Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 9 » Issue 1
Long-acting bronchodilator use after hospitalization for COPD: an observational study of health insurance claims data
Authors Baker CL , Zou KH , Su J
Received 17 December 2013
Accepted for publication 6 March 2014
Published 3 May 2014 Volume 2014:9(1) Pages 431—439
DOI https://doi.org/10.2147/COPD.S59322
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Christine L Baker,1 Kelly H Zou,1 Jun Su2
1Pfizer Inc., New York, NY, USA; 2Boehringer-Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA
Background: Treatment of stable chronic obstructive pulmonary disease (COPD) with long-acting bronchodilator (LABD) medications is recommended by the 2014 Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines. The primary objective of this study was to examine LABD prescription fills after a COPD-related hospitalization.
Methods: This retrospective observational study used claims from Truven Health MarketScan® Commercial and Medicare Supplemental databases. Patients (age ≥40, commercial; age ≥65, Medicare supplemental) had a first hospitalization with a primary COPD diagnosis between April 1, 2009 and June 30, 2011 (index hospitalization) and were continuously enrolled for 1 year before and 9 months after hospitalization. Patients were categorized according to pre-index and/or post-index pharmacy claims.
Results: A total of 27,738 patients had an index hospitalization and met inclusion/exclusion criteria. Of those, 19,783 patients had COPD as a primary or secondary diagnosis during the year before index hospitalization and were included in the analysis. Approximately one quarter of the patients (26.32%) did not fill a prescription for an LABD or short-acting bronchodilator both 90 days before and 90 days after hospitalization. During the 90-day pre-index period, 40.57% of patients filled an LABD (with or without a short-acting bronchodilator) prescription. Over half of the patients (56.88%) filled an LABD prescription at some point during the 180-day post-index period, but, of those, a significantly greater proportion of patients filled an LABD prescription in the 1- to 90-day post-index period than in the 91- to 180-day post-index period (51.27% versus 43.66%; P<0.0001).
Conclusion: A significant proportion of COPD patients in this study did not fill an LABD prescription before hospitalization for COPD. Moreover, hospitalization did not appear to greatly impact LABD initiation. Lastly, patients who did not fill an LABD prescription within the first 90 days posthospitalization were not likely to fill an LABD prescription later. Taken together, the results of this study suggest that many patients with COPD are undertreated.
Keywords: chronic obstructive pulmonary disease, non-interventional, retrospective, short-acting bronchodilator
Introduction
Chronic obstructive pulmonary disease (COPD) is a debilitating disease that is associated with an increased risk of death.1,2 A recent update on the state of health in the United States reported that the number of deaths attributed to COPD had increased from approximately 98,000 in 1990 to approximately 155,000 in 2010, making it the fourth leading disease contributing to years of life lost due to premature mortality.3 COPD was the fifth leading cause of death worldwide in 2002 and is projected to become the third leading cause of death worldwide by the year 2030.4 The economic burden of COPD is also projected to rise considerably over the next 15 to 20 years, driven primarily by the aging population.5
COPD is characterized by the symptoms of chronic cough, excessive sputum production, and wheeze. As the disease progresses, more severe symptoms are observed, such as dyspnea (breathlessness) and poor exercise tolerance,6 and intermittent acute exacerbations occur.7,8 Severe exacerbations contribute to the high rate of hospital admissions and readmission observed among COPD patients.9–16 A recent database analysis showed that COPD exacerbations were the primary event for initiation of medication.17 For patients with stable COPD, the 2014 Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines recommend maintenance therapy using inhaled long-acting bronchodilator (LABD) medications because they are convenient and are more effective at maintaining symptom relief than short-acting bronchodilator (SABD) medications.18 Some evidence suggests that treatment of COPD patients with inhaled long-acting β2-agonists can reduce the number of exacerbations leading to hospitalization.19 Thus, the present observational study examined patterns of prescription fills for bronchodilator medications, particularly LABD use at 3 months and 6 months after a hospitalization for COPD.
Methods
Data source
Anonymized claims data were extracted from the Truven Health MarketScan® Commercial and Medicare Supplemental Research Databases,20 which have previously been used for COPD research.9,21 Together, these databases include de-identified medical claims and prescription drug claims for individuals in the US with employer-sponsored health insurance, including individuals with Medicare supplemental coverage. The data are pooled from diverse points of care, including large employers, managed care organizations, hospitals, and public organizations, thus providing greater generalizability than single payer databases.20 Institutional review board approval was not required because only de-identified data were analyzed.
Study sample
This retrospective study included patients who were at least 40 years old (commercial) or at least 65 years old (Medicare supplemental), had a first hospitalization with COPD as the primary diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes: 491.x chronic bronchitis, 492.x emphysema, and 496 COPD, unspecified)22 between April 1, 2009 and June 30, 2011 (hereafter, index hospitalization), and were continuously enrolled 1 year before and 9 months after the index period (overall study period, April 1, 2008 through March 31, 2012). Only patients who had COPD as a primary or secondary diagnosis during the year before index hospitalization were included in the analysis. In addition, only patients who were alive at the time of index hospitalization discharge were included. Patients were excluded if they were diagnosed with cystic fibrosis (ICD-9-CM 277.0x) or tuberculosis (ICD-9-CM 010.xx – 018.xx) at any time during the study period on a non-diagnostic claim or were transferred to another inpatient facility after hospital discharge.
Comparison groups
The primary outcome was to compare the proportion of patients who had at least one pharmacy claim for an LABD (with or without an SABD) during the 90-day post-index period to the proportions of those who did not have any pharmacy claim for an LABD within the same period. An additional post-index period was included to understand prescription claim patterns during the period of 91 through 180 days after the index hospitalization.
LABDs included arformoterol, budesonide/formoterol, fluticasone/salmeterol, formoterol, indacaterol, salmeterol, and tiotropium. SABDs included albuterol, albuterol/ipratropium, ipratropium, levalbuterol, metaproterenol, pirbuterol, and salbutamol.
First, prescription claims were categorized into the following medication groups:
- no medication (no SABD or LABD pharmacy claims);
- SABD only (at least one pharmacy claim for an SABD, but none for an LABD);
- LABD only (at least one pharmacy claim for an LABD, but none for an SABD);
- and SABD plus LABD (at least one pharmacy claim for an LABD and at least one pharmacy claim for an SABD).
Second, to specifically examine prescription fills for LABD medications, the four categories above were combined into the following two broad groups:
- no LABD (no medication group combined with SABD only group);
- and LABD (LABD only group combined with LABD plus SABD group).
In order to account for prescriptions that a patient may have filled before the index hospitalization and continued using after hospital discharge, a grace period was included for post-index hospitalization prescription fills. The grace period was calculated, for each patient, as the length of time before a prescription refill was expected given the number of days dispensed in the fill immediately before the index hospitalization.
Baseline variables were also examined, and included age at index hospitalization, sex, geographic region within the US, urban or rural area, payer type, insurance plan type, month and year of index hospitalization, respiratory medications within 90 days before index hospitalization, and the Charlson Comorbidity Index, Deyo version (CCI),23 used to measure pre-index comorbid conditions. The CCI is a validated method of classifying comorbid conditions that takes into account the number and severity of comorbidities and has been adapted for research using ICD-9-CM codes in administrative databases.23
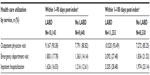
As secondary outcome measures, health care utilization for COPD, including outpatient (OP) physician visits, emergency department (ED) visits, or inpatient hospitalizations, within the 1- to 90-day and 1- to 180-day post-index periods are also shown for the LABD and no LABD groups.
Statistical analyses
For continuous variables, such as age and CCI, univariate descriptive statistics (mean, standard deviation, and median) were calculated. For discrete variables, counts (n) and percentage (%) were calculated. For independent samples (ie, in a two-sample setting between LABD and no LABD cohorts), we conducted two-sample tests including the chi-square test of proportions or the two-sample t-test of means. For correlated samples (ie, for the same patients across different time periods), McNemar’s chi-square test was conducted. These were within-patient comparisons across different patient cohorts because the data were correlated between time periods.24 Furthermore, the test of proportions was conducted to compare those in the LABD versus no LABD groups post-index. Two-sided P-values ≤0.05 were considered statistically significant. All statistical analyses were performed using SAS® Version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Of 77,367,886 individuals with claims within the MarketScan® database during the index period, 27,738 patients met inclusion/exclusion criteria (Figure 1). Of those, 19,783 patients had a primary or secondary COPD diagnosis during the 1-year pre-index period and were included in the final analysis.
  | Figure 1 Patient flow diagram. |
Baseline demographic characteristics overall and by post-index LABD use are shown in Table 1. The mean ± standard deviation of the age of the LABD group was 70.89±10.33 years with a median age of 71 years. Of 11,253 patients in the LABD group, approximately half (52.26%) were women, 80.73% resided in urban areas, and 41.22% resided in the North central region of the US. The most prevalent insurance plan type in the LABD group was comprehensive (43.58%). Medicare supplemental was the payer type for 70.52% of the patients in the LABD group and 67.06% in the no LABD group. Mean CCI was greater for the no LABD group (2.85±2.08) than the LABD group (2.49±1.87). Each of the pre-index respiratory medications was used by a larger percentage of patients in the LABD group than in the no LABD group. Besides an LABD (64.42%), an SABD was the most frequently used pre-index respiratory medication overall (44.66%), and was used by 57.36% of the LABD group and 27.91% of the no LABD group.
Figures 2 and 3 shows medication use patterns across different time periods for the four component medication groups (no medication, SABD only, LABD only, and SABD plus LABD). Of note, 5,206 patients (26.31%) did not fill any SABD or LABD prescription in the 90-day pre-index period or in the 1- to 90-day post-index period (Figure 2). Of the 6,499 patients who did not fill any SABD or LABD prescription in the 1- to 90-day post-index period, 5,461 patients (84.03%) did not fill any SABD or LABD prescription in the 91- to 180-day post-index hospitalization period (Figure 3).
When combined into the two broader groups based on LABD prescriptions, 11,253 patients (56.88%) filled an LABD prescription, but 8,530 (43.12%) did not fill an LABD prescription during the entire 1- to 180-day post-index period (Table 2). Of the 8,026 patients who filled an LABD prescription in the 90-day pre-index period, 6,855 (85.41%) maintained their prescription for an LABD in the 1- to 90-day post-index period. In contrast, of the 11,757 patients who did not fill an LABD prescription in the 90-day pre-index period, only 3,288 (27.97%) filled an LABD prescription in the 1- to 90-day post-index period. Significantly more patients filled an LABD prescription in the 1- to 90-day post-index period than in the 90-day pre-index period (51.27% versus 40.57%), and significantly more patients filled an LABD prescription in the 1- to 90-day post-index period than in the 91- to 180-day post-index period (51.27% versus 43.66%) (both, P<0.0001). Of the 11,757 patients who did not fill an LABD prescription in the 90-day pre-index period, 8,469 (72.03%) continued to not fill an LABD prescription in the 1- to 90-day post-index period. A similar trend was observed for the post-index time periods; of the 9,460 patients who did not fill an LABD prescription during the 1- to 90-day post-index period, 8,530 (88.49%) continued to not fill an LABD prescription during the 91- to 180-day post-index period.
  | Table 2 LABD prescription fills across various time periods |
During the 1- to 90-day post-index period, 90.38% of 10,143 patients in the LABD group and 80.82% of 9,640 patients in the no LABD group had at least one OP physician visit (Table 3). These proportions increased only slightly when expanded to include the entire 1- to 180-day post-index period. Similar trends were observed for the ED and inpatient visits.
Discussion
The results of this observational study of health insurance claims data from nearly 20,000 COPD patients suggest that many COPD patients are not filling prescriptions for LABD medications, even after being hospitalized for COPD. Approximately one quarter of the COPD patients did not fill any LABD or SABD prescription in the 90 days before or 180 days after hospitalization. In addition, only approximately one quarter of the COPD patients initiated an LABD following their hospitalization. These findings are important considering that pharmacological agents can reduce COPD exacerbation frequency and severity.17,25–29 It has also been shown that early treatment improves outcomes of COPD exacerbations and leads to a faster recovery, whereas a delay in medication initiation after an index hospitalization for COPD has been associated with an increased risk of a subsequent COPD-related hospitalization or ED visit.30,31 The results of the present analysis are consistent with a recent database analysis showing undertreatment (according to guidelines) in individuals with COPD who had an FEV1 <60% and an obstructive ratio defined as FEV1/FVC <70%.17 The previous database analysis also reported that most COPD treatment was initiated because of acute exacerbation, but this observation was not consistent with the results of the present study.17 Although we did not specifically examine acute COPD exacerbations, hospitalization was used as a proxy measure for patients who were experiencing an acute episode of COPD or an exacerbation that was severe enough to require hospitalization. Yet, only a little over half of the patients filled an LABD prescription within 180 days after hospitalization, and the majority of those were patients maintaining their LABD prescription. Thus, it appears that hospitalization did not largely impact use of LABD medications in the patient population studied.
The finding that patients in the no LABD group scored higher on the comorbidity index than the LABD group, and the finding that the no LABD group had fewer OP physician visits, ED visits, and hospitalizations in the 1- to 90- and 1- to 180-day post-index periods than the LABD group, both seem counterintuitive. One would assume that patients who are generally in poorer health would require more medications; however, this finding might suggest that patients in the no LABD group had more pressing health concerns that took precedence over control of COPD symptoms. In the aforementioned database analysis that showed undertreatment of COPD patients, comorbidity predicted lack of treatment.17 Furthermore, a possible reason for the lower health care utilization in the no LABD group could be that LABD use increases OP and/or ED visits. Alternatively, both of these findings might be because the patients who filled a prescription for an LABD had more severe COPD. However, disease severity could not be analyzed using the MarketScan® databases. Although the lack of disease severity information is a limitation of the current study, it should not have affected our primary outcome to examine patterns of prescription fills after hospitalization for COPD. Nonetheless, further research in this area would be of interest.
Another finding of this study worth noting was that patients who did not fill an LABD prescription within the first 90 days after hospitalization were not likely to fill an LABD prescription 91–180 days after hospitalization. These findings are consistent with other retrospective database studies that reported underuse of maintenance bronchodilators and/or inhaled corticosteroids following a hospitalization for COPD.32,33 In fact, a large database analysis reported that approximately 66% of COPD patients with commercial insurance did not receive any maintenance pharmacotherapy during the 1-year study period.34
This research had several strengths, including that the analyses were limited to patients who had COPD as a primary or secondary diagnosis during the year before the index hospitalization. Thus, these observational results reflect the experience of thousands of COPD patients in the real-world setting. Also, because of the possibility that a patient might have filled a prescription for a COPD medication before hospitalization and would likely have continued use after hospitalization, the study design included a prescription fill grace period that was calculated for each patient individually and added to the 90- or 180-day post-index period. Thus, the data more accurately include new prescription claims following the hospitalization.
This research also had some limitations. For example, codes on the insurance claims might have been recorded incorrectly or not at all, and it must be considered that a prescription claim for an LABD or other medication does not necessarily mean that the patient filled the prescription or was adherent by using the medication as prescribed. In addition, disease severity, COPD stage, mortality rate, and other clinical variables were unable to be recorded due to the nature of the database. The frequency and severity of exacerbations increase with the severity of the underlying COPD.35 Ideally, future research in this area will utilize different database types, such as electronic health records, to effectively control for factors such as disease severity and other potential confounders (ie, race, socioeconomic status, smoking history) during the study planning stage. It is also possible that a patient received a bronchodilator during their hospital stay or at the time of hospital discharge, which would not have been captured because the dataset only captures outpatient pharmacy claims. To help mitigate this limitation, this study included a 180-day post-index period, such that if a patient had filled a 3-month prescription for a bronchodilator during hospitalization or at discharge, they would likely have continued the medication and would have needed to refill the prescription at some time during the 180-day post-hospitalization window. It must also be acknowledged that a patient may have been prescribed another type of respiratory therapy during hospitalization. Finally, because these data were obtained from employer-sponsored insurance claims databases within the US, the results may not be applicable to the overall COPD patient population.9,21
Conclusion
In this observational study of claims data, a significant proportion of COPD patients who were hospitalized for COPD were not receiving an LABD before hospitalization, and, despite the negative impact of hospitalization on long-term outcome,36,37 hospitalization did not appear to greatly impact initiation of LABD medications. In fact, approximately one quarter of COPD patients in this study did not fill a prescription for any LABD or SABD during the entire 180-day period after hospitalization. In addition, patients who did not fill an LABD prescription within the first 90 days post-hospitalization were not likely to fill an LABD prescription in the second 90 days post-hospitalization. Taken together, these results suggest that many patients with COPD are undertreated. Whether treating COPD patients early in the course of the illness might avoid an initial hospitalization or whether the use of LABD medications following a hospitalization for COPD might prevent further hospitalizations needs to be further studied.
Acknowledgments
Editorial and medical writing support was provided by Cindy C Taylor, PhD and was funded by Pfizer Inc. and Boehringer-Ingelheim Pharmaceuticals Inc. Statistical programming oversight was conducted by the Clinical Informatics and Innovation, Information Strategy and Analytics, Pfizer Inc.
Disclosure
This research was supported by Pfizer Inc. and Boehringer-Ingelheim Pharmaceuticals Inc. All authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors and were fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development. CLB and KHZ are employees of Pfizer Inc., which cosponsored this analysis. JS is an employee of Boehringer-Ingelheim Pharmaceuticals Inc., which cosponsored this analysis.
References
Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance – United States, 1971–2000. Respir Care. 2002;47:1184–1199. | |
Shavelle RM, Paculdo DR, Kush SJ, Mannino DM, Strauss DJ. Life expectancy and years of life lost in chronic obstructive pulmonary disease: findings from the NHANES III Follow-up Study. Int J Chron Obstruct Pulmon Dis. 2009;4:137–148. | |
US Burden of Disease Collaborators. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. | |
Burden of COPD [webpage on the Internet]. Geneva: World Health Organization (WHO). Available from: http://www.who.int/respiratory/copd/burden/en/index.html. Accessed February 11, 2014. | |
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. | |
Qaseem A, Snow V, Shekelle P, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007; 147:633–638. | |
Makris D, Moschandreas J, Damianaki A, et al. Exacerbations and lung function decline in COPD: new insights in current and ex-smokers. Respir Med. 2007;101:1305–1312. | |
Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23:698–702. | |
Baker CL, Zou KH, Su J. Risk assessment of readmissions following an initial COPD-related hospitalization. Int J Chron Obstruct Pulmon Dis. 2013;8:551–559. | |
Elixhauser AA, Au DH, Podulka J. Readmission for Chronic Obstructive Pulmonary Disease, 2008. Rockville, MD: HCUP Statistical Brief #121: Healthcare Cost and Utilization Project; 2008. | |
National Quality Forum. Measure 1891: Hospital 30-Day, All-Cause, Risk-Standardized Readmission Rate (RSRR) Following Chronic Obstructive Pulmonary Disease (COPD) Hospitalization. Washington, DC: The National Quality Forum; 2012. Available from: http://www.qualityforum.org/Projects/n-r/Pulmonary_Endorsement_Maintenance/1891_30_Day_RSRR_COPD.aspx. Accessed February 11, 2014. | |
Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009; 360:1418–1428. | |
Agency for Healthcare Research and Quality [homepage on the Internet]. Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS). Washington, DC: US Department of Health and Human Services [updated February 18, 2014]. Available from: http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=226284BF0BAE1506&Form=SelLAY&JS=Y&Action=%3E%3ENext%3E%3E&_LAY=Researcher. Accessed February 11, 2014. | |
Garcia-Aymerich J, Barreiro E, Farrero E, Marrades RM, Morera J, Antó JM. Patients hospitalized for COPD have a high prevalence of modifiable risk factors for exacerbation (EFRAM study). Eur Respir J. 2000;16:1037–1042. | |
Perera PN, Armstrong EP, Sherrill DL, Skrepnek GH. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD. 2012;9:131–141. | |
Strassels SA, Smith DH, Sullivan SD, Mahajan PS. The costs of treating COPD in the United States. Chest. 2001;119:344–352. | |
Ingebrigtsen TS, Marott JL, Vestbo J, et al. Characteristics of undertreatment in COPD in the general population. Chest. 2013;144:1811–1818. | |
Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease [homepage on the Internet]. Global Initiative for Chronic Obstructive Lung Disease; 2014. Available from: http://www.goldcopd.com. Accessed February 11, 2014. | |
Kew KM, Mavergames C, Walters JA. Long-acting beta-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;10:CD010177. | |
Databases and Online Tools [webpage on the Internet]. Ann Arbor, MI: Truven Health Analytics Inc.; 2014. Available from: http://www.truvenhealth.com/your_healthcare_focus/pharmaceutical_and_medical_device/data_databases_and_online_tools.aspx. Accessed February 11, 2014. | |
Cao Z, Zou KH, Baker CL, et al. Respiratory-related medical expenditure and inpatient utilisation among COPD patients receiving long-acting bronchodilator therapy. J Med Econ. 2011;14:147–158. | |
Centers for Disease Control and Prevention; Classification of Diseases, Functioning, and Disability [webpage on the Internet]. International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). Hyattsville, MD: National Center for Health Statistics; 2013. Available from: http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed Febrary 11, 2014. | |
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. | |
Fagerland MW, Lydersen S, Laake P. The McNemar test for binary matched-pairs data: mid-p and asymptotic are better than exact conditional. BMC Med Res Methodol. 2013;13:91. | |
Bateman ED, Tashkin D, Siafakas N, et al. A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med. 2010;104:1460–1472. | |
Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. | |
Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093–1103. | |
Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1:199–209. | |
Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. | |
Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169:1298–1303. | |
Dalal AA, Shah MB, D’Souza AO, Dhamane AD, Crater GD. Outcomes associated with timing of maintenance treatment for COPD exacerbation. Am J Manag Care. 2012;18:e338–e345. | |
Nantsupawat T, Limsuwat C, Nugent K. Factors affecting chronic obstructive pulmonary disease early rehospitalization. Chron Respir Dis. 2012;9:93–98. | |
Yip NH, Yuen G, Lazar EJ, et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD. 2010;7:85–92. | |
Make B, Dutro MP, Paulose-Ram R, Marton JP, Mapel DW. Undertreatment of COPD: a retrospective analysis of US managed care and Medicare patients. Int J Chron Obstruct Pulmon Dis. 2012;7:1–9. | |
Trigueros Carrero JA. How should we define and classify exacerbations in chronic obstructive pulmonary disease? Expert Rev Respir Med. 2013;7:33–41. | |
Almagro P, Barreiro B, Ochoa de Echaguen A, et al. Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease. Respiration. 2006;73:311–317. | |
Bahadori K, FitzGerald JM. Risk factors of hospitalization and readmission of patients with COPD exacerbation – systematic review. Int J Chron Obstruct Pulmon Dis. 2007;2:241–251. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.