Back to Journals » OncoTargets and Therapy » Volume 14
Lobaplatin Enhances Radioactive 125I Seed-Induced Apoptosis and Anti-Proliferative Effect in Non-Small Cell Lung Cancer by Suppressing the AKT/mTOR Pathway
Received 21 October 2020
Accepted for publication 25 December 2020
Published 12 January 2021 Volume 2021:14 Pages 289—300
DOI https://doi.org/10.2147/OTT.S288012
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Nicola Silvestris
Jia-hui Rong,1 Dong Li,2 Yu-liang Li2
1Department of Cardiology, The Second Hospital of Shandong University, Jinan, People’s Republic of China; 2Department of Interventional Medicine, The Second Hospital of Shandong University, Jinan, People’s Republic of China
Correspondence: Dong Li; Yu-liang Li
Department of Interventional Medicine, The Second Hospital of Shandong University, 247 Beiyuan Street, Jinan 250000, People’s Republic of China
Email [email protected]; [email protected]
Introduction: In recent years, radioactive 125I seed implantation combined with chemotherapy has been regarded as a safe and effective treatment for advanced non-small cell lung cancer (NSCLC). However, the mechanism underlying this success is still unclear.
Methods: In this study, we investigated the apoptosis and anti-proliferative effect induced by 125I in A549, H1975, and H157 cells and determined whether a sensitizing concentration of lobaplatin (LBP) could enhance these effects. We performed in vitro experiments on A549, H1975, and H157 cells; we investigated the effects of 125I or lobaplatin (LBP) alone, or in combination, on cellular apoptosis and proliferation by performing flow cytometry, Bax/Bcl2 ratio, TUNEL, cell viability assay, cell cycle, and EdU. To further verify our findings, a subcutaneous tumor mouse model was established. Moreover, AKT/mTOR pathway was detected to determine whether this pathway was involved in the anti-cancer effect of 125I and LBP by up-regulating or down-regulating the expression of mTOR.
Results: Based on our results, the sensitizing concentration of LBP could enhance the 125I-induced apoptosis and anti-proliferation effect. Furthermore, the subcutaneous tumor mouse model obtained the consistent results. More importantly, the AKT/mTOR pathway was down-regulated after the treatment of 125I and LBP, and the anti-cancer effect of 125I and LBP could be compromised by up-regulating the mTOR expression.
Conclusion: Our study proved that LBP promotes the apoptotic and anti-proliferative effects of 125I in NSCLC cells by inhibiting the AKT/mTOR pathway and provides a foundation for future studies and enhanced combinatorial approaches for NSCLC in the clinical setting.
Keywords: radioactive 125I seed, lobaplatin, non-small cell lung cancer, apoptosis, proliferation, AKT/mTOR pathway
Introduction
Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers; it is one of the most commonly diagnosed cancers and a leading cause of cancer-related deaths worldwide.1,2 Although early diagnosis and treatment are effective, the majority of patients with NSCLC remain inoperable due to different metastases.3 Since the 1980s, radioactive 125I seed implantation has been regarded as an alternative treatment for NSCLC;4 it is considered a safe and effective approach for advanced NSCLC.5,6 Besides, combining 125I with chemotherapy can significantly improve the clinical efficacy and extend the overall survival of patients with NSCLC.7 Notably, 125I was reported to suppress tumor growth via Warburg effect inhibition in a dose-dependent manner in A549 xenografts.8 However, the mechanism by which 125I radiotherapy suppresses NSCLC cells in vitro and in vivo is unclear.
Lobaplatin (LBP), a third-generation platinum drug, was reported to induce cellular apoptosis and cell cycle arrest in various NSCLC cells in vitro.9 However, which phase of the cell cycle was blocked remains debatable; Zhang et al reported that LBP may induce G1 phase cell cycle arrest in A549 cells,9 whereas Xie et al revealed that an increase in S phase arrest, from 25.0% to 54.4%, was induced by LBP in a dose-dependent manner in A549 cells.3 Thus, further studies ought to be carried out to verify these controversial results. In addition, drug resistance remains an important issue that affects LBP clinical efficacy. Paclitaxel, a cytoskeletal antitumor drug, was reported to increase the sensitivity of lung cancer cell line NCI-H446 to LBP via downregulating the PI3K/AKT pathway.10 Therefore, identifying whether the AKT/mTOR pathway is involved in the regulation of LBP drug resistance would be beneficial for further clinical application of LBP.
The AKT/mTOR pathway is associated with cell proliferation, cell cycle progression, apoptosis, necrosis, and drug resistance;11–13 AKT regulates different biological processes such as cell survival, cell proliferation, and invasion.14 Continuously activated AKT can phosphorylate mammalian target of rapamycin complex 1 (mTORC1), which is an important regulator in gene expression and translation.15 In addition, phosphorylated AKT (p-AKT) promotes radiation resistance and positively relates to recurrence in cervical cancer.16 The AKT/mTOR pathway plays an essential role in various malignant tumors, including lung cancer, breast cancer, nasopharyngeal carcinoma, and bladder cancer.17–19 However, the role of AKT/mTOR signaling, especially p-AKT, in 125I irradiation and LBP treatment in NSCLC is rarely reported.
In this study, we adopted both in vivo and in vitro approaches to illustrate the potential of LBP in promoting 125I-induced apoptosis and proliferation inhibition in A549, H1975 and H157 cells. Besides, we also verified that 125I significantly downregulated the AKT/mTOR pathway, which was further facilitated by LBP. These findings may lay a foundation for future combinatorial approaches based on LBP and 125I in the clinical setting.
Materials and Methods
Mouse Subcutaneous Tumor Model Establishment
The mouse tumor model was established following the method we previously described.20 A549 cells (1×107/mL) were diluted in 0.9% saline solution and injected subcutaneously in the hind leg of BALB/c male nude mice (n=16, 4‒6 weeks) purchased from the Animal Research Center of Shandong University (Jinan, Shandong, China). Tumor diameter was measured every other day. According to the NIH Guide for the Care and Use of Laboratory, all mice were housed under specific pathogen-free conditions and the animal experiment was approved by the Shandong University Animal Care Committee.
The humane endpoint established in this study is that the diameter of the tumor is greater than 20mm and the mice were sacrificed by cervical dislocation.
Radioactive 125I Seed Implantation in Xenograft Mice
125I radioactive seeds were provided by Ningbo Junan Pharmaceutical Technology Company (Ningbo, Zhejiang, China). According to our previously described method, when the volume of the tumor reached 400mm2, 125I seeds were implanted into the mice under local anesthesia (lidocaine, 5mg/kg);20 tumors were punctured using a 18G needle (Kakko, Japan) under direct vision and the injections were made in the middle of the tumors. During 27-day 125I radioactive seed treatment, a few mice developed tumor ulceration, but no bleeding. For these mice, medical iodine volts can be used for disinfection.
Cell Culture and Transfection
The A549, H1975, and H157 cell lines were purchased from the Zhong Qiao Xin Zhou Biotechnology Company (Shanghai, China). The cells were cultured in Dulbecco’s modified Eagle’s medium (Corning, Inc., Corning, NY, USA) supplemented with 10% fetal calf serum (Gibco, Waltham, MA USA) and incubated in 5% CO2 at 37°C. Transfections of short interfering RNA (NT-siRNA/mTOR-siRNA) and plasmid (pcDNA/pcmTOR) were performed using lipofectamine 2000 (Invitrogen, CA, USA). The siRNA and plasmid were purchased from RiboBio (Guangzhou, Guangdong, China).
Sensitizing Concentration of LBP on NSCLC Cells
Based on the pilot experiment, the A549, H1975, and H157 cells were treated with different concentrations of LBP, ranging from 0 to 80 µg/mL, for 72 h. Cell proliferation was detected by the CCK-8 assay (Dojindo, Kumamoto, Japan) and the half-maximal inhibitory concentration (IC50) was calculated using Prism 7.0 software (GraphPad, San Diego, CA, USA). According to the previously published method, 10% of the IC50 was considered as a sensitizing concentration.20
Cell Viability Assay
After being treated with a sensitizing concentration of LBP (10% of IC50, 0.7867 µg/mL for A549 cells, 0.2119 µg/mL for H1975 cells, and 0.2907 µg/mL for H157 cells), 125I, or their combination, the A549, H1975, and H157 cells were seeded in 96-well plates at a density of 3×103 cells/well. After cell adherence, the CCK-8 reagent was added and incubated for 3 h to detect the optical density at 24, 48, and 72 h; measurements were performed using a microplate reader (Thermo, Waltham, MA, USA).
Flow Cytometry
Cells were seeded in 35 mm-dishes at 1×105 cells/dish and treated with sensitizing concentration of LBP, 125I, or a combination of both. The cell cycle assay was performed per the manufacture’s protocol (BD Biosciences, Franklin Lakes, NJ, USA). As for the apoptosis analysis, cells were stained with PI and Annexin V-FITC (BD Biosciences). Both the cell cycle and apoptosis assays were performed using a flow cytometer (BD Biosciences).
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
The TUNEL assay was performed as recommended by the manufacturer (Beyotime Biotechnology, Nanjing, Jiangsu, China). Fluorescence microscopy (Olympus, Tokyo, Japan) was used to observe the apoptotic cells and obtain images (at three different fields).
5-Ethynyl-2ʹ-Deoxyuridine (EdU) Staining
According to the manufacturer’s instructions, the Cell-Light EdU Apollo 567 in vitro kit (RiboBio, Guangdong, China) was used to examine cell proliferation. After the A549 cells were treated as indicated, the cell medium was replaced with 50 μM of EdU solution, diluted with DMEM, and incubated for 2 h. Fluorescence microscopy (Olympus, Tokyo, Japan) was used to visualize the cells and acquire images (at three different fields).
Western Blotting, Antibodies, and Other Reagents
The procedure was performed based on our previously reported method.21 The primary antibodies anti-Bax (ab32503), anti-Bcl-2 (ab692), anti-p-mTOR (ab109268), anti-mTOR (ab32028), anti-p-AKT (ab8805), and anti-AKT (ab105731) were purchased from Abcam (Cambridge, UK). The secondary antibodies goat anti-rabbit IgG (H&L) and goat anti-mouse IgG (H&L) were purchased from GenScript (Piscataway, NJ, USA). LBP was obtained from Hainan Changan International Pharmaceutical Co. (Haikou, Hainan, China).
Statistical Analysis
Data are presented as the mean ± SD of at least three experiments. The one-way ANOVA with Tukey’s multiple comparison were used to determine statistical significance; variations having a p < 0.05 were considered significant.
Results
LBP Enhanced 125I-Induced Apoptosis in NCSLC Cells
To investigate the effect of a sensitizing concentration of LBP, defined as 10% of the IC50, on 125I, we initially tested a range of LBP concentrations on NSCLC cells. Using the CCK-8 assay, we determined that the viability of the A549, H1975 and H157 cell lines was influenced by LBP in a dose-dependent manner; the IC50 values of LBP for A549, H1975, and H157 cells after a 72 h-treatment were 7.867 µg/mL, 2.119 µg/mL, and 2.907 µg/mL, respectively (Figure 1A).
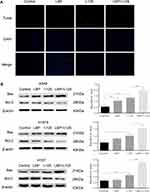
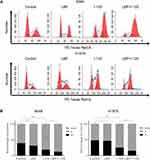
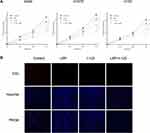
Moreover, flow cytometry analysis demonstrated that cellular apoptosis was significantly increased when NSCLC cells were concurrently treated with a sensitization concentration LBP and 125I compared with that in cells treated with either treatment alone (Figure 1B and C). The TUNEL assay results further confirmed that the combined treatment with a sensitization concentration of LBP and 125I could induce a more obvious cellular apoptosis than did either treatment alone in A549 cells (Figure 2A). Consistently, the Bax/Bcl-2 ratio of combined treatment was higher than that of single treatment in A549, H1975, and H157 cells (Figure 2B). Taken together, our results show that LBP enhanced 125I-induced apoptosis in NSCLC cells.
LBP Increased 125I-Induced Anti-Proliferative Effect in NSCLC Cells
The cell cycle analysis showed that G2/M cell cycle arrest was detected in all three treatment groups; however, this arrest was more intense after the combined treatment (Figure 3A and B). Moreover, the results of the CCK-8 assay revealed that although the proliferation of A549, H1975, and H157 cells was suppressed by single treatment with either 125I or LBP, their combination could induce a more significant cell growth inhibition (Figure 4A). Consistently, the EdU assay in A549 cells further supported the previous experiments and showed that fewer cells were considered to have a proliferative status in the combined treatment group (Figure 4B). Overall, the obtained results indicate that LBP increased 125I-induced anti-proliferative effect in NSCLC cells.
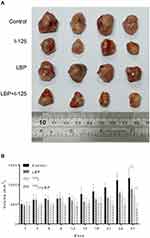
LBP Facilitated 125I-Induced Tumor Suppression in vivo
To investigate whether LBP could promote 125I-induced tumor suppression in vivo, an A549 xenograft nude mouse model was used. As shown in Figure 5A, the combination of 125I and LBP restrained tumor growth more effectively than did single 125I treatment. The tumor burden results are shown in Supplementary Figure S1. In addition, the tumor volume was more significantly decreased in the combined treatment group than in the 125I single treatment group (Figure 5B). Taken together, our data suggest that LBP facilitated 125I-induced tumor growth inhibition in vivo.
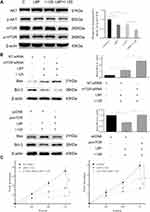
LBP Increased the 125I-Induced Down-Regulation of the AKT/mTOR Pathway
In our previous iTraq study, we found that the AKT/mTOR pathway was significantly down-regulated in HepG2, a hepatocellular carcinoma cell line, after treatment with 125I (Supplementary Figure S2). Based on our findings, we hypothesized that 125I could also downregulate the AKT/mTOR pathway in the A549 cell line. As shown in Figure 6A, to verify the potential mechanism by which LBP enhanced 125I-induced apoptosis and proliferation inhibition in A549 cells, AKT/mTOR pathway expression levels were detected after combined treatment or single treatment. The results demonstrated that combined treatment induced a more significant downregulation of the AKT/mTOR pathway than did single treatment. To further investigate whether AKT/mTOR pathway is involved in the anti-cancer of 125I and LBP, the Bax/Bcl2 ratio and cell viability in A549 cells were detected after the mTOR expression was up-regulated or down-regulated. The results showed that the down-regulation of mTOR could enhance the ant-cancer effect, while the up-regulation of mTOR could compromise the effect (Figure 6B and C). The original uncut Western blot bands are shown in Supplementary Figure S3–4.
Discussion
In recent years, radioactive 125I seed implantation has increasingly been regarded as an alternative and efficient treatment for advanced NSCLC.7 Besides, combining 125I with chemotherapy can significantly improve the clinical efficacy and extend the overall survival of NSCLC patients.6 However, the underlying mechanism of 125I brachytherapy in tumors is still unclear. Our previous research indicated that 125I induced apoptosis and proliferation inhibition in HCC by upregulating the PERK-eIF2α-ATF4-CHOP pathway.20 Based on our previous iTraq results, we found that the function of 125I is related to the AKT/mTOR pathway. In this study, we explored whether LBP could sensitize A549 cells to 125I radiation and determined the potential links among 125I, LBP, and the AKT/mTOR pathway. As indicated by our results, LBP could enhance 125I-induced apoptosis and anti-proliferative effect in A549 cells through the AKT/mTOR pathway.
LBP has been reported as the first-line treatment for NSCLC with minimal side effects and high specificity.9,20,22 Moreover, combining LBP with radiotherapy resulted in a better clinical efficacy than did single treatment alone.7 However, drug resistance is an important factor that affects LBP clinical efficacy. Thus, many studies have aimed at improving the sensitivity of LBP; paclitaxel, an antitumor drug that targets tubulin, was reported to increase the sensitivity of the lung cancer cell line NCI-H446 to LBP via the PI3K/AKT pathway.10 Furthermore, LBP could arrest the cell cycle at the G1 phase and induce apoptosis in A549 cells via the p53/ROS/p38MAPK signaling pathway.9 In contrast, another study demonstrated that LBP induce cell cycle arrest at G2/M phase.23 In our study, we also verified that LBP could induce apoptosis and inhibit the proliferation of A549 cells. And we found that LBP induced cell cycle arrest at the G2/M phase; we believe that these opposing results may be attributed to the difference in LBP concentration, considering that we used a sensitizing concentration (10% of the IC50). Moreover, our study suggested that LBP could enhance 125I-induced apoptosis and proliferation inhibition in A549 cells, which verified that the combination of LBP chemotherapy and 125I radiotherapy leads to a better outcome than either treatment alone.
Since the 1980s, radioactive 125I seed implantation has been widely used as an alternative treatment for NSCLC;4 radioactive 125I seeds could locally control tumors by continuously releasing a low dose of radiation to surrounding lesions for a long period of time.6 However, the underlying mechanism of 125I in NSCLC is still obscure; 125I was reported to suppress tumor growth via Warburg effect inhibition in a dose-dependent manner in A549 xenografts.8 Although the Warburg effect may be related to mTOR, c-Myc, HIF-1α, and GLUT1 downregulation, the exact mechanism remains unclear. From our previous study, we saw that 125I could induce cellular apoptosis and cell cycle arrest in PANC-1 cells;21 moreover, another recent study on HCC demonstrated that LBP promoted 125I-induced apoptosis and anti-proliferative effect via the PERK-eIF2α-ATF4-CHOP pathway, which we identified by iTraq.20 In our present study, we verified that 125I could significantly inhibit tumor growth in vivo and decrease proliferation with G2/M cell cycle arrest in vitro; interestingly, these anticancer effects could be enhanced by LBP.
Several studies have suggested that the AKT/mTOR signaling pathway plays an essential role in cell apoptosis, cell proliferation, cell cycle progression, necrosis, and drug resistance. A recent study revealed that xeroderma pigmentosum complementation group C (XPC) inhibition could reverse cisplatin resistance in A549 cells via downregulating the AKT/mTOR pathway.24 In addition, the inhibition of mTOR by rapamycin increased curcumin-induced apoptosis and autophagy, thereby inhibiting cell proliferation.25 Furthermore, the sensitivity of LBP was notably enhanced by paclitaxel via inhibiting the PI3K/AKT pathway.10 Another study demonstrated that casticin could cause anti-cancer effect in several human cancer, such as breast cancer, gastric cancer, and myeloma, through AKT/mTOR pathway.26 Moreover, the inhibitor of mTOR pathway combined with standard chemotherapy illustrated a positive effect in prevent and treatment of oral cancer.27 As for the resistance of radiotherapy, a review had concluded that the high expression level of mTOR is involved in antioxidant-related radioresistance of solid tumor, which indicated that mTOR pathway may be a target to promote tumor sensitivity to radiation.28 In our previous study, through the results of iTraq and preliminary data from our previous experiments, we demonstrated that 125I treatment significantly downregulated mTOR-related pathways in the HepG2 cell line;20 thus, we suggested a relationship between the expression level of AKT/mTOR signaling and 125I irradiation. In this study, we showed that 125I treatment could indeed significantly downregulate AKT/mTOR expression.
There are several limitations in our study, which need to be addressed in future investigations. We initially hypothesized the existence of a link between 125I irradiation and AKT/mTOR signaling based on the results of iTraq on HepG2. Thus, it is only fair that iTraq should be performed for the A549 cell line to identify the expression level of AKT/mTOR pathway after 125I treatment. Although we demonstrated that the combined treatment of LBP and 125I could induce cell cycle arrest at G2/M phase, the analysis of cyclin and CDK was not performed.
In conclusion, our results demonstrated that LBP enhanced 125I-induced apoptosis and proliferation inhibition in NSCLC cells and that these effects were closely related to the downregulation of the AKT/mTOR signaling pathway. Besides, we believe that our study lays a foundation for follow-up research and might have great clinical implications in the future.
Data Sharing Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Ethics Approval
The study was approved by the Shandong University Animal Care Committee.
Acknowledgments
We thank Yuan-hao Wang for supporting the experiments.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (Grant number 61671276).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.2192
2. Reck M, Rabe KF, Longo DL. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377(9):849–861. doi:10.1056/NEJMra1703413
3. Xie CY, Xu YP, Jin W, Lou LG. Antitumor activity of lobaplatin alone or in combination with antitubulin agents in non-small-cell lung cancer. Anticancer Drugs. 2012;23(7):698–705. doi:10.1097/CAD.0b013e328352cc10
4. Heelan RT, Hilaris BS, Anderson LL, et al. Lung tumors: percutaneous implantation of I-125 sources with CT treatment planning. Radiology. 1987;164(3):735–740. doi:10.1148/radiology.164.3.3615870
5. Jiang AG, Lu HY, Ding ZQ. Implantation of (125)I radioactive seeds via c-TBNA combined with chemotherapy in an advanced non-small-cell lung carcinoma patient. BMC Pulm Med. 2019;19(1):205. doi:10.1186/s12890-019-0974-8
6. Zhang W, Li J, Li R, Zhang Y, Han M, Ma W. Efficacy and safety of iodine-125 radioactive seeds brachytherapy for advanced non-small cell lung cancer-A meta-analysis. Brachytherapy. 2018;17(2):439–448. doi:10.1016/j.brachy.2017.11.015
7. Li J-R, Sun Y, Liu L. Radioactive seed implantation and lobaplatin chemotherapy are safe and effective in treating patients with advanced lung cancer. Asian Pac J Cancer Prev. 2015;16(9):4003–4006. doi:10.7314/apjcp.2015.16.9.4003
8. Zhang J, Zhu Y, Dong M, Yang J, Weng W, Teng L. Iodine-125 interstitial brachytherapy reduces tumor growth via Warburg effect inhibition in non-small cell lung cancer A549 xenografts. Oncol Lett. 2018;16(5):5969–5977. doi:10.3892/ol.2018.9346
9. Zhang H, Chen R, Wang X, Zhang H, Zhu X, Chen J. Lobaplatin-induced apoptosis requires p53-mediated p38MAPK activation through ROS generation in non-small-cell lung cancer. Front Oncol. 2019;9:538. doi:10.3389/fonc.2019.00538
10. Ma D, Li S, Cui Y, et al. Paclitaxel increases the sensitivity of lung cancer cells to obaplatin via PI3K/Akt pathway. Oncol Lett. 2018;15(5):6211–6216. doi:10.3892/ol.2018.8086
11. Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018;18(12):744–757. doi:10.1038/s41568-018-0074-8
12. Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10(6):594–601. doi:10.1038/nm1052
13. LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 2016;34(31):3803–3815. doi:10.1200/JCO.2014.59.0018
14. Schettino C, Bareschino MA, Sacco PC, et al. New molecular targets in the treatment of NSCLC. Curr Pharm Des. 2013;19(30):5333–5343. doi:10.2174/13816128113199990343
15. Yang Y, Luo J, Zhai X, et al. Prognostic value of phospho-Akt in patients with non-small cell lung carcinoma: a meta-analysis. Int J Cancer. 2014;135(6):1417–1424. doi:10.1002/ijc.28788
16. Kim TJ, Lee JW, Song SY, et al. Increased expression of pAKT is associated with radiation resistance in cervical cancer. Br J Cancer. 2006;94(11):1678–1682. doi:10.1038/sj.bjc.6603180
17. Li X, Shang D, Shen H, Song J, Hao G, Tian Y. ZSCAN16 promotes proliferation, migration and invasion of bladder cancer via regulating NF-kB, AKT, mTOR, P38 and other genes. Biomed Pharmacother. 2020;126:110066. doi:10.1016/j.biopha.2020.110066
18. Zhang W, Zhang Y, Xi S. Expression of Concern: upregulation of lncRNA HAGLROS enhances the development of nasopharyngeal carcinoma via modulating miR-100/ATG14 axis-mediated PI3K/AKT/mTOR signals. Artif Cells Nanomed Biotechnol. 2020;48(1):717. doi:10.1080/21691401.2020.1741909
19. Wang Y, Liu Y, Du X, Ma H, Yao J. Berberine reverses doxorubicin resistance by inhibiting autophagy through the PTEN/Akt/mTOR signaling pathway in breast cancer. Onco Targets Ther. 2020;13:1909–1919. doi:10.2147/OTT.S241632
20. Li D, Wang WJ, Wang YZ, Wang YB, Li YL. Lobaplatin promotes (125)I-induced apoptosis and inhibition of proliferation in hepatocellular carcinoma by upregulating PERK-eIF2alpha-ATF4-CHOP pathway. Cell Death Dis. 2019;10(10):744. doi:10.1038/s41419-019-1918-1
21. Li D, Jia YM, Cao PK, Wang W, Liu B, Li YL. Combined effect of (125)I and gemcitabine on PANC-1 cells: cellular apoptosis and cell cycle arrest. J Cancer Res Ther. 2018;14(7):1476–1481. doi:10.4103/jcrt.JCRT_43_18
22. Larsen JE, Minna JD. Molecular biology of lung cancer: clinical implications. Clin Chest Med. 2011;32(4):703–740. doi:10.1016/j.ccm.2011.08.003
23. Yang F, Yu Y, Lei Q, et al. Lobaplatin arrests cell cycle progression, induces apoptosis and impairs migration and invasion in B16-F10 melanoma cell line in vitro. Biomed Pharmacother. 2015;69:402–408. doi:10.1016/j.biopha.2014.12.011
24. Teng X, Fan XF, Li Q, et al. XPC inhibition rescues cisplatin resistance via the Akt/mTOR signaling pathway in A549/DDP lung adenocarcinoma cells. Oncol Rep. 2019;41(3):1875–1882. doi:10.3892/or.2019.6959
25. Liu F, Gao S, Yang Y, et al. Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol Rep. 2018;39(3):1523–1531. doi:10.3892/or.2018.6188
26. Lee JH, Kim C, Um JY, Sethi G, Ahn KS. Casticin-induced inhibition of cell growth and survival are mediated through the dual modulation of Akt/mTOR signaling cascade. Cancers (Basel). 2019;11(2). doi:10.3390/cancers11020254
27. Harsha C, Banik K, Ang HL, et al. Targeting AKT/mTOR in oral cancer: mechanisms and advances in clinical trials. Int J Mol Sci. 2020;21(9). doi:10.3390/ijms21093285
28. Woo Y, Lee HJ, Jung YM, Jung YJ. mTOR-mediated antioxidant activation in solid tumor radioresistance. J Oncol. 2019;2019:5956867. doi:10.1155/2019/5956867
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.