidation of the critical roles of lncRNAs and miRNAs in breast cancer may provide insight into cancer diagnosis and therapy.
Long intergenic non-coding RNA 922 (LINC00922) has been identified as an important regulator of lung cancer progression.25 To the best of our knowledge, the role of LINC00922 in regulating the aggressive nature of breast cancer and its underlying mechanisms are largely unknown. In this study, LINC00922 expression was detected in breast cancer tissues and cell lines. A series of functional assays were performed to assess the influence of LINC00922 on the malignant behavior of breast cancer cells in vitro and in vivo. Finally, experiments were conducted to assess the mechanism by which LINC00922 affects breast cancer progression.
Materials and Methods
Patient Samples and Cell Lines
This study was approved by the Ethics Committee of The First People's Hospital of Jingzhou (JZFPH. #20,170,405) and adhered to the tenets of the Declaration of Helsinki. Informed consent was provided by all participants prior to their participation in the study. Human breast cancer tissues and matched normal tissues were acquired from 58 patients with breast cancer admitted to The First People's Hospital of Jingzhou. No preoperative radiotherapy, chemotherapy, or other anticancer therapies were given to these patients. Fresh tissues were snap frozen in liquid nitrogen and stored in liquid nitrogen until further use.
The human immortalized breast epithelial cell line MCF-10A and breast cancer cell line BT-474 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). MCF-10A cells were cultured in Mammary Epithelial Cell Growth Medium BulletKitTM (Lonza/Clonetics Corporation, Walkersville, MD, USA) supplemented with 100 ng/mL cholera toxin. BT-474 cells were cultured in Hybri-Care Medium (ATCC) containing 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA), 100 U/mL penicillin (Gibco), and 100 mg/mL streptomycin (Gibco). Three other breast cancer cell lines, MCF-7, SKBR3, and MDA-MB-231, were obtained from the Shanghai Institute for Biological Sciences, Chinese Academy of Sciences (Shanghai, China), and grown in Eagle’s minimum essential medium (ATCC), Dulbecco’s modified Eagle’s medium (Gibco), and Leibovitz’s L-15 medium (Gibco). The other culture conditions of these three cell lines were the same as those used to culture BT-474 cells. All cells were maintained at 37°C in a humidified incubator supplied with 5% CO2.
RNA Oligonucleotides, Plasmids, and Cell Transfection
The LINC00922 small interfering (si)RNA (si-LINC00922) and scrambled siRNA [si-negative control (NC)] were designed and synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). miR-424-5p mimic, NC miRNA mimic (miR-NC), miR-424-5p inhibitor (anti-miR-424-5p), and NC inhibitor were purchased from Guangzhou RiBoBio Co., Ltd. (Guangzhou, China). The pcDNA 3.1 plasmid specific to brain-derived neurotrophic factor (BDNF) (termed pc-BDNF) and empty pcDNA3.1 plasmid were obtained from GeneChem Company (Shanghai, China). Cells were seeded into 6-well plates, and transfection of RNA oligonucleotides or plasmids was performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Nuclear–Cytoplasmic Fractionation
Breast cancer cells were separated into nuclear and cytoplasmic fractions using the PARIS kit (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was isolated from both fractions and subjected to reverse transcription-quantitative PCR (RT-qPCR) to measure the levels of LINC00922, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and U6 small nuclear RNA expressions. GAPDH was used as the cytoplasmic loading control and U6 small nuclear expression was used as the nuclear loading control.
RT-qPCR
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to isolate total RNA from tissues or cells. The quality and quantity of total RNA was determined using NanoDrop 2000 (Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed into first-strand complementary DNA (cDNA) using the PrimeScript RT Reagent Kit (Takara, Dalian, China). Thereafter, RT-qPCR was performed to measure the levels of LINC00922 and BDNF expressions using the SYBR Premix Ex Taq (Takara). GADPH served as the endogenous control for LINC00922 and BDNF.
For the determination of miR-424-5p expression, total RNA was extracted using the miRNeasy Mini Kit (Qiagen GmbH, Hilden, Germany) and was used for the synthesis of cDNA using the miScript Reverse Transcription kit (Qiagen GmbH). Next, the produced cDNA was subjected to qPCR using the miScript SYBR Green PCR kit (Qiagen GmbH). Expression of miR-424-5p was normalized to that of U6. All reactions were conducted using an Applied Biosystems 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA), and relative gene expression was analyzed using the 2−ΔΔCt method.
Cell Counting Kit-8 (CCK-8) Assay
After 24 h of cultivation, transfected cells were harvested and suspended in complete culture medium. Then, 100 μL of cell suspension containing 2 × 103 cells was added to each well of the 96-well plates, after which cells were cultured at 37°C in a humidified incubator supplied with 5% CO2 for 0, 24, 48, and 72 h. At the indicated time points, cells were incubated with 10 μL of CCK-8 solution (Sigma-Aldrich; Merck KGaA) at 37°C for another 2 h. The optical density of each well was detected at a wavelength of 450 nm using a Tecan microplate reader (Tecan Group, Ltd.).
Flow Cytometry
After 48 h of incubation, the transfected cells were removed from the 6-well plates by treatment with a trypsin reagent without ethylenediaminetetraacetic acid, followed by rinsing with pre-cooled phosphate-buffered saline solution. After centrifugation, the phosphate-buffered saline solution was discarded and the apoptotic status of the obtained cells was tested using an annexin V–fluorescein isothiocyanate (FITC) apoptosis detection kit (BioLegend, Inc.). The cells were resuspended in 100 μL of 1× binding buffer and additionally treated with 5 μL of annexin V–FITC and 5 μL of propidium iodide in the dark at room temperature for 15 min. Finally, double-stained cells were analyzed using the FACSCalibur Flow Cytometry system (BD Biosciences, San Jose, CA, USA) and the proportion of apoptotic cells was evaluated using CellQuest version 2.9 (BD Biosciences).
Transwell Migration and Invasion Assays
To determine cell migration, transfected cells were trypsinized after 48 h of incubation, centrifuged, and resuspended in serum-free culture medium. The upper chambers of Transwell inserts (BD Biosciences) were seeded with 100 μL of cell suspension containing 5 × 104 cells, whereas 500 μL of culture medium containing 20% FBS was added to the lower chambers. Twenty-four hours later, the non-migrated cells that remained on the upper surface of the membrane were gently scraped with a cotton tip, whereas the migrated cells were fixed with 4% cold paraformaldehyde and stained with 0.05% crystal violet. After imaging, five fields were randomly selected and the average number of migrated cells was counted. The experimental steps of the Transwell invasion assays were the same as those described for the Transwell migration assays, except that the insets were precoated with Matrigel matrix (BD Biosciences).
Tumor Model Assay
LINC00922 short hairpin (sh)RNA (sh-LINC00922) and scrambled shRNA (sh-NC) were obtained from Shanghai GenePharma Co., Ltd. and inserted into the GenePharma SuperSilencing Vector. Following lentivirus packaging, MCF-7 cells were transfected with lentivirus expressing sh-LINC00922 or sh-NC. The sh-LINC00922- or sh-NC-stably transfected MCF-7 cells were selected by incubation with puromycin.
All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee of The First People's Hospital of Jingzhou (JZFPH. #20,190,115) and performed following the NIH guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85–23 Rev.1985). Four- to six-week-old female BALB/c nude mice were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and assigned to two groups: sh-LINC00922 or sh-NC. The sh-LINC00922 group was subcutaneously injected with 1 × 106 MCF-7 cells stably expressing sh-LINC00922, whereas the same number of MCF-7 cells with sh-NC was injected into the sh-NC group. The dimensions of tumor xenografts were recorded once every 5 days, and their volume was calculated using the following equation: tumor volume (mm3) = 1/2 × length (mm) × width2 (mm2). All mice were euthanized at post-injection day 35, and tumor xenografts were extracted and weighed.
Bioinformatics Analysis
Gene Expression Profiling Interactive Analysis (GEPIA; http://gepia.cancer-pku.cn/) was used to determine the expression status and clinical value of LINC00922 in breast cancer. The interaction between lncRNA and miRNA was assessed using StarBase version 3.0 (http://starbase.sysu.edu.cn/). Three online databases, ie, StarBase version 3.0, miRDB (http://mirdb.org/), and TargetScan (http://www.targetscan.org/), were employed to identify the potential targets of miR-424-5p.
Luciferase Reporter Assay
The potential miR-424-5p-binding site in LINC00922 wild-type (wt) fragments and the corresponding mutant (mut) fragments were synthesized and inserted into the pmirGLO Dual-Luciferase vector (Promega, Madison, WI). The constructed luciferase reporter plasmids were termed LINC00922-wt and LINC00922-mut, respectively. BDNF-wt and BDNF-mut reporter plasmids were designed and constructed using the same experimental steps. For the luciferase reporter assay, cells were seeded into 24-well plates and then cotransfected with miR-424-5p mimic or miR-NC and either the wt or mut reporter plasmids using Lipofectamine 2000. After 48 h, the Dual-Luciferase Reporter Assay System (Promega) was used to detect firefly and Renilla luciferase activities. Renilla luciferase activity was used for normalization.
RNA Immunoprecipitation (RIP) Assay
The Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) was used for the RIP assay. Breast cancer cells were scraped off from 6-well plates and incubated with the RIPA lysis buffer. The cell lysate was treated with RIPA lysis buffer containing magnetic beads conjugated with a human antibody against Ago2 (Millipore) or negative control immunoglobulin G (IgG; Millipore). After incubation overnight at 4°C, the beads were harvested and washed with pre-cold NT2 buffer, followed by incubating with proteinase K for protein digestion. RT-qPCR was performed to test the enrichment of LINC00922 and miR-424-5p in the extracted immunoprecipitated RNA.
Western Blotting
Total protein was extracted using the RIPA lysis buffer with 1% phenylmethylsulfonyl fluoride and 1% protease inhibitor cocktail (KeyGEN BioTECH; Nanjing, China). Protein concentration was detected using the BCA Protein Assay Kit (KeyGEN BioTECH). After adding equal amounts of protein to each lane, the separation of total protein was conducted using 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis. The separated proteins were then transferred onto polyvinylidene difluoride membranes and blocked with 5% fat-free milk at room temperature for 2 h. Primary antibodies against BDNF (cat. No. ab205067; Abcam, Cambridge, UK) or GAPDH (cat. No. ab8245; Abcam) each at a dilution of 1:1000, were incubated with the membranes at 4°C overnight. After washing once, the membranes were further incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:5000 dilution; cat. No. ab205719; Abcam). Protein signals on the membranes were developed after the addition of an enhanced chemiluminescence Western blotting detection reagent (GE Healthcare Life Sciences, Little Chalfont, UK).
Statistical Analysis
All statistical analyses were conducted using SPSS version 18.0 (SPSS, Inc.). Student’s t-test was performed to test the differences between two groups, and one-way analysis of variance followed by Dunnett’s post hoc test was performed to compare differences among three or more groups. Pearson’s correlation coefficient was used to evaluate the correlation between the expression levels of LINC00922 and miR-424-5p. All results are presented as mean ± SD, and a P-value of <0.05 was considered to indicate a statistically significant difference.
Results
Downregulation of LINC00922 Restricts Breast Cancer Cell Proliferation, Facilitates Cell Apoptosis, and Impedes Cell Migration and Invasion in vitro
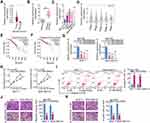
The GEPIA database was used to assess LINC00922 expression in breast cancer. LINC00922 was found to be highly expressed in breast cancer tissues compared with that in normal tissues (Figure 1A). To confirm this observation, LINC00922 expression was quantified in 58 pairs of breast cancer and matched adjacent normal tissues by RT-qPCR. LINC00922 was found to be upregulated in breast cancer tissues compared with that in adjacent normal tissues (Figure 1B). RT-qPCR was also performed to determine LINC00922 expression in four breast cancer cell lines: BT-474, MCF-7, SKBR3, and MDA-MB-231. MCF-10A served as the control. Results showed that LINC00922 expression was higher in these breast cancer cell lines than in MCF-10A (Figure 1C). We also evaluated the clinical value of upregulation of LINC00922 expression in breast cancer using the GEPIA database. Patients with breast cancer in the high-LINC00922 group had no significant correlation with tumor stage (Figure 1D), overall survival (Figure 1E), and disease-free survival (Figure 1F).
To determine whether LINC00922 is associated with the progression of breast cancer, we transfected MCF-7 and MDA-MB-231 cells with si-LINC00922 to silence endogenous LINC00922 expression. si-LINC00922#2 presented the highest efficiency in decreasing endogenous LINC00922 expression (Figure 1G) and was therefore used in the subsequent experiments. CCK-8 assay indicated that the transfection of si-LINC00922 decreased proliferation of MCF-7 and MDA-MB-231 cells (Figure 1H). In addition, flow cytometry was performed to assess the influence of LINC00922 knockdown on breast cancer cell apoptosis. Apoptosis of MCF-7 and MDA-MB-231 cells was enhanced by LINC00922 silencing (Figure 1I). Furthermore, the migratory and invasive abilities of LINC00922-deficient MCF-7 and MDA-MB-231 cells were characterized using Transwell migration and invasion assays. LINC00922 depletion notably reduced the migratory (Figure 1J) and invasive (Figure 1K) abilities of MCF-7 and MDA-MB-231 cells. Collectively, these findings suggest that LINC00922 functions as an oncogenic lncRNA that facilitates the malignant progression of breast cancer.
LINC00922 Serves as a Molecular Sponge for miR-424-5p in Breast Cancer Cells
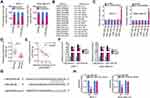
LncRNAs perform specific functions by serving as ceRNAs of miRNAs that abrogate miRNA-mediated degradation of the direct targets of miRNAs.26 Cytoplasmic and nuclear RNA fractions were analyzed using RT-qPCR to determine the localization of LINC00922 in breast cancer cells. The results indicated that LINC00922 was primarily concentrated in the cytoplasm of MCF-7 and MDA-MB-231 cells (Figure 2A), suggesting that LINC00922 serves as a ceRNA in breast cancer. Accordingly, bioinformatics analysis was performed to predict the putative miRNA(s) that bind to sequence(s) within LINC00922.
A total of 15 miRNAs were predicted to have a potential binding site on LINC00922 (Figure 2B). Among these candidates, miR-346,27 miR-15b-5p,28 and miR-513a-5p29 were upregulated in breast cancer and were therefore excluded from this study cohort. Among the remaining identified miRNAs, miR-383-5p,30 miR-195-5p,31 miR-497-5p,32 miR-16-5p,33 miR-15a-5p,34 and miR-424-5p35 were selected for experimental verification because these miRNAs have been widely reported to be downregulated in breast cancer and to have an anticancer role. We subsequently determined whether LINC00922 is involved in the regulation of the expressions of these miRNAs in breast cancer. RT-qPCR indicated that transfection with si-LINC00922 resulted in an obvious increase in the expression of miR-424-5p, whereas the expressions of other miRNAs remained unaltered in response to LINC00922 knockdown (Figure 2C). Relative miR-424-5p expression in the 58 pairs of breast cancer tissues and adjacent normal tissues was then evaluated using RT-qPCR. miR-424-5p expression was lower in the breast cancer tissues than in the adjacent normal tissues (Figure 2D). In the 58 breast cancer tissues, there was an inverse correlation between the expression levels of LINC00922 and miR-424-5p (Figure 2E; r = −0.7114, P < 0.0001), as assessed using Pearson’s correlation coefficient.
Ago2 is a crucial component of the RNA-induced silencing complex and downregulates mRNA through specific miRNAs. Hence, the anti-Ago2 antibody was used in the RIP assay of MCF-7 and MDA-MB-231 cell lysates to test whether LINC00922 can directly bind to Ago2. As shown in Figure 2F, LINC00922 and miR-424-5p were enriched in the Ago2 immunoprecipitates compared with that in the control IgG group. This suggests that there is a direct interaction between LINC00922 and miR-424-5p in breast cancer cells. The wt and mutant binding sites for miR-424-5p on LINC00922 are presented in Figure 2G. Luciferase reporter assay results revealed that miR-424-5p directly binds to LINC00922 and thereby decreases the luciferase activity of LINC00922-wt (Figure 2H) but not that of LINC00922-mut. These observations conclusively indicated that LINC00922 directly interacts with and serves as a molecular sponge for miR-424-5p in breast cancer cells.
LINC00922 Positively Regulates BDNF Expression in Breast Cancer Cells by Competitively Binding to miR-424-5p
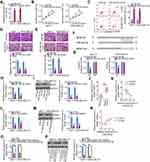
To further assess the activity of miR-424-5p in breast cancer cells, miR-424-5p mimic was transfected into MCF-7 and MDA-MB-231 cells and RT-qPCR was performed to assess the efficiency of miR-424-5p overexpression. Transfection with miR-424-5p mimic evidently increased miR-424-5p expression in MCF-7 and MDA-MB-231 cells (Figure 3A). CCK-8 assay and flow cytometry were performed to assess proliferation and apoptosis of miR-424-5p-overexpressing MCF-7 and MDA-MB-231 cells. miR-424-5p upregulation significantly inhibited the proliferation (Figure 3B) and promoted the apoptosis (Figure 3C) of MCF-7 and MDA-MB-231 cells. In addition, the migratory (Figure 3D) and invasive (Figure 3E) abilities of MCF-7 and MDA-MB-231 cells decreased following miR-424-5p overexpression.
Three online databases (miRDB, StarBase version 3.0, and TargetScan) were used to search for an miR-424-5p target. BDNF was predicted as a candidate target gene of miR-424-5p; the binding sequences of miR-424-5p in the 3′-UTR of BDNF mRNA are illustrated in Figure 3F. Next, luciferase reporter assay showed that exogenous miR-424-5p expression drastically decreased BDNF-wt-driven luciferase activity; however, this suppressive effect was abrogated by mutation of the predicted miR-424-5p-binding sequence (Figure 3G). BDNF mRNA (Figure 3H) and protein (Figure 3I) expression levels were reduced with miR-424-5p overexpression in MCF-7 and MDA-MB-231 cells, as indicated by RT-qPCR and Western blotting, respectively. Next, to examine the correlation between miR-424-5p and BDNF expressions in breast cancer cells, RT-qPCR was performed to quantify the BDNF mRNA expression levels in the 58 pairs of breast cancer tissues and matched adjacent normal tissues. BDNF mRNA expression was upregulated in breast cancer tissues (Figure 3J), and this overexpression was inversely correlated with miR-424-5p expression (Figure 3K; r = −0.6577, P < 0.0001). These results suggest that there is an miRNA–target mRNA relationship between miR-424-5p and BDNF mRNA in breast cancer.
Thus, LINC00922 serves as a molecular sponge for miR-424-5p in breast cancer cells and BDNF is a direct target gene of miR-424-5p. Hence, we hypothesized that LINC00922 functions as a ceRNA of miR-424-5p and consequently positively regulates BDNF expression in breast cancer. To test this hypothesis, BDNF expression was quantified following LINC00922 downregulation in MCF-7 and MDA-MB-231 cells. LINC00922 knockdown evidently decreased the BDNF mRNA (Figure 3L) and protein (Figure 3M) expression levels in MCF-7 and MDA-MB-231 cells. A significant positive correlation was identified between BDNF mRNA and LINC00922 expression levels in the 58 breast cancer tissues (Figure 3N; r = 0.7041, P < 0.0001). A rescue experiment was performed to determine whether the miR-424-5p sponge is required for the positive modulation of LINC00922 on BDNF expression in breast cancer cells. MCF-7 and MDA-MB-231 cells were cotransfected with si-LINC00922 and miR-424-5p inhibitor (anti-miR-424-5p) or NC inhibitor. After transfection, Western blotting and RT-qPCR were conducted to determine the changes in BDNF expressions. The si-LINC00922-induced decreases in BDNF mRNA (Figure 3O) and protein (Figure 3P) expression levels were effectively reversed after cotransfection with anti-miR-424-5p. These findings suggest that LINC00922 is a ceRNA that can directly interact with miR-424-5p in breast cancer cells and participate in the stimulation of BDNF expression.
Increasing the miR-424-5p/BDNF Axis Output Neutralizes the Effects of LINC00922 Knockdown on the Proliferation, Apoptosis, Migration, and Invasion of Breast Cancer Cells
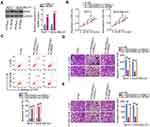
Rescue experiments were performed to evaluate whether the stimulatory effects of LINC00922 on the oncogenicity of breast cancer cells are dependent on the miR-424-5p/BDNF axis. To this end, either anti-miR-424-5p or NC inhibitor was introduced into MCF-7 and MDA-MB-231 cells in the presence of si-LINC00922. The efficiency of anti-miR-424-5p transfection was evaluated using RT-qPCR (Figure 4A). After cotransfection, breast cancer cell proliferation, apoptosis, migration, and invasion in each group were quantified using the CCK-8 assay, flow cytometry, Transwell migration, and Transwell invasion assays, respectively. The reduction in the proliferation (Figure 4B) and induction of apoptosis (Figure 4C) of MCF-7 and MDA-MB-231 cells with LINC00922 silencing were partially reversed by miR-424-5p inhibition. Similarly, LINC00922 expression interference impaired the migratory (Figure 4D) and invasive (Figure 4E) abilities of MCF-7 and MDA-MB-231 cells, and these effects were abrogated by miR-424-5p inhibition.
Similarly, MCF-7 and MDA-MB-231 cells transfected with si-LINC00922 were cotransfected with BDNF-overexpressing pc-BDNF or empty pcDNA3.1 plasmid. Western blotting analysis confirmed that transfection with pc-BDNF increased BDNF protein expression in MCF-7 and MDA-MB-231 cells (Figure 5A). CCK-8 assay and flow cytometry analysis revealed that loss of LINC00922 suppressed MCF-7 and MDA-MB-231 cell proliferation (Figure 5B) and promoted cell apoptosis (Figure 5C), but these impacts were abrogated in response to BDNF overexpression. In addition, the si-LINC00922-mediated inhibition of migratory (Figure 5D) and invasive (Figure 5E) abilities of MCF-7 and MDA-MB-231 cells was reversed after transfection with pc-BDNF. These results collectively suggest that LINC00922 performs pro-oncogenic actions during breast cancer progression via the miR-424-5p/BDNF axis.
Depletion of LINC00922 Attenuates Tumor Growth in vivo
The biological role of LINC00922 in breast cancer tumor growth was studied in vivo using a xenograft model. MCF-7 cells stably transfected with sh-LINC00922 or sh-NC were injected subcutaneously into mice. The knockdown efficiency of sh-LINC00922 in MCF-7 cells was analyzed using RT-qPCR (Figure 6A). Consistent with the results obtained in vitro, LINC00922 depletion significantly slowed the growth of MCF-7 xenograft cells in vivo (Figure 6B and C). In addition, the tumor xenografts derived from the LINC00922-deficient MCF-7 cells had a lower weight than those in the sh-NC group (Figure 6D). Next, miR-424-5p was proven to be upregulated (Figure 6E) and the BDNF protein to be downregulated (Figure 6F) in sh-LINC00922-expressing tumor xenografts. Therefore, we identified that LINC00922 promotes tumor growth of breast cancer cells in vitro via the miR-424-5p/BDNF axis.
Discussion
In recent years, lncRNAs have been identified to play a critical role in the genesis and progression of multiple human cancer types.36,37 In breast cancer, various lncRNAs are dysregulated and implicated in many malignant processes.38–40 Hence, lncRNAs may be an important target for the diagnosis, treatment, and prognosis of breast cancer. Although multiple lncRNAs have been validated,41 further research is necessary to identify their roles in breast cancer. Therefore, we aimed to determine the expression and functions of LINC00922 in breast cancer. We also aimed to determine how LINC00922 regulates the oncogenicity of breast cancer cells in vitro and in vivo.
LINC00922 is upregulated in lung cancer and is correlated with poor clinical outcomes of patients with lung cancer.25 Functional research affirmed that LINC00922 overexpression promotes cell proliferation, migration, and invasion in lung cancer.25 To date, no relevant studies have investigated the expression and roles of LINC00922 in breast cancer. In this study, the TCGA and GTEx databases were used to evaluate LINC00922 expression in breast cancer. The results indicated that LINC00922 was highly expressed in breast cancer. To confirm this observation, RT-qPCR was performed on 58 pairs of human breast cancer tissues and adjacent normal tissues to quantify LINC00922 expression. A high LINC00922 expression level was verified in breast cancer tissues. Additionally, the four breast cancer cell lines used had relatively higher LINC00922 expression than MCF-10A. Functionally, the interference of LINC00922 facilitated apoptosis but suppressed the proliferation, migration, and invasion of breast cancer cells in vitro. Furthermore, LINC00922 knockdown restricted tumor growth of breast cancer cells in vivo.
Next, a series of experiments was performed to identify the underlying mechanisms by which LINC00922 performs its pro-oncogenic roles in breast cancer. Previous studies have reported that the roles of lncRNAs were closely related to their cellular localization.42,43 Hence, the distribution of LINC00922 was assessed in breast cancer cells using nuclear–cytoplasmic fractionation, followed by RT-qPCR analysis, which confirmed that LINC00922 was primarily distributed in the cytoplasm of breast cancer cells. Recent research has proposed the ceRNA hypothesis, which states that lncRNAs possess miRNA-responsive elements and serve as molecular sponges for miRNAs in human cancers, thereby protecting the target mRNAs from suppression and/or degradation.44–46 This lncRNA/miRNA crosstalk regulates gene expression and consequently drives pathological processes during cancer genesis and progression.47
In the present study, using an online database for lncRNA target prediction, StarBase 3.0, miR-424-5p was identified as a putative miRNA target of LINC00922. To confirm this, luciferase reporter assay was performed, which demonstrated the direct binding interaction between LINC00922 and miR-424-5p in breast cancer. This was also confirmed using the RIP assay. In addition, miR-424-5p was identified to have reduced expression in breast cancer tissues and to exert tumor-inhibiting activities. Furthermore, the direct binding of miR-424-5p and BDNF 3′-UTR was demonstrated by several mechanistic experiments. Interestingly, LINC00922 silencing improved miR-424-5p expression levels but decreased BDNF expression via miR-424-5p sponging in breast cancer cells. LINC00922 levels were inversely related to miR-424-5p expression but were positively correlated with BDNF mRNA expression in the 58 breast cancer tissues. Collectively, these results identified a novel LINC00922-mediated ceRNA network in breast cancer involving LINC00922, miR-424-5p, and BDNF.
Abnormal miR-424-5p expression has been previously reported in various human cancers including breast cancer.35,48,49 miR-424-5p was confirmed as an anti-oncogenic miRNA in breast cancer cells and was shown to inhibit cancer progression.35,48,49 Mechanistically, BDNF, which is located on the short arm of chromosome 11 (11p13),50 was verified as a direct target of miR-424-5p in breast cancer. In this study, BDNF expression was shown to be regulated by the LINC00922/miR-424-5p axis in breast cancer cells. The results of rescue experiments further demonstrated that the influences on breast cancer cell proliferation, apoptosis, migration, and invasion induced by LINC00922 silencing were abrogated by increasing the output of the miR-424-5p/BDNF axis, implying that miR-424-5p/BDNF was required for LINC00922-mediated breast cancer progression. Overall, oncogenic LINC00922 aggravates the malignancy of breast cancer by targeting the miR-424-5p/BDNF axis.
Conclusion
LINC00922 was overexpressed in breast cancer tissues and cell lines. LINC00922 could function as an miR-424-5p sponge and was implicated in breast cancer progression by promoting BDNF expression. The LINC00922/miR-424-5p/BDNF pathway may offer a promising novel molecular target in breast cancer therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi:10.1002/ijc.29210
3. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
4. Castrellon AB. Novel strategies to improve the endocrine therapy of breast cancer. Oncol Rev. 2017;11(1):323.
5. Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem. 2017;17(2):152–163. doi:10.2174/1871520616666160502122724
6. Arnaiz E, Sole C, Manterola L, Iparraguirre L, Otaegui D, Lawrie CH. CircRNAs and cancer: biomarkers and master regulators. Semin Cancer Biol. 2019;58:90–99. doi:10.1016/j.semcancer.2018.12.002
7. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. doi:10.1016/j.cell.2009.02.006
8. Sanchez Calle A, Kawamura Y, Yamamoto Y, Takeshita F, Ochiya T. Emerging roles of long non-coding RNA in cancer. Cancer Sci. 2018;109(7):2093–2100. doi:10.1111/cas.13642
9. Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–1109. doi:10.1016/j.bbagrm.2014.08.012
10. Renganathan A, Felley-Bosco E. Long noncoding RNAs in cancer and therapeutic potential. Adv Exp Med Biol. 2017;1008:199–222.
11. Liu F, Hu L, Pei Y, et al. Long non-coding RNA AFAP1-AS1 accelerates the progression of melanoma by targeting miR-653-5p/RAI14 axis. BMC Cancer. 2020;20(1):258. doi:10.1186/s12885-020-6665-2
12. Quan M, Chen J, Zhang D. Exploring the secrets of long noncoding RNAs. Int J Mol Sci. 2015;16(3):5467–5496. doi:10.3390/ijms16035467
13. Watabe K. Roles of lncRNA in breast cancer. Front Biosci. 2015;7(1):94–108. doi:10.2741/s427
14. Soudyab M, Iranpour M, Ghafouri-Fard S. The role of long non-coding RNAs in breast cancer. Arch Iran Med. 2016;19(7):508–517. doi:0161907/AIM.0011
15. Lo PK, Wolfson B, Zhou X, Duru N, Gernapudi R, Zhou Q. Noncoding RNAs in breast cancer. Brief Funct Genomics. 2016;15(3):200–221. doi:10.1093/bfgp/elv055
16. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/S0092-8674(04)00045-5
17. Tufekci KU, Oner MG, Meuwissen RL, Genc S. The role of microRNAs in human diseases. Methods Mol Biol. 2014;1107:33–50.
18. Thyagarajan A, Tsai KY, Sahu RP. MicroRNA heterogeneity in melanoma progression. Semin Cancer Biol. 2019;59:208–220. doi:10.1016/j.semcancer.2019.05.021
19. Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi:10.1038/nrc1840
20. Hao NB, He YF, Li XQ, Wang K, Wang RL. The role of miRNA and lncRNA in gastric cancer. Oncotarget. 2017;8(46):81572–81582. doi:10.18632/oncotarget.19197
21. Abdollahzadeh R, Daraei A, Mansoori Y, Sepahvand M, Amoli MM, Tavakkoly-Bazzaz J. Competing endogenous RNA (ceRNA) cross talk and language in ceRNA regulatory networks: a new look at hallmarks of breast cancer. J Cell Physiol. 2019;234(7):10080–10100. doi:10.1002/jcp.27941
22. Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505(7483):344–352. doi:10.1038/nature12986
23. Ye Y, Shen A, Liu A. Long non-coding RNA H19 and cancer: a competing endogenous RNA. Bull Cancer. 2019;106(12):1152–1159. doi:10.1016/j.bulcan.2019.08.011
24. Chen DL, Lu YX, Zhang JX, et al. Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression. Theranostics. 2017;7(19):4836–4849. doi:10.7150/thno.20942
25. Liang T, Wang B, Li J, Liu Y. LINC00922 accelerates the proliferation, migration and invasion of lung cancer via the miRNA-204/CXCR4 axis. Med Sci Monit. 2019;25:5075–5086. doi:10.12659/MSM.916327
26. Dong BS, Shi MJ, Su SB, Zhang H. Insight into long noncoding competing endogenous RNA networks in hepatic fibrosis: the potential implications for mechanism and therapy. Gene. 2019;687:255–260. doi:10.1016/j.gene.2018.11.063
27. Guo Z, Li J, Sun J, Sun L, Zhou Y, Yu Z. miR-346 promotes HCC progression by suppressing breast cancer metastasis suppressor 1 expression. Oncol Res. 2018;26(7):1073–1081. doi:10.3727/096504017X15145088802439
28. Wu B, Liu G, Jin Y, et al. miR-15b-5p promotes growth and metastasis in breast cancer by targeting HPSE2. Front Oncol. 2020;10:108. doi:10.3389/fonc.2020.00108
29. Muti P, Donzelli S, Sacconi A, et al. MiRNA-513a-5p inhibits progesterone receptor expression and constitutes a risk factor for breast cancer: the hormone and diet in the etiology of breast cancer prospective study. Carcinogenesis. 2018;39(2):98–108. doi:10.1093/carcin/bgx126
30. Zhang J, Kong X, Shi Q, Zhao B. MicroRNA-383-5p acts as a potential prognostic biomarker and an inhibitor of tumor cell proliferation, migration, and invasion in breast cancer. Cancer Biomark. 2020;27(4):423–432. doi:10.3233/CBM-190704
31. Yang R, Xing L, Zheng X, Sun Y, Wang X, Chen J. The circRNA circAGFG1 acts as a sponge of miR-195-5p to promote triple-negative breast cancer progression through regulating CCNE1 expression. Mol Cancer. 2019;18(1):4. doi:10.1186/s12943-018-0933-7
32. Li X, Wang Q, Rui Y, et al. HOXC13-AS promotes breast cancer cell growth through regulating miR-497-5p/PTEN axis. J Cell Physiol. 2019;234(12):22343–22351. doi:10.1002/jcp.28800
33. Ruan L, Qian X. MiR-16-5p inhibits breast cancer by reducing AKT3 to restrain NF-kappaB pathway. Biosci Rep. 2019;39(8). doi:10.1042/BSR20191611
34. Yuan JH, Li WX, Hu C, Zhang B. Upregulation of SNHG12 accelerates cell proliferation, migration, invasion and restrain cell apoptosis in breast cancer by enhancing regulating SALL4 expression via sponging miR-15a-5p. Neoplasma. 2020. doi:10.4149/neo_2020_190808N731
35. Wang J, Wang S, Zhou J, Qian Q. miR-424-5p regulates cell proliferation, migration and invasion by targeting doublecortin-like kinase 1 in basal-like breast cancer. Biomed Pharmacother. 2018;102:147–152. doi:10.1016/j.biopha.2018.03.018
36. Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–5667. doi:10.1038/onc.2017.184
37. Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965–3981. doi:10.1158/0008-5472.CAN-16-2634
38. Zhang Y, Li Z, Chen M, et al. Identification of a new eight-long noncoding RNA molecular signature for breast cancer survival prediction. DNA Cell Biol. 2019;38(12):1529–1539. doi:10.1089/dna.2019.5059
39. Zheng P, Dong L, Zhang B, et al. Long noncoding RNA CASC2 promotes paclitaxel resistance in breast cancer through regulation of miR-18a-5p/CDK19. Histochem Cell Biol. 2019;152(4):281–291. doi:10.1007/s00418-019-01794-4
40. Thomas PB, Seim I, Jeffery PL, et al. The long non-coding RNA GHSROS facilitates breast cancer cell migration and orthotopic xenograft tumour growth. Int J Oncol. 2019;55(6):1223–1236. doi:10.3892/ijo.2019.4891
41. Bin X, Hongjian Y, Xiping Z, Bo C, Shifeng Y, Binbin T. Research progresses in roles of LncRNA and its relationships with breast cancer. Cancer Cell Int. 2018;18(1):179. doi:10.1186/s12935-018-0674-0
42. Beckedorff FC, Amaral MS, Deocesano-Pereira C, Verjovski-Almeida S. Long non-coding RNAs and their implications in cancer epigenetics. Biosci Rep. 2013;33(4). doi:10.1042/BSR20130054
43. Caley DP, Pink RC, Trujillano D, Carter DR. Long noncoding RNAs, chromatin, and development. ScientificWorldJournal. 2010;10:90–102. doi:10.1100/tsw.2010.7
44. Qi X, Zhang DH, Wu N, Xiao JH, Wang X, Ma W. ceRNA in cancer: possible functions and clinical implications. J Med Genet. 2015;52(10):710–718. doi:10.1136/jmedgenet-2015-103334
45. Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19(5):1310. doi:10.3390/ijms19051310
46. Wang L, Cho KB, Li Y, Tao G, Xie Z, Guo B. Long noncoding RNA (lncRNA)-mediated competing endogenous RNA networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int J Mol Sci. 2019;20(22).
47. Zhang Y, Xu Y, Feng L, et al. Comprehensive characterization of lncRNA-mRNA related ceRNA network across 12 major cancers. Oncotarget. 2016;7(39):64148–64167. doi:10.18632/oncotarget.11637
48. Du H, Xu Q, Xiao S, et al. MicroRNA-424-5p acts as a potential biomarker and inhibits proliferation and invasion in hepatocellular carcinoma by targeting TRIM29. Life Sci. 2019;224:1–11. doi:10.1016/j.lfs.2019.03.028
49. Rodriguez-Barrueco R, Nekritz EA, Bertucci F, et al. miR-424(322)/503 is a breast cancer tumor suppressor whose loss promotes resistance to chemotherapy. Genes Dev. 2017;31(6):553–566. doi:10.1101/gad.292318.116
50. Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi:10.1002/j.1460-2075.1982.tb01207.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.