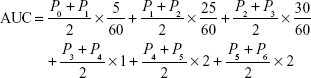Back to Journals » OncoTargets and Therapy » Volume 11
Local analgesic effect of a bioadhesive barrier-forming oral liquid in cancer patients with oral mucositis caused by chemotherapy and/or radiotherapy: a randomized multicenter, single-use, positive-controlled, open-label study
Authors Cheng Y, Qin SK , Chen YP, Dong LH, Sun XD, Yu SY, Wu SK
Received 30 August 2018
Accepted for publication 31 October 2018
Published 30 November 2018 Volume 2018:11 Pages 8555—8564
DOI https://doi.org/10.2147/OTT.S185915
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Yuan Cheng,1 Shu Kui Qin,1 Yan Ping Chen,2 Li Hua Dong,3 Xiang Dong Sun,4 Shi Ying Yu,5 Shi Kai Wu6
1Department of Oncology, Bayi Hospital Affiliated to Nanjing University of Chinese Medicine, Nanjing 210002, China; 2Department of Stomatology, Fourth Hospital of Hebei Medical University, Shijiazhuang 050011, China; 3Department of Radiotherapy, First Hospital of Jilin University, Changchun 130021, China; 4Department of Radiotherapy, Bayi Hospital Affiliated to Nanjing University of Chinese Medicine, Nanjing 210002, China; 5Department of Oncology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430012, China; 6Department of Radiation Oncology, Affiliated Hospital of Academy of Military Medical Sciences, Beijing 10071, China
Objective: CAM2028 (Episil®; Camurus AB, Lund, Sweden) is a liquid for use in the oral cavity to treat various pains associated with mouth injuries. Upon contact with the swollen oral mucosa, the oral liquid forms a thin protective film that acts as a mechanical barrier to relieve pain. This study was the first in China to evaluate the local analgesic effect of oral liquid in cancer patients who developed oral mucositis following chemotherapy and/or radiotherapy.
Methods: A total of 60 patients were randomized in a 1:1 ratio to the CAM2028 group (the pump device was firmly pressed three times and the fluid was distributed to the painful area of the oral cavity) or KS (a mucoadhesive oral wound rinse, Kangsu™; Luye Pharmaceutical Co. Ltd, Nanjing, China) group (5 mL of the oral rinse was poured into and kept in the oral cavity for at least 1 minute). The primary endpoint was the area under the oral mucosal pain score–time curve (AUC) within 6 hours of treatment in the trial and control groups. Medical device adverse events were assessed according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0. Statistical analyses were performed using the chi-squared test (Fisher’s exact test), independent-samples t-test, and analysis of covariance.
Results: Sixty patients were included in the per-protocol set population analysis. The average (mean ± SD) 6-hour AUC of the CAM2028 group and the KS group was 14.20±10.29 and 24.46±14.15, respectively. The difference between the groups was statistically significant (P=0.0022). The incidence of adverse events in the trial group and the control group was 16.67% and 30.0%, respectively, and there was no statistical difference.
Conclusions: CAM2028 displayed an efficacious local analgesic effect in cancer patients who developed oral mucositis following chemotherapy and/or radiotherapy. The results demonstrated its potential value in clinical applications.
Keywords: oral mucositis, radiotherapy, chemotherapy, analgesic effect, episil
Introduction
Oral mucositis (OM) is a serious and common adverse event associated with chemotherapy and/or radiotherapy used in the treatment of cancers or prior to bone marrow transplantation. OM occurs in 20%–40% of patients with cancer who receive conventional chemotherapy, 80% of patients who receive high-dose chemotherapy (for hematopoietic stem cell transplantation), and nearly all patients who receive radiotherapy in the head and neck region.1,2 OM is often a dose-limited toxicity condition that leads to dose reduction, interruption, or delays in chemotherapy and/or radiotherapy. It may cause severe pain in some patients who are unable to eat and will require the need for more supportive care, parenteral or enteral nutrition, opioid analgesics, and hospitalization. Many patients who receive opioid treatment still experience severe pain and are unable to eat, drink, and speak. In addition to analgesics, some medical devices are approved for use to alleviate the pain caused by OM.
CAM2028 (Episil®; Camurus AB, Lund, Sweden) is a preservative-free oral liquid gel that can be used for the management of pain associated with oral lesions of various etiologies. The oral liquid forms a protective film upon contact with the swollen oral mucosa. The film can act as a mechanical barrier to relieve pain. The oral liquid has been registered and approved as a medical device in the European Union, Israel, the United Arab Emirates, and the USA.
KS (Kangsu™; Luye Pharmaceutical Co. Ltd, Nanjing, China) is an aqueous polymer gel. It is approved in China as a class II medical device for the prevention and treatment of stomatitis caused by radiotherapy and chemotherapy, as well as the prevention and treatment of various OM and oral ulcers.
The purpose of this study was to evaluate the best medical device for relieving pain associated with OM to improve patients’ compliance with and tolerability to treatment as well as to enhance their quality of life. This was a randomized multicenter, single-use, positive-controlled, open-label study. The local analgesic effects of CAM2028 and KS after at least 6 hours of a single use were compared in cancer patients who developed OM following chemotherapy and/or radiotherapy. The safety and mucoadhesiveness of CAM2028 after a single use were also evaluated.
Materials and methods
Study design
This study was led by Professor Qin Shu Kui and there were seven participating institutions: Bayi Hospital Affiliated Nanjing University of Chinese Medicine, First Affiliated Hospital of Nanjing Medical University, Fourth Hospital of Hebei Medical University, First Hospital of Jilin University, Fudan University Cancer Center, Tongji Hospital of Tongji Medical College, Huazhong University of Science, and Affiliated Hospital of Academy of Military Medical Sciences. This was a randomized multicenter, single-use, positive-controlled, open-label study conducted from September 2017 to May 2018.
Inclusion/exclusion criteria
The main inclusion criteria were as follows: participants with histopathologically and/or cytologically confirmed malignant tumors; patients who exhibited symptomatic OM (WHO grade 2 or above) when receiving chemotherapy and/or radiotherapy at screening; patients with pain scores of at least 6 on a Likert scale of 0–10 at screening and on the first day of enrolment; and patients who had received chemotherapy and/or radiotherapy and, prior to the first day of enrolment, had received at least one cycle of chemotherapy with a multicycle regimen and/or multifraction radiotherapy. The main exclusion criteria were as follows: patients with a tumor in the oral cavity; patients with confirmed or suspected metastases or confirmed invasion of the central nervous system; and patients who had received drugs that promoted oral mucosal healing within 4 weeks before screening (eg, granulocyte-macrophage colony-stimulating factor and palifermin).
Study procedures
Participants were administered CAM2028 or KS, at random, on treatment day 1. The treatment duration was 9 consecutive days for all patients (7 days of screening, 1 day for treatment [day 1], and 1 day for follow-up observation [day 2]). The trial group (CAM2028) treatment procedure was as follows: patients firmly pressed the pump device three times; distributed the fluid to the painful areas of the oral cavity, eg, by using the tongue; and waited for 5 minutes until a protective film had formed in the oral cavity. For the control group (KS), the procedure was as follows: participants slowly poured 5 mL of the oral rinse into the oral cavity and kept the rinse in the mouth for at least 1 minute. Oral mucosal pain was assessed by the patients using numerical scoring (ie, 0–10 on a Likert scale, consisting of 11 points from 0 to 10; numbers from low to high indicate pain from painless to the most painful, with 0 for painless, 10 for severe pain, and the middle scores for varying degrees of pain; and patients choose a number that best represents their own pain) at screening, before dosing, and at 5 and 30 minutes and 1, 2, 4, and 6 hours post-dose. The efficacy evaluation was the area under the oral mucosal pain score–time curve (AUC) within 6 hours after a single use. Safety assessment was performed at screening and on treatment day 2, including medical device adverse events, laboratory tests (complete blood count and blood biochemistry), vital signs, physical examination, and oral mucosal examination (assessed according to the WHO Oral Toxicity Scale). Adverse events and rescue medications were recorded throughout the trial. Mucoadhesiveness was evaluated at 5 and 30 minutes and at 1, 2, 4, and 6 hours postdose in the trial group.
Efficacy and safety evaluations
Primary efficacy objective
The primary efficacy objective was the AUC of oral mucosal pain score–time within 6 hours after a single use. Baseline was defined as the oral mucosal pain assessed using a numerical score (ie, 0–10 on the Likert scale) before a single use of the study medical device. Pt (t=0, 1, …, 6 hours) was used to indicate oral pain scores at baseline and at 5 and 30 minutes and 1, 2, 4, and 6 hours after a single use of the study medical device. A trapezoidal method was used to calculate the AUC and the formula is as follows (the unit of time was hours):
|
Secondary efficacy objectives
The secondary efficacy objectives were as follows: 1) pain intensity difference (PID) 6 hours after a single use; 2) peak pain intensity difference (PPID); 3) the use of rescue medications; and 4) the mucoadhesiveness of CAM2028.
Safety evaluations
Medical device adverse events were assessed according to the five-grade scale of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 4.0.
Ethical review
The study protocol was approved by the ethics committees of each center and was registered at ClinicalTrials.gov (identification number: NCT03546985). The trial was conducted in accordance with the Declaration of Helsinki and all patients signed a written informed consent form prior to entering the study.
Statistical analysis
Referring to the HS-05-161 study,3 a total of 60 subjects were enrolled with the aim of a final sample of 50 evaluable patients. All 60 subjects were randomized in a 1:1 ratio to the trial group or the control group. Random tables were generated using SAS 9.4 software. Randomization was done by block randomization.
Statistical analysis was performed in the full analysis set (FAS), the per-protocol set (PPS), and the safety analysis set (SAS). The FAS was defined as all patients randomized to a group who had used the study device; the PPS was a subset of the FAS, including all patients who met the eligibility criteria in the protocol, completed the evaluations for the primary efficacy endpoint, and had not received other drugs or treatments that might affect the efficacy evaluation during the trial; and the SAS was all patients randomized to a group who had used the study device at least once. The FAS and PPS populations were used for the analyses of primary and secondary efficacy measures, and the SAS population was used for safety analysis.
Statistical analyses were performed using the chi-squared test, Fisher’s exact test, independent samples t-test, and analysis of covariance. The measurement data are presented as mean and SD. The count data are presented as number of cases and frequency. The baseline was defined as the oral mucosal pain score before the single use of the study medical device. SAS version 9.4 software was used to process the data. Statistical significance was set to a P-value of <0.05.
Results
Baseline characteristics
In this study, 61 patients were screened and 60 patients were enrolled. All enrolled patients completed the trial, and none discontinued their participation in the study. The reason for the screening failure in one patient was that the patient had used a drug that promoted oral mucosal healing within 4 weeks prior to the screening period. All enrolled patients were included in the FAS data set, PPS data set, and SAS data set. Sixty subjects were randomized in a 1:1 ratio to the trial group (30 patients in the CAM2028 group) or the control group (30 patients in the KS group). The oldest patient in the trial group was 78 years old and the youngest was 20 years old; the median age of the trial group was 51 years. In the control group, the oldest patient was 81 years old and the youngest was 35 years old; the median age of the control group was 55 years. The two groups were comparable, with no statistically significant differences in age, sex, height, weight, smoking history, drinking history, allergic history, Eastern Cooperative Oncology Group (ECOG) score, or oral pain score at baseline (Table 1).
Primary efficacy analysis
In the comparison of oral pain scores at baseline, the patients in both the test and control groups had a maximum and minimum oral pain score of 10 and 6 points, respectively. The pain scores of the test and control groups were 6.7±1.12 and 7.23±1.19, respectively. By independent samples t-test, the difference was not statistically significant (P=0.0795), indicating that the baseline pain scores of the two groups were comparable.
The mean AUC value for the measure of oral mucosal pain score within 6 hours after a single use of the study medical device in the trial group was 14.20±10.29, with a maximum value of 39.79 and a minimum value of 1.00. The mean AUC value of the control group was 24.46±14.15, with a maximum value of 59.54 and a minimum value of 0.33. The comparison between the groups indicated that the difference was statistically significant (P=0.0022).
Furthermore, analysis of covariance revealed that the difference between the groups was statistically significant (FAS: P=0.0004; PPS: P=0.0004). The difference among centers showed a consistent trend (FAS: P=0.8872; PPS: P=0.8872). The adjusted mean differences in the FAS and PPS of the trial and control groups were both −11.71 (95% CI −17.89 to −5.53), and the difference between the groups was statistically significant (P<0.05).
The adjusted mean AUC in the FAS and PPS of the trial group was 9.73 for both sets (95% CI 4.79 to 14.67). The adjusted mean AUC in the FAS and PPS of the control group was 21.44 for both sets (95% CI 16.46 to 26.42).
Secondary efficacy analysis
To determine the PID within 6 hours after a single use of the study medical device, pain scores in the two groups were measured 5 minutes after the use of the study device. The pain score of the trial group decreased from 6.7 to 3.57, with a reduction in median pain intensity of approximately 46.7%. The pain score of the control group decreased from 7.23 to 4.67, with a reduction in median pain intensity of approximately 35.4%. The pain intensity of the trial group continued to decrease, and the reduction was maintained up to 6 hours post-treatment. The lowest pain score was 2.0. In the control group, the pain intensity decreased to a minimum at 2 hours post-treatment, at which time the score was 3.93. Subsequently, the pain intensity rebounded and the pain score at 6 hours post-treatment returned to 4.33. There was no significant difference in the baseline pain scores between the two groups, whereas significant differences were observed at 1, 2, 4, and 6 hours post-treatment (P<0.05) (Figure 1 [the results for FAS and PPS were the same] and Table 2).
  | Figure 1 Oral pain scores at various time points after a single use of the study device. |
Independent samples t-tests indicated that compared with the baseline values, the reductions in oral pain scores at 5 minutes, 30 minutes, 1 hour, and 6 hours after the use of the study devices were not statistically different between the trial and control groups (P>0.05), whereas at 2 and 4 hours, the differences between the groups were statistically significant (P<0.05) (Table 2).
Analysis of covariance revealed a statistically significant difference in the PID at 6 hours after treatment between the groups (FAS/PPS: P=0.0081), and the difference among centers showed a consistent trend (FAS/PPS: P=0.9587). The adjusted mean differences in the oral pain scores in the FAS and PPS of the trial and control groups were both −1.63 (95% CI −2.82 to −0.44), and the difference between the groups was statistically significant (P<0.05).
The adjusted mean PIDs at 6 hours after treatment in the FAS and PPS of the trial group were both −5.08 (95% CI −6.03 to −4.13), and those of the control group were both −3.45 (95% CI −4.41 to −2.49).
The PPIDs within 6 hours after a single use of the study medical device for the trial and the control groups were 4.33±2.07 and 5.27±2.16, respectively. An independent samples t-test indicated P=0.0934; the difference was not statistically significant. Analysis of covariance revealed that the difference between the groups was not statistically significant (FAS/PPS: P=0.1122), and the difference among centers showed a consistent trend (FAS/PPS: P=0.9436).
The differences between the PPID and baseline values in the trial and control groups were −2.37±1.71 and −1.97±2.04, respectively. An independent samples t-test indicated P=0.4144; the difference was not statistically significant. Analysis of covariance revealed that the difference between the groups was not statistically significant (FAS/PPS: P=0.1122), and the difference among centers showed a consistent trend (FAS/PPS: P=0.9436).
The use of rescue medications within 6 hours after a single use of the study device was evaluated. Among the 30 patients in the trial group, three required the use of rescue medications for a total of five times. The rescue medications used were oxycodone tablets, morphine tablets, Kangfuxin Ye, and lidocaine injection (all used to relieve oral pain). The incidence rate was 10.00%. Among the 30 patients in the control group, two required the use of rescue medications for a total of three times. The rescue medications used were tramadol, oxycodone tablets, and morphine tablets. The incidence rate was 6.67%. There was no statistically significant difference in the usage rate as determined by Fisher’s exact probability test (P=1.0000).
After excluding the patients who had used rescue medications, the mean AUC value of the trial group was 13.72±10.37, with a maximum value of 39.79 and a minimum value of 1.00. The mean AUC value of the control group was 23.14±12.94, with a maximum value of 47.54 and a minimum value of 0.33. The intergroup difference was statistically significant (P=0.0045). Analysis of covariance revealed that the difference between the groups was statistically significant (FAS: P=0.0003; PPS: P=0.0003). The adjusted mean differences in the FAS and PPS of the trial and control groups were both −11.45 (95% CI −17.0 to −5.49), and the difference between the groups was statistically significant (P<0.05).
The adjusted mean AUC in the FAS and PPS of the trial group was 9.36 for both sets (95% CI 4.69 to 14.04). The adjusted mean AUC in the FAS and PPS of the control group for both sets was 20.81 (95% CI 16.04 to 25.58).
Mucoadhesiveness in the trial group within 6 hours after a single use of the study device (CAM2028) was evaluated. Regarding mucoadhesiveness after 5 minutes of a single use, 29 patients (96.67%) reported that the oral liquid adhered easily, and one patient (3.33%) reported some difficulties in adhesion. All 30 patients (100.00%) reported that application of the oral liquid was acceptable. The time from the use of the oral liquid to film formation was <1 minute in three patients (10.00%), 1–5 minutes in 24 patients (80.00%), and >5 minutes in three patients (10.00%).
At 5 and 30 minutes and at 1, 2, 4, and 6 hours after a single use of the study device, most patients reported the taste as pleasant or tasteless, without irritation, and the device was comfortable or relatively comfortable to use (Table 3).
Six hours after a single use of the study device, 28 patients (93.33%) were willing to accept a repeated treatment, and two patients (6.67%) refused to accept a repeated treatment. Specifically, 93.33% of the patients were willing to repeat the treatment using this product.
Adverse events
In this study, 18 cases of medical device adverse events occurred in 14 patients in the trial and control groups. Six cases of medical device adverse events occurred in five patients in the trial group (the incidence rate was 16.67%), and 12 cases of medical device adverse events occurred in nine patients in the control group (the incidence rate was 30.0%). The difference in incidence rates between the groups was not statistically significant (P=0.3604) (Table 4).
  | Table 4 Cases and frequency of medical device adverse events |
There were seven cases of medical device adverse events, which occurred in six patients, that were considered to be related to the study device, including three cases of medical device adverse events in three patients (10.00%) in the trial group and four cases of medical device adverse events in three patients (10.00%) in the control group. The difference in incidence rates between the groups was not statistically significant (P=1.0000). The device-related adverse events that occurred in the trial group included elevated C-reactive protein (one case), abdominal discomfort (one case), and gastrointestinal irritation (one case); device-related adverse events in the control group included elevated C-reactive protein (two cases), nausea (one case), and retching (one case). These were all mild events.
There were no medical device adverse events or serious adverse events that led to discontinuation during the entire trial. In addition, this study did not identify any defects in the device that could lead to serious adverse events.
Discussion
The purpose of this study was to compare the analgesic effects of CAM2028 and KS on pain associated with OM. Both devices in the study could relieve pain rapidly and effectively 5 minutes after use. The AUC of the trial group was significantly smaller than that of the control group. The difference between the two groups was statistically significant, indicating that the local analgesic effect at 0–6 hours was significantly better in the trial group than in the control group. There was no significant difference between the two groups in the use of rescue medications. To exclude the impact of rescue medications, the AUC values after excluding the patients who received rescue medications were calculated. The results showed that the AUC of the trial group remained significantly smaller than that of the control group.
Pain in the trial group was rapidly relieved after treatment, and relief was maintained for up to 6 hours post-treatment. In contrast, pain in the control group was relieved and relief was maintained for 2 hours post-treatment, but pain subsequently returned. Compared with the baseline values, the reductions in pain scores at 2 and 4 hours post-treatment were more significant in the trial group than in the control group, and the differences between the groups were statistically significant at both time points. The reduction in the oral pain score at 6 hours post-treatment was compared with the baseline. After adjustment for the analysis of covariance, the difference between the two groups was statistically significant, and the difference among centers showed a consistent trend. These results indicated that the analgesic effects lasted longer in the trial group.
The device-related adverse events in this study were all mild events, and symptoms resolved spontaneously without treatment. There was no significant difference in the incidence rates of adverse events between the two groups. This study did not identify any defects in the device that could lead to serious adverse events. These results showed that both CAM2028 and KS had a good safety profile. However, the incidence rates of adverse events in the two groups were 16.67% and 30.0%, respectively. The high incidence rates may be due to the small number of patients enrolled. Future studies should include a larger sample size and continue to monitor adverse events. During the study, a total of seven patients had elevated C-reactive protein, and three of these events (one in the trial group and two in the control group) were considered to be device related as assessed by the investigator. Both devices in the present study were used for local treatment, and no chemical reactions occurred during the formation of the protective films. The mechanical film surface alleviated mucosal swelling and inflammation, including exposure of nerve endings, which reduced pain and discomfort. As there was no active substance entering the blood, the device should not be associated with elevated C-reactive protein. The potential reason for the elevated C-reactive protein may be related to local pain and inflammatory stimuli.
Within 6 hours of CAM2028 treatment, most patients reported that the oral liquid adhered easily and the application was acceptable. The film-formation time was generally 1–5 minutes. The taste was pleasant (tasteless), and it was non-irritating. Most patients reported that CAM2028 was (relatively) comfortable to use and were willing to repeat treatment.
OM occurs not only during conventional radiotherapy and chemotherapy,1,4–6 but novel targeted therapies are also associated with treatment-related OM, with the highest incidence occurring during treatment with everolimus (78%),7 sunitinib (37%),8 and trastuzumab (24%).9 Pain is one of the most serious symptoms of OM and has a significant impact on patients’ compliance with treatment and quality of life. There are currently limited approaches to treat OM-related pain effectively. In 2014, the Multinational Association of Supportive Care in Cancer and the International Society of Oral Oncology (MASCC/ISOO) published clinical practice guidelines for the management of mucositis secondary to cancer therapy. The guidelines recommend morphine, transdermal fentanyl, morphine mouth rinse, and doxepin mouth rinse for the management of OM pain.2 In addition, medical devices approved by the US Food and Drug Administration for the treatment of OM include GelClair®, Episil®, Mucotrol®, Caphosol®, and MuGard®. In 2015, Episil and MuGard were recommended by UK Oral Mucositis in Cancer Care (UKOMiC) to treat OM of all grades and to relieve the associated pain. There is currently no relevant diagnosis and treatment consensus or guidelines in China.
MuGard is an oral mucoadhesive protective agent that forms a protective layer of hydrogel over the oral mucosal surface. A study10 has confirmed that it can alleviate pain symptoms of OM and delay the progression of OM. However, the drug has not yet been approved in China. The KS approved in China is a product similar to MuGard. Its main components are carbomer homopolymer type A, glycerine, benzyl alcohol, sodium saccharin, phosphoric acid, citric acid, potassium hydroxide, and polysorbate 60. Therefore, the oral rinse was chosen as the control treatment in this study.
As an approved medical device, CAM2028 is a lipid-based, preservative-free liquid that does not contain pharmaceutically active ingredients. It is composed of six inactive ingredients (soy phosphatidylcholine, glycerol diolein, ethanol, propylene glycol, polysorbate 80, and peppermint oil) and is harmless to swallow. Using Camurus’ proprietary FluidCrystal® technology, the lipid component of the oral liquid forms a protective film in a physically self-assembled manner upon contact with a small amount of water-soluble liquid in the mouth (ie, saliva). The film adheres firmly to and covers the injured oral mucosa.11 Importantly, no chemical reaction occurs during the formation of the protective film. The mechanical film surface formed can alleviate mucosal swelling and inflammation, including exposure of nerve endings, which reduces pain and discomfort. As a result of this technology, the oral liquid displays strong adhesion on the surface of biological tissues with a long residence time, which prolongs its analgesic effects.
A randomized multicenter, double-blind, crossover study3 that included 38 patients with head and neck cancer who received radiotherapy and developed WHO grade 2–3 OM showed that CAM2028 rapidly relieved pain 5 minutes after treatment, and the analgesic effect lasted for up to 8 hours. The incidence rate of adverse events was 10.5%; not all adverse events were related to the oral liquid. Between January 2012 and March 2013, a post-marketing observational study was conducted in patients with OM in Germany.12 The study enrolled a total of 146 patients, 85% of whom had grade 2–3 OM. The results showed that CAM2028 significantly relieved pain during treatment. The effect was observed after the first application, and the efficacy was even more pronounced after 5 days of treatment. The median time to the onset of effect was 5 minutes. The maximum effect was observed after a median of 10 minutes. The median duration of the effect was 4 hours, with approximately 85% of patients reporting an improvement in their quality of life.
A limitation of this trial is the lack of sample size calculation. Future clinical research should include a better sample size assumption, accumulate more data, and carry out prospective analyses. At the same time, stratified analysis should be conducted according to tumor type, and chemotherapy and radiotherapy regimens.
Conclusion
In summary, both CAM2028 and KS could quickly relieve the pain associated with OM. Both devices showed a good safety profile and caused few adverse events. However, the local analgesic effect of CAM2028 within 6 hours post-treatment was significantly better than that of KS. In addition, the analgesic effect of CAM2028 lasted for 6 hours after treatment, which was longer than that of KS. Furthermore, CAM2028 adhered easily and the film formed rapidly. It was also non-irritating and was comfortable to use. The patients were willing to repeat the treatment. Therefore, CAM2028 achieved satisfactory therapeutic effects in cancer patients who developed OM pain after receiving radiotherapy and chemotherapy. CAM2028 provided a novel means by which to relieve the pain caused by chemotherapy and radiotherapy, as well as to improve quality of life and treatment compliance in clinical settings.
Data sharing statement
The authors agree to share all of the individual participant data collected during the trial, after deidentification. The study protocol, statistical analysis plan, informed consent form, clinical study report, and analytic code can also be shared. These will be available immediately following publication, with no end date. Anyone who wishes to access the data for any purpose should directly email Yuan Cheng: [email protected].
Disclosure
The authors report no conflicts of interest in this work.
References
Fang W. Combined Chemoradiotherapy for Locally Advanced Nasopharyngeal Carcinoma: A Multicenter, Open, Randomized Controlled Trial [dissertation]. Nanning: Guangxi Medical University; 2014. | ||
Lalla RV, Bowen J, Barasch A, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120(10):1453–1461. | ||
Hadjieva T, Cavallin-Ståhl E, Linden M, Tiberg F. Treatment of oral mucositis pain following radiation therapy for head-and-neck cancer using a bioadhesive barrier-forming lipid solution. Support Care Cancer. 2014;22(6):1557–1562. | ||
Fernandes LL, Torres SR, Garnica M, et al. Oral status of patients submitted to autologous hematopoietic stem cell transplantation. Support Care Cancer. 2014;22(1):15–21. | ||
Nishimura N, Nakano K, Ueda K, et al. Prospective evaluation of incidence and severity of oral mucositis induced by conventional chemotherapy in solid tumors and malignant lymphomas. Support Care Cancer. 2012;20(9):2053–2059. | ||
Nonzee NJ, Dandade NA, Patel U, et al. Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis: results from a Northwestern University Costs of Cancer Program pilot study with head and neck and nonsmall cell lung cancer patients who received care at a county hospital, a Veterans Administration hospital, or a comprehensive cancer care center. Cancer. 2008;113(6):1446–1452. | ||
Afinitor [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015. | ||
Sternberg CN, Calabrò F, Bracarda S, et al. Safety and efficacy of sunitinib in patients from Italy with metastatic renal cell carcinoma: final results from an expanded-access trial. Oncology. 2015;88(5):273–280. | ||
Herceptin [package insert]. South San Francisco, CA: Genentech; 2015. | ||
Allison RR, Ambrad AA, Arshoun Y, et al. Multi-institutional, randomized, double-blind, placebo-controlled trial to assess the efficacy of a mucoadhesive hydrogel (MuGard) in mitigating oral mucositis symptoms in patients being treated with chemoradiation therapy for cancers of the head and neck. Cancer. 2014;120(9):1433–1440. | ||
Barauskas J, Christerson L, Wadsäter M, Lindström F, Lindqvist AK, Tiberg F. Bioadhesive lipid compositions: self-assembly structures, functionality, and medical applications. Mol Pharm. 2014;11(3):895–903. | ||
Seidenspinner I, Adamietz IA, Strohm GL, Tiberg F. Effects of episil® oral liquid in cancer patients with oral mucositis: an observational study. Poster presented at the MASCC/ISOO Annual Meeting on Supportive Care in Cancer. June 25–27, 2015; Copenhagen, Denmark. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.