Back to Journals » Cancer Management and Research » Volume 12
Long-Chain Non-Coding SOX21-AS1 Promotes Proliferation and Migration of Breast Cancer Cells Through the PI3K/AKT Signaling Pathway
Authors Sheng XY, Wang CH, Wang CF, Xu HY
Received 2 July 2020
Accepted for publication 23 September 2020
Published 2 November 2020 Volume 2020:12 Pages 11005—11014
DOI https://doi.org/10.2147/CMAR.S270464
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Xiu-Yun Sheng,1,* Cheng-Hong Wang,2,* Chun-Feng Wang,3 Hong-Yan Xu4
1Department of Hematology and Oncology, The Second People’s Hospital of Liaocheng, Liaocheng, Shandong Province, 252600, People’s Republic of China; 2Department of Radiotherapy, The Second People’s Hospital of Liaocheng, Liaocheng 252600, Shandong Province, People’s Republic of China; 3Department of Thyroid and Breast Surgery, The Second People’s Hospital of Liaocheng, Liaocheng 252600, Shandong Province, People’s Republic of China; 4Department of Oncology, The Second People’s Hospital of Liaocheng, Liaocheng 252600, Shandong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong-Yan Xu
Department of Oncology, The Second People’s Hospital of Liaocheng, Linqing City, Liaocheng 252600, Shandong Province, People’s Republic of China
Tel +86-15166580828
Email [email protected]
Aim: This study aimed to investigate the effect of long-chain non-coding SOX21-AS1 on the proliferation and migration of breast cancer (BC) cells through the PI3K/AKT signaling pathway.
Methods: Eighty-eight BC and adjacent tissues were collected, and BC cells and normal breast epithelial cells were purchased. LncRNA SOX21-AS1 expression in tissues and cells was detected by RT-PCR. miR-NC, si-SOX21-AS1, and Sh-SOX21-AS1 were transfected into BC cells. The PI3K/AKT signaling pathway was interfered with L740Y-P (activator of the PI3K/AKT pathway) and LY294002 (inhibitor of the PI3K/AKT pathway) in BC cells. The SOX21-AS1 expression in BC tissues and cells was tested by qRT-PCR, and the expression levels of p-PI3K, p-AKT, N-cadherin, E-cadherin, and vimentin were detected by WB.
Results: SOX21-AS1 was highly expressed in BC, and the p-PI3K and p-AKT levels were also high. Cell experiments showed that inhibiting SOX21-AS1 expression could inhibit the proliferation, invasion, migration, and EMT of BC cells, and up-regulating its expression could promote the proliferation, invasion, migration, and EMT of ovarian cancer cells. The tumor-forming experiment in nude mice was consistent with the results in vitro. 740Y-P intervention could reverse the inhibition effect of Si-SOX21-AS1 on BC cell proliferation, invasion, migration, and EMT, while LY294002 intervention could reverse the promotion effect of Sh-SOX21-AS1 on BC cell proliferation, invasion, migration, and EMT.
Conclusion: SOX21-AS1 is highly expressed in BC tissues. Silencing BC expression can inhibit the proliferation, invasion, migration, and EMT of cells by inhibiting the PI3K/AKT signaling pathway, which may be a new target for diagnosis and treatment.
Keywords: LncRNA SOX21-AS1, PI3K/AKT, breast cancer cells, proliferation, migration
Introduction
Breast cancer (BC) is one of the major frequent malignancies among women all over the world at present. Every year, a large number of new cases appear continuously, and its mortality has remained high, posing a serious threat to the life safety of women in the world.1,2 At present, BC clinical treatment mainly adopts the method of surgery combined with radiotherapy and chemotherapy. However, due to the heterogeneity of BC tumors and the characteristics of easy metastasis of tumor cells, many patients still have poor efficacy even after treatment, and drug resistance or metastasis occurs, resulting in poor prognosis.3,4 Therefore, exploring BC pathogenesis has important clinical significance for finding possible therapeutic targets.
Long-chain non-coding RNA (LncRNA) is a non-coding RNA with a length greater than 200 nt, which will not be translated into protein.5 In recent years, continuous research6,7 has found that LncRNA has imbalance in various tumor diseases, including BC, and is effective in disease development and progression. For example, a recent study8 has found that LncRNA GAS5 is down-regulated in BC and has a regulatory effect on the apoptosis of BC cells. LncRNA SOX21 antisense RNA1 (SOX21-AS1) is an LncRNA located at chromosome 13q32.1, which has also been found to play an important part in various diseases including tumor diseases in the past.8 For example, research9 has discovered that SOX21-AS1 is related to the progress of hepatocellular carcinoma, and it can predict the prognosis of patients by epigenetically silencing p21 expression. Besides, another research study10 has also verified that SOX21-AS1 can regulate the VDAC1 expression through competitive binding of miR-7 to promote the development of cervical cancer. However, SOX21-AS1 expression in BC and related mechanisms have not been studied and discussed.
In our research, we confirmed that SOX21-AS1 had effects in BC development and progression, and explored its potential mechanism in BC, in order to provide new directions and insights for its clinical diagnosis and treatment.
Materials and Methods
Clinical Data
We collected 88 patients who underwent BC resection in our hospital from March 2014 to March 2016. Eighty-eight BC tissues and 88 adjacent tissues were taken for detection during the operation on the basis of patients’ consent. Inclusion criteria were as follows: patients diagnosed as BC by pathological diagnosis; patients diagnosed as BC for the first time. Exclusion criteria were as follows: patients who received radiotherapy and chemotherapy; patients with other malignancies; patients with serious liver and kidney dysfunction; patients with blood system diseases; patients who refused to participate in the study. All patients agreed to participate in the experiment and provided their written informed consent prior to study inclusion. This experiment was approved by the Medical Ethics Committee of the Second People’s Hospital of Liaocheng and is in line with the Declaration of Helsinki.
Experimental Methods
We drew lessons from some experimental methods of Huang et al11 and others to regulate LncRNA and the PI3K/AKT pathway, as follows.
Experimental Materials and Reagents
The main experimental materials and reagents were as follows: human BC cell lines MCF-7, BT-20, MDA-MB-231, and MCF-10A and human normal breast epithelial cells Hs 278Bst (purchased from ATCC), qRT-PCR and reverse transcription kits (TransGen Biotech, Beijing, China), CCK-8 kits (Promega, USA), Transwell kit (Beijing Xinshengyuan Biomedical Technology Co., Ltd), PBS, fetal bovine serum (FBS) (Gibco, Rockville, MD, USA), Trizol reagent (Beijing Baiaolaibo Technology Co., Ltd), Dual-Lucy Assay kit (Beijing Baiaolaibo Technology Co., Ltd), p-PI3K, p-AKT, N-cadherin, E-cadherin, vimentin, and β-Actin antibodies (Cell Signaling Technology, Boston, MA, USA), goat anti-rabbit IgG secondary antibody (Wuhan Boster Biology Co., Ltd), RIPA, BCA protein kit (Thermo Fisher Scientific, Waltham, MA, USA), ECL developer (Thermo Fisher Scientific, Waltham, MA, USA), PCR instrument (ABI, USA). All primers were designed and compounded by Shanghai Sangon Bioengineering Technology Service Co., Ltd.
Cell Culture and Transfection
Human BC cell lines MCF-7, BT-20, MDA-MB-231, and MCF-10A and normal breast epithelial cells Hs 278Bst were put in DMEM medium containing 10% PBS and cultured at 37 °C, 5% CO2. When cell adherent growth and fusion were observed to reach 85%, 25% pancreatin was added for digestion. After that, they were placed in the medium for continuous culture and passage. Then, the SOX21-AS1 expression in each cell line was detected. MCF-7 and BT-20 were selected for transfection, and targeted inhibition SOX21-AS1 sequence (si-SOX21-AS1), targeted overexpression SOX21-AS1 plasmid (sh-SOX21-AS1), negative control RNA (miR-NC), and Lipofectamine™ 2000 kits were respectively used for cell transfection, and the operation steps were strictly conducted in the light of the kit instructions.
Real-Time Quantitative PCR
Firstly, the total RNA in tissues and cells was extracted with Trizol reagent, then 5 μg was drawn respectively for reverse transcription of cDNA according to the instructions of the kit, and 1 μL synthesized cDNA was collected for amplification. The amplification system was as follows: cDNA 1 μL, upstream and downstream primers 0.4 μL each, 2× TransScript® Tip Green qPCR SuperMix 10 μL, passive reference dye (50×) 0.4 μL, nuclease-free water added to 20 μL. GAPDH was employed as internal reference and the data were analyzed by 2–ΔΔct. (Table 1).
 |
Table 1 Primer Sequence Table |
Cell Proliferation Test
The proliferation of MCF-7 and BT-20 cells was evaluated by CCK-8 kit. Cells 48 h after transfection were collected and diluted to 3×104 cell/mL. Then, they were inoculated into 96-well plates, and each well was inoculated with 100 μL cells and cultured at 37 °C with 5% CO2. A total of 10 μL CCK8 solution was supplemented to each well at 0, 24, 48, and 72 h after they adhered to the wall. After the reagent was added, the cells were continuously fostered for 2 h in an incubator at 37 °C, 5% CO2. Finally, the OD value was measured at 450 nm via an enzyme reader to detect the cell proliferation and draw the growth curve. The experiment was repeated three times.
Cell Migration and Invasion Test
The ability of cell migration and invasion was estimated by scratch healing test and Transwell test. For wound healing determination, the cells were divided into a cell-free area by a 200 μL sterile loading gun head, the divided cells were washed by PBS, and a new culture medium was supplemented for culture. At 0 (W0) and 24 h (W24) after cell division, the cell migration was evaluated by microscope for scratches at three different positions. Transwell assay: firstly, 200 μL DMEM culture solution containing 1x105 cells was supplemented to the upper chamber, and 500 mL DMEM containing 20% FBS was supplemented to the lower one. Substrate and cells that did not cross over the membrane surface in the upper chamber were wiped off 48 h after they were cultured at 37 °C, cleaned via PBS three times, fixed for 10 min with paraformaldehyde, and then were cleaned via double-distilled water three times and dyed for 10 min through 0.1% crystal violet after it was dried, and cell invasion was observed with a microscope.
Western Blot Test
After cells of each group were collected and cultured, RIPA lysate was added into the cells, and the total protein was extracted. Next, the protein concentration was detected by BCA method and adjusted to 4 μg/μL, and 12% SDS-PAGE electrophoresis separation was carried out. Afterward, it was transferred to PVDF membrane that was sealed by 5% defatted milk for 2 h. Then, p-PI3K (1:1000), p-AKT (1:1000), N-cadherin (1:500), E-cadherin (1:500), vimentin (1:500), and β-Actin (1:1000) primary antibody were added and sealed overnight at 4 °C. The membrane was cleaned to remove primary antibody, horseradish peroxidase-labeled goat anti-rabbit secondary antibody (1:1000) was supplemented, and then it was incubated for 1 h at 37 °C and cleaned three times by PBS, each time for 5 min. The protein bands on the membrane were developed in a dark room using the enhanced chemiluminescence reagent, and the excess liquid was absorbed with filter paper.
Tumorigenesis Experiment in Nude Mice in vivo
Female BALB/c nude mice aged 5 weeks (bought from Shanghai Experimental Animal Center, Chinese Academy of Sciences) were raised in a sterile environment. Then, 3x106 MCF-7 cells transfected with stable Si-SOX21-AS1 and its control plasmid were suspended in 100 μL phosphate buffer and injected subcutaneously into the dorsal subcutaneous layer of nude mice. Five nude mice in each group were tested for tumor growth every 7 days. The tumor growth was determined by the formula of volume=length×width2×0.52. Twenty-eight days after injection, the mice were euthanized (rats were anesthetized with 4% isoflurane (Nanjing KEW Biotechnology Co., Ltd, C002) and killed for cervical dislocation) and the size and quality of tumors in vivo were accurately measured. The research was carried out based on the proposals in the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Second People’s Hospital of Liaocheng. All animal experiments took place at the Second People’s Hospital of Liaocheng.
Statistical Methods
In our research, the collected data were statistically analyzed via SPSS 20.0, and the required pictures were drawn by GraphPad 7. The inter-group comparison was analyzed by independent-samples t-test, multi-group comparison was assessed via one-way analysis of variance (ANOVA), post hoc pairwise comparison was evaluated by LSD t-test, multi-time point expression was tested by repeated-measures ANOVA, and Bonferroni was employed for backtesting. A p-value lower than 0.05 was considered to be statistically remarkable.
Results
SOX21-AS1 is Up-Regulated in BC
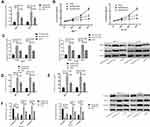
qRT-PCR quantitative detection of SOX21-AS1 expression in BC tissues revealed that the expression in BC tissues was dramatically up-regulated compared with adjacent normal tissues, and in those cells it was also dramatically up-regulated compared with normal those epithelial cells (P<0.05). ROC analysis of the working curve of the subjects found that the area under the SOX21-AS1 curve was 0.930. According to the median expression of SOX21-AS1 (1.67), the patients were divided into the SOX21-AS1 high expression group (40 cases) and low expression group (48 cases). The results signified that SOX21-AS1 expression was tied to the tumor size, pathological stage, differentiation degree, and lymph node metastasis of BC patients, and the 3-year survival rate of the SOX21-AS1 high expression group was markedly lower than that of the low expression group (P<0.05) (Table 2 and Figure 1).
 |
Table 2 Relationship Between SOX21-AS1 and Pathological Data of BC Patients |
SOX21-AS1 Promotes Proliferation, Invasion, and EMT of BC Cells
To explore SOX21-AS1’s effect on the biological function of BC cells, we intervened in SOX21-AS1 expression in MCF-7 and BT-20 cells. The results displayed that, compared with the miR-NC group, knocking-down SOX21-AS1 expression could remarkably inhibit the proliferation, invasion, migration, and EMT of BC cells. Moreover, the N-cadherin and vimentin expression levels in transfected miR-NC cells reduced dramatically and the E-cadherin expression increased dramatically after Si-SOX21-AS1 transfection, while the N-cadherin and vimentin expression levels in transfected miR-NC cells increased markedly and E-cadherin expression decreased markedly after Sh-SOX21-AS1 transfection (Figure 2).
Effect of SOX21-AS1 on the PI3K/AKT Signaling Pathway in BC
The PI3K/AKT signaling pathway in BC cells was tested via western blot. The results suggested that knocking-down SOX21-AS1 expression reduced the phosphorylation level of PI3K and AKT obviously, but overexpression stimulated it obviously (Figure 3).
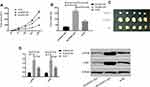
Effect of SOX21-AS1 on Tumor Growth in vivo
To further confirm the growth of SOX21-AS1 on breast tumors in vivo, MCF-7 cells stably transfected with Si-SOX21-AS1, Sh-SOX21-AS1, and miR-NC were subcutaneously injected into mice, and tumor growth was observed. The results displayed that compared with the miR-NC group, SOX21-AS1 expression in nude mice tumors injected with Si-SOX21-AS1 reduced markedly, as was the case on the tumor volume and mass. However, in nude mice tumor injected with Sh-SOX21-AS1 it increased obviously, as was the case on the tumor volume and mass. Further detection of the PI3K/AKT signal pathway in nude mice tumor tissue manifested that the expression of p-PI3K and p-Akt proteins in it injected with Si-SOX21-AS1 was obviously inhibited, but that injected with Sh-SOX21-AS1 increased dramatically (Figure 4).
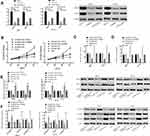
Effect of Stimulating or Inhibiting the PI3K/AKT Signaling Pathway on BC
In order to further prove that SOX21-AS1 affects the function of BC cells by regulating the PI3K/AKT signaling pathway, we treated the untransfected MCF-7 and BT-20 cells with 5 μL 740Y-P (activator of the PI3K/AKT pathway) and 5 μL LY294002 (inhibitor of the PI3K/AKT pathway) respectively for 48 h. Then, it was found that the p-Akt and p-PI3K expression levels in MCF-7 and BT-20 cells treated with 740Y-P increased markedly (p<0.05), and those in MCF-7 and BT-20 cells treated with LY294002 decreased markedly (p<0.05), suggesting that 740Y-P could activate the PI3K/AKT signaling pathway while LY294002 could inhibit it. Subsequently, the cells transfected with Si-SOX21-AS1 were exposed to 5 μL 740Y-P, and those transfected with Sh-SOX21-AS1 were exposed to 5 μL LY294002 for 48 h. The results indicated that the intervention of 740Y-P could reverse the inhibition of Si-SOX21-AS1 on BC cell proliferation, invasion, migration, and EMT, while that of LY294002 could reverse the promotion of Sh-SOX21-AS1 in the same way (Figure 5).
Discussion
BC is one of the major familiar malignancies in women. Its high morbidity and mortality have posed a serious threat to the life and health of women all over the world.12 However, its specific pathogenesis is still not completely clear at present, and many patients still have poor prognosis after treatment due to its high invasiveness and metastasis, so it is quite significant for those patients to ulteriorly make a thorough inquiry for BC pathogenesis and find new target therapeutic directions.13,14
In the past, many studies have proved that LncRNA is abnormally expressed in various malignancies including BC and plays a vital biological function.15 SOX21-AS1 has been verified to be out of balance in many human cancers in the past. For example, research16 found that SOX21-AS1 was found to be down-regulated in oral squamous cell carcinoma, and correlation analysis also found that SOX21-AS1 was relevant to the clinical stage of patients and tumor size. However, more data signified that SOX21-AS1 was abnormally expressed in human tumors. For instance, research17 confirmed that SOX21-AS1 expression in lung cancer tissues was markedly higher than that in normal non-tissues after TCGA data analysis of lung cancer. In addition, studies18 also found that SOX21-AS1 expression was significantly up-regulated in cervical cancer tissues, and discovered that it was strongly linked to the prognosis of patients. In our study, we also found that SOX21-AS1 expression in BC tissue was obviously up-regulated compared to that in adjacent tissues, and its clinical significance revealed that SOX21-AS1 was not only of high value in the diagnosis of BC, but also tied to the pathological stage, differentiation degree, tumor metastasis, and prognosis of those patients, which indicated that SOX21-AS1 might be effective in it. In the past, some studies also pointed out that SOX21-AS1 was concerned with the prognosis of patients and other clinicopathologic factors in many tumors. For example, some studies19 showed clearly that the high expression of SOX21-AS1 indicated a poor prognosis for colorectal cancer, similar to our conclusion. In order to further explore the biological function of SOX21-AS1 in BC, we regulated its expression in those cells. The results exhibited that inhibiting the SOX21-AS1 expression could greatly inhibit BC cell proliferation, invasion, and EMT; but after it was further up-regulated, their proliferation, invasion, and EMT capabilities were significantly enhanced. The results of in vivo nude mice tumorigenesis experiments were consistent with those of in vitro experiments, which indicated that SOX21-AS1 worked as oncogene in BC. This was in accordance with the conclusions of many previous studies; for instance, a study20 found that knocking-down SOX21-AS1 expression could inhibit the proliferation and colony formation of nephroblastoma cells.
In the process of tumor occurrence, apart from LncRNA regulation, there are many signal pathways involved, and PI3K/AKT is one of the major crucial signal pathways.21 For example, research22 pointed out that PI3K/AKT could promote MET by regulating the expression of snail family transcription repressor 1. In our research, we found that SOX21-AS1 had a certain effect on activation of the PI3K/AKT signaling pathway in BC. When we inhibited SOX21-AS1 expression, we found that its activation was also inhibited significantly; on the contrary, it was also enhanced significantly after increasing SOX21-AS1 expression. This suggested that SOX21-AS1 might regulate its occurrence and development by regulating this pathway. Previously, many studies on the role of the PI3K/AKT signaling pathway in tumors pointed out its important role; for example, some studies23 verified that the proliferation and migration of lung cancer cells could be promoted by activating this pathway. In order to further confirm that SOX21-AS1 regulates BC cells through the PI3K/AKT signaling pathway, we conducted rescue experiments, and inhibited or activated it for BC cells after up-regulating or down-regulating SOX21-AS1. The results signified that the intervention of 740Y-P could reverse the inhibition of Si-SOX21-AS1 on BC cell proliferation, invasion, and EMT. Nevertheless, LY294002 intervention could reverse Sh-SOX21-AS1’s promotion on proliferation, invasion, and EMT of BC cells, which further proved that SOX21-AS1 regulated those cells through the PI3K/AKT signaling pathway. Also, many studies have been conducted on the role of this pathway in BC. For instance, research24 pointed out that MRG3 could inhibit the proliferation and glycolysis of BC cells by inhibiting the PI3K/AKT signaling pathway, which was analogous to our conclusion.
Overall, LncRNA SOX21-AS1 can promote BC cell proliferation, invasion, and EMT by regulating the PI3K/AKT signaling pathway, which may be a new target for diagnosis and treatment. However, there are still some limitations. For example, there may be other regulatory targets for LncRNA SOX21-AS1 in BC. However, we have not carried out further in-depth discussion on this. What is more, we will carry out further basic research in future studies to further elaborate the role and mechanism of LncRNA SOX21-AS1 in BC.
Conclusion
Overall, LncRNA SOX21-AS1 can promote BC cell proliferation, invasion, and EMT by regulating the PI3K/AKT signaling pathway, which may be a new target for diagnosis and treatment. However, there are still some limitations. For example, there may be other regulatory targets for LncRNA SOX21-AS1 in BC. However, we have not carried out further in-depth discussion on this. What is more, we will carry out further basic research in future studies to further elaborate the role and mechanism of LncRNA SOX21-AS1 in BC.
Disclosure
Xiu-Yun Sheng and Cheng-Hong Wang are co-first authors for this study. The authors report no conflicts of interest in this work.
References
1. Ennour-Idrissi K, Ayotte P, Diorio C. Persistent organic pollutants and breast cancer: a systematic review and rigorous review of the literature. Cancer (Basel). 2019;11.
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimated the morbidity and mortality of 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Cutress RI, McIntosh SA, Potter S. Opportunities and priorities for breast surgery research. Lancet Oncol. 2018;19(10):e521–e533. doi:10.1016/S1470-2045(18)30511-4
4. De Cicco P, Catani MV, Gasperi V, Sibilano M, Quaglietta M, Savini I. Nutrition and breast cancer: a review of the literature on prevention, treatment and relapse. Nutrients. 2019;11.
5. Brosnan CA, Voinnet O. The length of non-coding RNA. Curr Opin Cell Biol. 2009;21(3):416–425. doi:10.1016/j.ceb.2009.04.001
6. Gibb EA, Brown CJ, Lam WL. Functional role of long non-coding RNA in human cancer. Moore Cancer. 2011;10(1):38. doi:10.1186/1476-4598-10-38
7. Hauptman N, Glavac D. Long non-coding RNA in cancer. Int J Mol Sci. 2013;14(3):4655–4669. doi:10.3390/ijms14034655
8. Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5 is a non-protein-encoding RNA that controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28(2):195–208. doi:10.1038/onc.2008.373
9. Congxin W, Hong W, Fei X, et al. LncRNA SOX21-AS1 is associated with progression of hepatocellular carcinoma and predicts prognosis through epigenetically silencing p21. Biomed Pharmacother. 2018;104:137–144. doi:10.1016/j.biopha.2018.05.010
10. Xiaoyan Z, Xianlan Z, Yan L, et al. Long noncoding RNA SOX21-AS1 promotes cervical cancer progression by competitively sponging miR-7/VDAC1. J Cell Physiol. 2019;234(10):17494–17504. doi:10.1002/jcp.28371
11. Huang Y, Yongqing X, Feng SY, Zhang X, Jingdong N. LncRNA TDRG1 promotes the proliferation, invasion and epithelial-mesenchymal transition of osteosarcoma through PI3K/AKT signaling pathway. Cancer Manag Res. 2020;12:4531–4540. doi:10.2147/CMAR.S248964
12. Li Z, Hou P, Fan D. lncRNA ANCR-mediated degradation of EZH2 attenuates the ability of breast cancer to invade and metastasize. Cell Death Differ. 2017;24(1):59–71. doi:10.1038/cdd.2016.95
13. Savva C, Adhikaree J, Madhusudan S, Chokkalingam K. Carcinogenic osteomalacia and metastatic breast cancer: a case report and review of the literature. Diabetes Metab Disord. 2019;18(1):267–272. doi:10.1007/s40200-019-00398-y
14. Honkela A, Peltonen J, Topa H, et al. Genome-wide modeling of transcription kinetics reveals patterns of delayed RNA production. Proc Natl Acad Sci. 2015;112(42):13115–13120. doi:10.1073/pnas.1420404112
15. Eastlack Steven C, Shengli D, Mo Yin Y, et al. Expression of long noncoding RNA MALAT1 correlates with increased levels of nischarin and inhibits oncogenic cell functions in breast cancer. PLoS One. 2018;13(6):e0198945. doi:10.1371/journal.pone.0198945
16. Cheng-Mei Y, Tsung-Han W, Hung-Chih C. Aberrant DNA hypermethylation-silenced SOX21-AS1 gene expression and its clinical importance in oral cancer. Clin Epigenetics. 2016;8(1):129. doi:10.1186/s13148-016-0291-5
17. Xiyi L, Chenjun H, He X. A novel long non-coding RNA, SOX21-AS1, indicates a poor prognosis and promotes lung adenocarcinoma proliferation. Cell Physiol Biochem. 2017;42(5):1857–1869. doi:10.1159/000479543
18. Ruijie W, Li Y, Du P, Zhang X, Li X, Cheng G. Hypomethylation of the lncRNA SOX21-AS1 has clinical prognostic value in cervical cancer. Life Sci. 2019;233:116708. doi:10.1016/j.lfs.2019.116708
19. Wenjie W, Li H-T, Yu J-P. A competitive endogenous RNA network reveals novel potential lncRNA, miRNA and mRNA biomarkers in the prognosis of human colon adenocarcinoma. J Surg Res. 2019;235:22–33. doi:10.1016/j.jss.2018.09.053
20. Jingxiu Z, Tianzhao H. SOX21-AS1 is associated with clinical stage and regulates cell proliferation in nephroblastoma. Biosci Rep. 2019;39.
21. Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial-to-mesenchymal transition and cell migration. J Biochem. 2000;275:36803–36810.
22. Dong J, Zhai B, Sun W, Hu F, Cheng H, Xu J. Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced sorafenib on liver cancer cells multidrug resistance. PLoS One. 2017;12(9):e185088. doi:10.1371/journal.pone.0185088
23. Wen-Long Z, Ya-Nan Z, Zhang-Zhen S, Cong D, Bai Y-S. Lutein inhibits cell growth and activates apoptosis via the PI3K/AKT/mTOR signaling pathway in A549 human non-small-cell lung cancer cells. J Environ Pathol Toxicol Oncol. 2018;37(4):341–350. doi:10.1615/JEnvironPatholToxicolOncol.2018027418
24. Mingzhi Z, Xiaochun W, Gu Y, Wang F, Li L, Qiu X. MEG3 overexpression inhibits the tumorigenesis of breast cancer by downregulating miR-21 through the PI3K/Akt pathway. Arch Biochem Biophys. 2019;661:22–30. doi:10.1016/j.abb.2018.10.021
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.