Back to Journals » Journal of Pain Research » Volume 9
Local analgesic effect of tramadol is mediated by opioid receptors in late postoperative pain after plantar incision in rats
Authors Oliveira Junior JO, de Freitas MF, Bullara de Andrade C, Chacur M, Ashmawi HA
Received 20 July 2016
Accepted for publication 8 September 2016
Published 17 October 2016 Volume 2016:9 Pages 797—802
DOI https://doi.org/10.2147/JPR.S117674
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
José Oswaldo de Oliveira Junior,1 Milena Fernandes de Freitas,2 Carolina Bullara de Andrade,3 Marucia Chacur,2 Hazem Adel Ashmawi1
1Laboratório de Anestesiologia Experimental, Faculdade de Medicina da Universidade de São Paulo, 2Departamento de Anatomia do Instituto de Ciências Biomédicas da Universidade de São Paulo, 3Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil
Abstract: Tramadol is a drug used to treat moderate to severe pain. It is known to present a peripheral effect, but the local mechanisms underlying its actions remain unclear. The role of peripheral opioid receptors in postoperative pain is not well understood. In the present study, we examined the peripheral opioid receptors to determine the local effect of tramadol in a plantar incision pain model. Rats were subjected to plantar incision and divided into four groups on postoperative day (POD) 1: SF_SF, 0.9% NaCl injected into the right hindpaw; SF_TraI, 0.9% NaCl and tramadol injected into the right hindpaw; SF_TraC, 0.9% NaCl and tramadol injected into the contralateral hindpaw; and Nal_Tra, naloxone and tramadol injected into the ipsilateral hindpaw. To determine the animals’ nociceptive threshold, mechanical hyperalgesia was measured before incision, on POD1 before treatment and at 15, 30, 45, and 60 minutes after the incision. The same procedure was repeated on the POD2. The expression levels of μ-opioid receptor (MOR) and δ-opioid receptor (DOR) were obtained through immunoblotting assays in the lumbar dorsal root ganglia (L3–L6) in naïve rats and 1, 2, 3, and 7 days after the incision. Our results showed that the plantar incision was able to cause an increase in mechanical hyperalgesia and that tramadol reversed this hyperalgesia on POD1 and POD2. Tramadol injections in the contralateral paw did not affect the animals’ nociceptive threshold. Naloxone was able to antagonize the tramadol effect partially on POD1 and completely on POD2. The DOR expression increased on POD2, POD3, and POD7, whereas the MOR expression did not change. Together, our results show that tramadol promoted a local analgesic effect in the postoperative pain model that was antagonized by naloxone in POD2, alongside the increase of DOR expression.
Keywords: tramadol, postoperative pain, opioid receptors, naloxone, rats
Introduction
Tramadol is a drug used for the treatment of moderate to severe pain in acute and chronic pain conditions.1,2 Tramadol demonstrates different mechanisms of action, such as central and peripheral. It exhibits a weak opioid effect through the decrease of the reuptake of monoamines (serotonin and noradrenaline) in the central nervous system and also shows a peripheral and local analgesic effect, which is, in part, local-anesthetic-like.3–5
The peripheral analgesic effect of tramadol has also been demonstrated in humans after infiltration surgical procedures and as adjuvants to local anesthetics in postoperative pain management.6–9 Opioids are potent analgesics that exert pharmacological and physiological effects due to their interaction with receptors distributed in several regions. In the literature, the following three subtypes of opioid receptors are described: μ-opioid receptors (MOR), which are widely distributed in different parts of the system, and δ-opioid receptor (DOR) and κ-opioid receptor (KOR), both distributed in different systems such as the immune system, the neuroendocrine system, the peripheral nervous system, and the central nervous system, modulating pain and inflammation. Peripheral opioid receptors have long been described in studies and their roles in analgesia in animals and humans have been extensively demonstrated, but little is known about the peripheral opioid-mediated effect of tramadol.10–14
Previous data published from our group have shown that the local analgesic effect of tramadol was not mediated by the opioid in the first hour after plantar incision in rats.15 We hypothesized that opioid receptors could have been expressed later on in the same model, possibly affecting local analgesia produced by tramadol under similar circumstances.
Based on our previous work and due to the lack of information about the possible role of opioid receptors in the postoperative pain model, our goal in this work was to evaluate the role of opioid receptors in the analgesic effects of intraplantar tramadol on plantar incision-induced postoperative pain. In addition, we also analyzed the expression of MOR and DOR in dorsal root ganglia (DRGs).
Materials and methods
Animals
All experiments were performed under the approval of the Bioethics Committee of the Hospital of Clinics of the Faculty of Medicine, University of São Paulo (approval number: 188/10) and followed Brazilian regulations for the care of the animals according to the Committee for Research and Ethical Issues of the International Association for the Study of Pain (IASP).16 All experiments were performed on Wistar male rats, weighing 250 g, supplied by the Faculty of Medicine, University of São Paulo, breeding facilities. A total 75 animals were used in this study, 35 animals in the behavioral study and 40 animals in the immunoblotting study. All behavioral experiments were conducted between 9:00 am and 12:00 noon. All animals were housed in pairs in cages (wood shavings) with free access to food and water.
Plantar incision
Plantar incision was performed on the right hindpaw of the rats, as previously described.17 Rats were anesthetized using 2–3% isoflurane delivered through a nose cone. The plantar surface of the right hindpaw was prepared in a sterile manner with a 10% povidone–iodine solution and draped. A 1 cm longitudinal incision was made with a number 11 blade through the skin and fascia of the plantar surface of the rat paw, starting 0.5 cm from the heel proximal edge and extending toward the toes. The flexor muscle was elevated and incised longitudinally, while the muscle origin and insertion were kept intact. After hemostasis with gentle pressure, the skin was sutured with two simple knots of 5–0 mononylon filaments.
Mechanical hyperalgesia
Rats were placed in compartments over an elevated plastic mesh floor covered with a clear plastic top. The animals were allowed to ambulate, explore, and eventually rest lying on the mesh. For assessing the animals’ nociceptive behavior, the electronic Von Frey device was used (Insight, Brazil) and applied to the ipsi/contralateral paw surface, adjacent to the wound, underneath the mesh.18,19 In this device, a tip was adapted to a force transducer and pressed against the animal’s plantar surface, the force increasing constantly until the paw withdrew. This force was considered as the animal’s nociceptive threshold.
Experimental design
Behavioral experiments
After baseline assessments of the withdrawal thresholds for mechanical hyperalgesia, animals were subjected to plantar incision on the right hindpaw. On postoperative day (POD) 1 (24 hours after incision), the animals were tested again for mechanical hyperalgesia before interventions. Afterward, the animals were divided into four experimental groups of 8–10 animals each: 1) SF_SF, ie, under general anesthesia, 50 μL of 0.9% NaCl solution was injected in the plantar surface of the right hindpaw, and 15 minutes later another 50 μL of 0.9% NaCl solution was reinjected and the animal was awakened; 2) SF_TraI, ie, tramadol was injected ipsilaterally, 50 μL of 0.9% NaCl solution was injected in the plantar surface of the hindpaw, and 15 minutes later 5 mg (20 mg/kg) of tramadol dissolved in 50 μL of 0.9% NaCl was administered in the plantar surface of the hindpaw; 3) SF_TraC, ie, tramadol was injected contralaterally, 50 μL of 0.9% NaCl solution was injected in the plantar surface of the contralateral hindpaw, and 15 minutes later 5 mg (20 mg/kg) of tramadol was administered in the plantar surface of the contralateral hindpaw; 4) Nal_Tra, ie, naloxone/tramadol (naloxone [1 mg/kg] dissolved in 50 μL of 0.9% NaCl) was injected in the plantar surface of the ipsilateral hindpaw, and 15 minutes later 5 mg (20 mg/kg) of tramadol was injected in the plantar surface of the ipsilateral hindpaw. As soon as the animals recovered, they were tested for behavioral assessments at 15, 30, 45, and 60 minutes after recovery from anesthesia. The same procedure was repeated on POD2. The doses of tramadol and naloxone were chosen according to previous study.15 The timeline of the experimental protocol is shown in Figure 1. At the end of the experiments, the animals received a lethal injection of sodium thiopental.
Western blot analysis
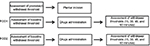  | Figure 1 Experimental design scheme. Note: Timelines of injections and assessments or experimental groups are shown. Abbreviation: POD, postoperative day. |
Western blotting analyses were performed on samples from individual animals. Animals were subjected to plantar incision under isoflurane anesthesia. Tissue collection was performed under isoflurane anesthesia at POD1, POD2, POD3, and POD7, and one group of animals was used as control (naïve). DRGs were quickly removed and homogenized in an extraction buffer containing 100 mM Tris, pH 7.4, 10 mM EDTA, 2 mM PMSF, and 10 μg/mL aprotinin. After extraction, the homogenates were centrifuged at 11,500 × g for 20 minutes, and the protein concentration of the supernatant was determined using the Bradford protein assay with albumin as standard (Bio-Rad, USA).20 Samples containing 30 μg protein were loaded on a 12% polyacrylamide gel and electrophoretically transferred to nitrocellulose membranes using a Bio-Rad miniature transfer apparatus for 1.5 hours at a steady 120 V. After transferring, the membranes were treated for 2 hours at room temperature with a blocking solution containing 5% powdered milk, washed three times with Tris-buffered saline + Tween 20 (TBST), and incubated overnight at 4°C with rat monoclonal antibodies against anti-MOR or (μ), anti-KOR or (κ), and anti-DOR or (δ) (all 1:250; Santa Cruz Biotechnology, Inc, USA). Membranes were then washed three times with TBST and incubated for 2 hours at room temperature with a biotin-conjugated goat anti-rabbit secondary antibody (to MOR and DOR) diluted 1:5000 (Jackson ImmunoResearch Laboratories, Inc, USA). In all immunoblotting experiments, β-actin (mouse, 1:15,000; Sigma-Aldrich, USA) was used as internal control. The specific antibody binding was visualized using a chemoluminescence kit (Amersham Biosciences). The membranes were revealed in a UVITEC® device (Alliance Chroma, UK), and the results were densitometrically analyzed using the NIH-Scion Image 4.0.2 software (Scion Corporation, USA).
Drugs
Tramadol hydrochloride and naloxone hydrochloride were provided by Cristália Prod. Quim. Farm (São Paulo, Brazil).
Statistical analysis
All data were analyzed by the Prism 5.0 software (GraphPad Software, Inc., USA). Withdrawal thresholds to mechanical stimuli were analyzed using two-way analysis of variance (ANOVA) with repeated measures, followed by post hoc Bonferroni tests. The data are expressed as mean ± standard error. Opioid receptor expression values were compared using ANOVA multiple comparison and post hoc analysis using the Tukey test.
Results
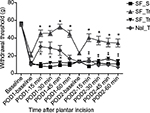
Behavioral experiments
For this part of the study, 35 rats were used and divided into four groups. All animals exhibited mechanical hyperalgesia in their baseline values on POD1 and POD2, before drug injections (Figure 2). The SF_SF group showed mechanical hyperalgesia at all time points of the experiment. The SF_TraI group exhibited mechanical hyperalgesia on POD1 and POD2 at baseline; after tramadol injection, the animal’s withdrawal thresholds increased throughout 60 minutes in both days. In the SF_TraC group, animals exhibited hyperalgesia at every time point analyzed on both days. In the Nal_Tra group, a partial decrease in mechanical hyperalgesia was observed on POD1; on POD2, naloxone antagonized the anti-hyperalgesic effect of tramadol (Figure 2).
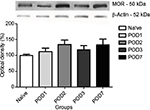
Expression of opioid receptors in the DRGs
Analysis of the opioid receptor protein levels was done to evaluate their expression levels over time. Our results showed an increase in DOR levels on the 2nd, 3rd, and 7th day after plantar incision compared to the naive group (Figure 3). MOR protein expression did not change during the 7 days after the injury, compared to the naive group (Figure 4). KOR protein expression was also analyzed, but we did not detect any trace of this receptor in DRG at any time point (data not shown). No significant statistical difference was observed in the β-actin expression.
Discussion
The use of classical experimental pain models is under scrutiny due to the partial failure of the translation of results obtained experimentally to the clinical environment and real situations.21,22 The development and search for models that mimic clinical scenarios is very important. In our study, the main aim was to deepen the understanding of the peripheral analgesia of tramadol. For that, we chose plantar incision as the experimental pain model,17 which has been used for the last two decades and brings to the experimental environment a relevant clinical situation, namely the postoperative pain.23–26
The peripheral analgesic effect of tramadol has been demonstrated in the last 14 years,5,15,27,28 mostly its local anesthetic effect. As tramadol is an opioid agonist, the hypothesis of its mechanism of action in analgesia is apparent. The use of tramadol has been tested before in the formalin pain model, and it did not show a significant effect in the first hour after plantar incision.5,6 Ji et al found an increase of opioid expression in DRG, raising the possibility of a late opioid mediation in analgesia.29 Our results showed a partial reversal of analgesia after the use of opioid on day 1 and complete reversal on day 2. It is interesting to note that in our previous study no opioid effect was observed and only the local anesthetic effect was found.15 In this study, we detected a decrease in the local anesthetic effect and an increase in the analgesia mediated by endogenous opioids. A plausible explanation for the decrease in the local anesthetic effect might be related to the local environment of the incision, where the pH level is low, lactate level is high, and hypoxia occurs. All these factors interfere with the effect of tramadol, which has a high pKa (9.4), influencing its local anesthetic properties.30–32
Opioid receptors expression in DRG showed a significant increase of DOR, but no change in MOR levels was observed. DOR expression increased from POD2 to POD7, which corroborates with the observation of antagonism of the opioid effect on the 2nd day after incision. The lack of involvement of MOR and KOR is still not clear. However, studies showed different results in relation to various types of opioid receptors. Studies of Ji et al,29 Porreca et al,33 and Truong et al,34 where opioid receptor expression in different pain models were studied (carrageenan, complete Freund’s adjuvant, sciatic nerve constriction, and spinal nerve ligation), showed no definite pattern of expression for inflammatory or neuropathic pain models, but it seems that DOR expression increases in neuropathic pain and MOR increases in inflammation. Mechanisms of pain in the plantar incision model comprise ischemia (hypoxia, local acidosis, and lactate elevation) and neuronal sensitization, involving both neuropathic and inflammatory components.35 The involvement of KOR in the anti-nociceptive effect induced by prostaglandin E2, carrageenan, and crotalphine has been shown.36 Other study has shown differences in the expression of opioids, with the increase in KOR and DOR in a cancer pain model,37 but both groups did not observe the participation of MOR receptor in their studies. These data confirm our findings that there was no participation of MOR in our model.
This study is the first to demonstrate the role of opioid mediation in the peripheral analgesic effect of tramadol in the plantar incision model and report the expression of opioid receptors in the same model in rats.
Disclosure
The authors report no conflicts of interest in this work.
References
Dayer P, Desmeules J, Collart L. Pharmacology of tramadol. Drugs. 1997;53(Suppl 2):18–24. | ||
Lewis KS, Han NH. Tramadol: a new centrally acting analgesic. Am J Health Syst Pharm. 1997;54(6):643–652. | ||
Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992;260(1):275–285. | ||
Tsai YC, Chang PJ, Jou IM. Direct tramadol application on sciatic nerve inhibits spinal somatosensory evoked potentials in rats. Anesth Analg. 2001;92(6):1547–1551. | ||
Sousa AM, Ashmawi HA, Costa LS, Posso IP, Slullitel A. Percutaneous sciatic nerve block with tramadol induces analgesia and motor blockade in two animal pain models. Braz J Med Biol Res. 2012;45(2):147–152. | ||
Prakash S, Tyagi R, Gogia AR, Singh R, Prakash S. Efficacy of three doses of tramadol with bupivacaine for caudal analgesia in paediatric inguinal herniotomy. Br J Anaesth. 2006;97(3):385–388. | ||
Kargi E, Isikdemir A, Tokgoz H, et al. Comparison of local anesthetic effects of tramadol with prilocaine during circumcision procedure. Urology. 2010;75(3):672–675. | ||
Demiraran Y, Albayrak M, Yorulmaz IS, Ozdemir I. Tramadol and levobupivacaine wound infiltration at cesarean delivery for postoperative analgesia. J Anesth. 2013;27(2):175–179. | ||
Polat F, Tuncel A, Balci M, et al. Comparison of local anesthetic effects of lidocaine versus tramadol and effect of child anxiety on pain level in circumcision procedure. J Pediatr Urol. 2013;9(5):670–674. | ||
Hervera A, Negrete R, Leanez S, Martin-Campos JM, Pol O. Peripheral effects of morphine and expression of μ-opioid receptors in the dorsal root ganglia during neuropathic pain: nitric oxide signaling. Mol Pain. 2011;7:25. | ||
Saloman JL, Niu KY, Ro JY. Activation of peripheral delta-opioid receptors leads to anti-hyperalgesic responses in the masseter muscle of male and female rats. Neuroscience. 2011;190:379–385. | ||
Auh QS, Ro JY. Effects of peripheral κ opioid receptor activation on inflammatory mechanical hyperalgesia in male and female rats. Neurosci Lett. 2012;524(2):111–115. | ||
Stein C, Yassouridis A. Peripheral morphine analgesia. Pain. 1997;71(2):119–121. | ||
Likar R, Schafer M, Paulak F, et al. Intraarticular morphine analgesia in chronic pain patients with osteoarthritis. Anesth Analg. 1997;84(6):1313–1317. | ||
Sousa AM, Ashmawi HA. Local analgesic effect of tramadol is not mediated by opioid receptors in early postoperative pain in rats. Braz J Anesthesiol. 2015;65(3):186–190. | ||
Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. | ||
Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64(3):493–501. | ||
Möller KÄ, Johansson B, Berg OG. Assessing mechanical allodynia in the rat paw with a new electronic algometer. J Neurosci Methods. 1998;84(1–2):41–47. | ||
Vivancos GG, Verri WA Jr, Cunha TM, et al. An electronic pressure-meter nociception paw test for rats. Braz J Med Biol Res. 2004;37(3):401–409. | ||
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. | ||
Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10(4):283–294. | ||
Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151(1):12–17. | ||
Villarreal CF, Del Bel EA, Prado WA. Involvement of the anterior pretectal nucleus in the control of persistent pain: a behavioral and c-Fos expression study in the rat. Pain. 2003;103(1–2):163–174. | ||
Obata H, Conklin D, Eisenach JC. Spinal noradrenaline transporter inhibition by reboxetine and Xen2174 reduces tactile hypersensitivity after surgery in rats. Pain. 2005;113(3):271–276. | ||
Reichl S, Augustin M, Zahn PK, Pogatzki-Zahn EM. Peripheral and spinal GABAergic regulation of incisional pain in rats. Pain. 2012;153(1):129–141. | ||
Ghasemlou N, Chiu IM, Julien JP, Woolf CJ. CD11b+Ly6G− myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 2015;112(49):E6808–E6817. | ||
Mert T, Gunes Y, Guven M, Gunay I, Ozcengiz D. Comparison of nerve conduction blocks by an opioid and a local anesthetic. Eur J Pharmacol. 2002;439(1–3):77–81. | ||
Mert T, Gunes Y, Ozcengiz D, Gunay I, Polat S. Comparative effects of lidocaine and tramadol on injured peripheral nerves. Eur J Pharmacol. 2006;543(1–3):54–62. | ||
Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hokfelt T. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci. 1995;15(12):8156–8166. | ||
Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology. 2004;101(2):468–475. | ||
Kim TJ, Freml L, Park SS, Brennan TJ. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. J Pain. 2007;8(1):59–66. | ||
Kang S, Lee D, Theusch BE, Arpey CJ, Brennan TJ. Wound hypoxia in deep tissue after incision in rats. Wound Repair Regen. 2013;21(5):730–739. | ||
Porreca F, Tang QB, Bian D, Riedl M, Elde R, Lai J. Spinal opioid mu receptor expression in lumbar spinal cord of rats following nerve injury. Brain Res. 1998;795(1–2):197–203. | ||
Truong W, Cheng C, Xu QG, Li XQ, Zochodne DW. Mu opioid receptors and analgesia at the site of a peripheral nerve injury. Ann Neurol. 2003;53(3):366–375. | ||
Brennan TJ. Pathophysiology of postoperative pain. Pain. 2011;152(3 Suppl):S33–S40. | ||
Brigatte P, Sampaio SC, Gutierrez VP, et al. Walker 256 tumor-bearing rats as a model to study cancer pain. J Pain. 2007;8(5):412–421. | ||
Konno K, Picolo G, Gutierrez VP, et al. Crotalphine, a novel potent analgesic peptide from the venom of the South American rattlesnake Crotalus durissus terrificus. Peptides. 2008;29(8):1293–1304. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.