Back to Journals » Cancer Management and Research » Volume 12
lncRNA ZFAS1 Is Involved in the Proliferation, Invasion and Metastasis of Prostate Cancer Cells Through Competitively Binding to miR-135a-5p
Received 5 November 2019
Accepted for publication 7 January 2020
Published 13 February 2020 Volume 2020:12 Pages 1135—1149
DOI https://doi.org/10.2147/CMAR.S237439
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Jiaqiang Pan,1,* Xingyan Xu,2,* Guangliang Wang3
1Department of Urology, The Second Affiliated Hospital of Guilin Medical University, Guilin, Guangxi, People’s Republic of China; 2Department of Ophthalmology, The Second Affiliated Hospital of Guilin Medical University, Guilin, Guangxi, People’s Republic of China; 3Department of Histology and Embryology, Guilin Medical University, Guilin, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guangliang Wang
Department of Histology and Embryology, Guilin Medical University, No. 109, North Huancheng Second Road, Guilin, Guangxi Zhuang Autonomous Region 541004, People’s Republic of China
Tel + 86-773-5895162
Email [email protected]
Background: Prostate cancer (PCa) is a common malignant tumor in men. lncRNA ZFAS1 plays a carcinogenic role in many types of cancer; however, its potential role in PCa remains unclear. The current study aimed to determine the expression and function of ZFAS1 in PC.
Methods: The ZFAS1 expression in PC tissues and cells was determined by quantitative polymerase chain reaction (qPCR). SiZFAS1, miR-135a-5p mimic and miR-135a-5p inhibitor were transfected into PCa cells. The direct target of ZFAS1 was predicted by Starbase and verified by dual-luciferase reporter. Cell viability, proliferation, apoptosis, migration and invasion of the PCa cells were determined by cell counting kit-8, clone formation assay, flow cytometer, scratch and Transwell assay, respectively. The expression levels of related proteins and mRNAs were determined by Western blotting and qPCR.
Results: ZFAS1 expression was up-regulated in PCa cells and tissues. ZFAS1 could competitively bind to miR-135a-5p in PCa cells, and down-regulation of ZFAS1 inhibited cell viability, proliferation, migration, invasion of PCa cells and the occurrence of epithelial–mesenchymal transformation (EMT) and promoted apoptosis of PCa cells and increased the miR-135a-5p expression. Moreover, the function of miR-135a-5p mimic in PCa cells was consistent with ZFAS1 knockdown, while the function of miR-135a-5p inhibitor was opposite to that of miR-135a-5p mimic in PCa cells. The results showed that knocking down ZFAS1 could attenuate the effects of miR-135a-5p inhibitor on cell proliferation, invasion and EMT of PCa cells.
Conclusion: Knocking down ZFAS1 could inhibit the proliferation, invasion and metastasis of PCa cells through regulating miR-135a-5p expression.
Keywords: lncRNA ZFAS1, prostate cancer, miR-135a-5p, epithelial-mesenchymal transition
Introduction
Morbidity and mortality of prostate cancer (PCa) are increasing1; however, obstacles still remain in clinical diagnosis and treatment of PCa due to its unspecific early symptoms and complex pathogenesis.2–4 More importantly, many patients are at advanced PCa stage of development when diagnosed, thus resulting in poor prognosis for patients with the disease5,6; moreover, PCa patients also suffer from fractures, severe pain and other complications.7–9 Thus, early diagnosis and intervention of PCa could reduce suffering to patients and improve therapeutic effects. The discovery of molecular biology indicators specific to PCa and possible molecular biological mechanisms may improve the prevention and early diagnosis of PCa.10,11
Long non-coding RNAs (lncRNAs) are RNAs with structural characteristics of mRNAs in their cytoplasms or nuclei, but do not have protein-coding function.12,13 Recent studies showed that abnormally expressed lncRNAs contribute to the pathogenesis of a variety of diseases including cancers.14–16 Similarly, lncRNAs, such as SChLAP1,17 NEAT1,18 and HOTTIP,19 have been found to play critical roles in the development of PCa. Zinc finger antisense 1 (ZFAS1) is a member of lncRNA family associated with cancer,20 and lncRNA ZFAS1 is reported to play a tumorigenic role in stomach cancer,21 glioma,22 nasopharyngeal carcinoma23 and colorectal cancer.24 At present, the specific role of ZFAS1 in PCa is not clear, the role of ZFAS1 has been recognized. Thus, the current study investigated the role of ZFAS1 in the growth and metastasis of PCa cells, aiming to improve the current understanding and clinical diagnosis of PCa.
Materials and Methods
Patient Samples
Cancer tissues and paired adjacent tissues were obtained from PCa patients (n=30) who received surgical resection at the Second Affiliated Hospital of Guilin Medical University. All patients were diagnosed as having PCa by postoperative pathology diagnosis. The patients did not receive radiotherapy or chemotherapy prior to the surgery. The collected cancer tissues and paired adjacent tissues were immediately frozen in liquid nitrogen before use. The study was approved by the Ethics Committee of the Second Affiliated Hospital of Guilin Medical University, and written informed consent was obtained from the patients.
Cell Culture
Human normal prostate epithelial cell line RWPE-1 and PC cell lines, PC3, DU145, 22RV1 and LNCAP were purchased from American Type Culture Collection (ATCC, http://www.atcc.org/). The cell lines were cultured in RPMI-1640 medium (Gibco, USA) containing 10% fetal bovine serum (FBS, Gibco, USA) and 1% penicillin/streptomycin solution in a humidified incubator at 37°C with 5% CO2.
Transfection
miR-135a-5p mimic (M), miR-135a-5p inhibitor (I), miR-135a-5p mimic control (MC) and miR-135a-5p inhibitor control (IC) were synthesized by Ribobio (Guangzhou, Guangdong, China). Small interfering RNA (siRNA) against lncRNA ZFAS1 (siZFAS1) and negative control siRNA (siNC) was synthesized by GenePharma Co., Ltd. (China). To investigate the effects of mir-135a-5p and ZFAS1 on the proliferation and metastasis of PCa cells, the cells were divided into five groups, namely, MC group (PCa cells transfected with MC), M group (PCa cells transfected with M), IC group (PCa cells transfected with IC and siNC), I group (PCa cells transfected with I and siNC), and siZFAS1+ I group (PCa cells transfected with I and siZFAS1). Briefly, according to the manufacturer’s protocol of Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA, USA), OPTIM-MEM medium was used to dilute the miR-135a-5p products and Lipofectamine 2000 solution, respectively. Then, diluted Lipofectamine 2000 was mixed with diluted miR-135a-5p products to generate transfection reagent mixtures. The cells (at 5×104 cells/well) were inoculated into a 96-well plate and, respectively, transfected with M (50 nM), I (50 nM), MC (50 nM) and IC (50 nM), siZFAS1 (50 nM) and siNC (50 nM) on the next day. The transfection reagent mixture was then added into the cells, incubated together in an incubator at 37°C with 5% CO2 for 48 h and then collected. The sequences are listed in Table 1.
 |
Table 1 The Sequences Used for Transfection |
Cell Counting Kit-8 (CCK-8)
Cell viability was determined by CCK-8. The transfected cells were seeded into 96-well plates at 5×103 cells/well, and incubated for 24 h, 48 h and 72 h with 5% CO2 at 37°C. Next, CCK-8 reagent was added into the wells containing treated cells and cultured for another 2 h. The absorbance was measured at OD 450 nm using a microplate (Model 680, Bio-Rad, Hercules, CA, USA).
Clone Formation Assay
Briefly, transfected cells were seeded into 6-well plates, and cultured in medium containing 10% FBS for 14 days at 37°C with 5% CO2, and the medium was refreshed every 2–3 days. The cells were fixed by 4% methanol and stained by 0.1% crystal violet, and stained colonies were counted under an inverted microscope (Eclipse TS100, Nikon, Japan).
Cell Apoptosis
Transfected cells were harvested, cultured for 48 h, digested by trypsin, and then centrifuged (at 1000×g for 5 min) and precipitated at room temperature. After the supernatant was discarded, the cells were suspended in binding buffer, and co-stained by Annexin V-FITC and propidium iodide for 15 min according to the instructions of Annexin V-FITC Apoptosis Detection kit (KeyGEN, Shanghai, China). Next, cell apoptosis was detected by FACSCalibur flow cytometer of BD (BD Biosciences, USA).
Scratch Assay
Transfected cells were seeded at a density of 5×105 (cells/mL) into a 24-well plate, scratches were created using a sterilized pipette tip, and the floating cells were removed by phosphate-buffered saline (PBS). After incubation for 0 and 48 h at 37°C with 5% CO2 in an incubator, the images were captured under an inverted microscope (Eclipse TS100, Nikon, Japan), and migrated distance was calculated using ImageJ-1.38x software (National Institutes of Health, USA).
Transwell Assay
Transfected cells (5×103 cells/mL) suspended in proper free-serum RPMI-1640 medium were seeded into upper chambers (8 μm pore size, Millipore, Billerica, MA, USA) coated with Matrigel, while in the lower chambers, the medium containing 10% FBS served as a chemotaxis factor. After incubation for 48 h, unmigrated cells were removed using wet cotton swabs, while invaded cells were fixed by methanol and stained by 0.2% crystal violet and air-dried. Invaded cells were calculated under an inverted microscope (Eclipse TS100, Nikon, Japan).
Dual-Luciferase Reporter Assay
miRNAs targeted by ZFAS1 and their binding regions were predicted by StarBase (http://starbase.sysu.edu.cn/index.php). The luciferase reporter vectors containing the 3′-UTR sequences of ZFAS1, wild-type ZFAS1 3′-UTR (ZFAS1-WT) and the mutant 3′-UTR (ZFAS1-MUT) were synthesized by Shanghai GenePharma Co., Ltd., China). ZFAS1-WT and ZFAS1-MUT were inserted into pMIR-report miRNA plasmid (AM5795, ThermoFisher, Waltham, MA, USA) containing a firefly luciferase gene. pRL-TK (E2241, Promega, Fitchburg, WI, USA), which contains a renilla luciferase gene, served as an internal control. The cells were co-transfected with miR-135a-5p mimic control (20 nM), miR-135a-5p mimic (20 nM) and reporter plasmids ZFAS1-WT (50 ng) and ZFAS1-MUT (50 ng) using Lipofectamine 2000 Transfection Reagent (Invitrogen). Next, 48 h after the transfection, the cells were harvested for luciferase detection, and the luciferase activities were measured by dual-luciferase reporter assay system (Promega Corp) following the manufacturer’ instructions. The firefly luciferase activity was normalized against renilla luciferase activity.
Quantitative Polymerase Chain Reaction (qPCR)
Total RNAs from PC tissues and cells were extracted using Trizol reagent (Invitrogen). NanoDrop 2000 (Thermo Fisher Scientific, USA) was used to determine the purity and concentration of RNAs. CDNAs were obtained by reverse transcription from RNAs (2 μg) using a PrimeScript RT Master Mix kit (Takara, China). PCR reaction was performed in an Mx3005P QPCR System (Stratagene, USA) using SYBR miRNA detection assay (Takara, China). The reaction was set at 94°C for 5 min, at 94°C for 15 s, at 60°C for 60 s for 40 cycles. The expression level of each gene was calculated by the 2−ΔΔCT method.25 U6 and GAPDH served reference genes and the sequences of primers are listed in Table 2.
 |
Table 2 The Primers Used for qPCR |
Western Blot
Total proteins were extracted from the cells using RIPA buffer (Beyotime, China) and protein concentration was determined using a Bio-rad DC Assay kit (Hercules, USA). Equal amounts of protein (20 μg) were separated on 10% SDS-PAGE (Invitrogen) and electroblotted onto a PVDF membrane (Merck, Germany), which was then blocked by 5% non-fat milk for 1 h and further incubated with E-cadherin (E-Cad, Cat#14472, 1:1000, CST, USA), N-Cadherin (N-Cad, Cat #14215, 1:1000, CST, USA), Snail (ab53519, 1:1000, Abcam, Cambridge, MA, USA), and GAPDH (ab8245, 1:2000, Cambridge, MA, Abcam) overnight at 4°C. Secondary antibodies (donkey anti-goat IgG H&L (HRP) (ab205723, 1:2000, Cambridge, MA, Abcam) or goat anti-mouse IgG H&L (HRP) (ab205719, 1:2000, Cambridge, MA, Abcam)) were incubated with the membrane for 2 h. The membrane was developed by ECL chemiluminescence (Thermo Scientific, USA) and Band Scan 5.0 system (Bio-Rad). GAPDH served as an internal reference.
Statistical Analysis
The experiments were conducted in triplicate. The data were shown as mean ±standard deviation (SD), and the differences were evaluated by student’s tests or one-way ANOVA and processed by SPSS 20.0 system (SPSS Inc., USA). A P value less than 0.05 was considered as statistically significant.
Results
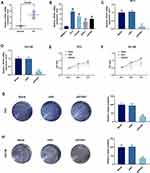
ZFAS1 Was Increased in PCa Tissues and Cell Lines
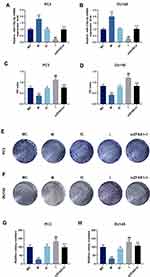
The results of qPCR showed increased expression of ZFAS1 in PC tissues (Figure 1A, P<0.05). Expression of ZFAS1 was also determined by qPCR in RWPE-1 cell line and four PC cell lines, compared with RWPE-1 cells, it was found that the expression of ZFAS1 in PC cell lines was greatly up-regulated (Figure 1B, P<0.05). In PC cell lines, ZFAS1 was high-expressed in PC3 and DU145 cells, therefore PC3 and DU145 cells were selected to be used in later experiments.
Proliferation, Migration, Invasion and Epithelial–Mesenchymal Transformation (EMT) of PCa Cells Were Inhibited by siZFAS1, While Apoptosis Was Increased
To study the biological role of ZFAS1 in PCa cells, the ZFAS1 siRNA was transfected into PC3 and DU145 cells, and the transfection efficiency of siZFAS1 was determined by qPCR. The data revealed that the ZFAS1 levels in PC3 (Figure 1C) and DU145 (Figure 1D) cells were reduced (P<0.05), suggesting that the ZFAS1 expression was successfully down-regulated in PC3 and DU145 cells.
Furthermore, functional experiments were performed to investigate the role of ZFAS1 in proliferation and invasion of PCa cells. CCK-8 analysis demonstrated that the cell viabilities of PC3 (Figure 1E) and DU145 (Figure 1F) transfected with siZFAS1 were lower than that of cells without siZFAS1 transfection (P<0.05). Moreover, compared with blank, the results of clone formation assay revealed that the colony numbers of PC3 (Figure 1G) and DU145 (Figure 1H) cells were significantly reduced after knocking down ZFAS1 (P<0.05).
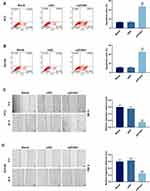
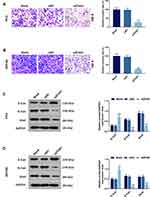
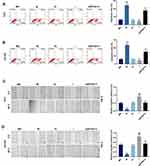
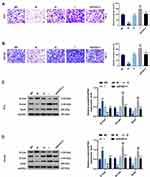
Subsequently, apoptosis was determined by flow cytometry to investigate whether ZFAS1 affects cell apoptosis, and we found that apoptosis rates of PC3 (Figure 2A) and DU145 (Figure 2B) cells were increased in siZFAS1 group as compared with blank group (P<0.05). Furthermore, the results from scratch assay and Transwell assay showed that knocking down ZFAS1 in PC3 and DU145 cells significantly shortened the migration distance (Figure 2C and D) and reduced invasion (Figure 3A and B) of PCa cells compared with blank group (P<0.05).
To determine the EMT transformation of PCa cancer cells, Western blotting was performed. The data demonstrated that compared with blank group, the E-cad level was increased significantly in PC3 (Figure 3C) and DU145 (Figure 3D) cells transfected with siZFAS1 (P<0.05), whereas the levels of N-Cad and Snail were reduced in the cells transfected with siZFAS1 (P<0.05).
The above results indicated that knocking down ZFAS1 expression could inhibit the proliferation, migration and invasion of PCa cells and EMT, whereas knocking down ZFAS1 expression promotes cell apoptosis.
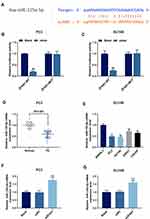
ZFAS1 Bound to miR-135a-5p in PC Cell Lines
The interaction of ZFAS1 with miR-135a-5p in nasopharyngeal carcinoma has been reported.23 As shown in Figure 4A, StarBase predicted that there were potential binding sites between ZFAS1 and miR-135a-5p, suggesting that miR-135a-5p may be a potential target of ZFAS1. Next, miR-135a-5p mimic, ZFAS1-WT and ZFAS1-MUT were, respectively, transfected into PC3 and DU145 cells. Dual-luciferase assay showed that the luciferase activity of ZFAS1-WT in PC3 (Figure 4B) and DU145 (Figure 4C) cells was significantly inhibited by miR-135a-5p mimic (P<0.05), while that of ZFAS1-MUT was not affected (P>0.05). In addition, the expression of miR-135a-5p in PCa tissues and cell lines were detected by qRT-PCR. The result showed that miR-135a-5p expression was lower in PCa tissues than that in normal tissues, and miR-135a-5p was low-expressed in PCa cell lines (Figure 4D and E, P<0.01). We also measured the miR-135a-5p expression in PCa cell lines after the transfection with siZFAS1, the results from qPCR showed that miR-135a-5p level was up-regulated in siZFAS1 group compared with blank group (Figure 4F and G, P<0.01). Thus, these data indicated that miR-135a-5p could specifically bind to ZFAS1.
SiZFAS1 May Regulate Progression of PCa Cells Through Mediating miR-135a-5p Expression
To better understand the regulatory relationship between ZFAS1 and miR-135a-5p in PCa cell lines, PC3 and DU145 cells were transfected with siZFAS1 and miR-135a-5p mimic/inhibitor, and qPCR was performed to detect the miR-135a-5p expression. As shown in Figure 5A and B, miR-135a-5p level was increased in PC3 and DU145 cells transfected with miR-135a-5p mimic (P<0.05), while the level of miR-135a-5p was reduced in cells transfected with miR-135a-5p inhibitor (P<0.05). Moreover, the level of miR-135a-5p was higher in cells transfected with siZFAS1 and miR-135a-5p inhibitor than that in I group (P<0.05). Therefore, these results suggested that miR-135a-5p expression has been successfully regulated in PCa cells.
The data from CCK-8 and clone formation assay showed that compared with the cells treated by miR-135a-5p mimic/inhibitor control, miR-135a-5p mimic inhibited cell viability (Figure 5C and D) and reduced colony numbers (Figure 5E–H) of PC3 and DU145 cells (P<0.05), while miR-135a-5p inhibitor increased the cell viability and colony numbers (P<0.05). However, silencing ZFAS1 reduced the viability and colony numbers of PC3 and DU145 cells previously increased by miR-135a-5p inhibitor (P<0.05).
Flow cytometry was performed to detect cell apoptosis, and we found that the apoptosis rates of PC3 (Figure 6A) and DU145 (Figure 6B) cells were increased by miR-135a-5p mimic but reduced by miR-135a-5p inhibitor (P<0.05). In the siZFAS1+I group, the apoptosis rates were higher than that in I group (P<0.05). Scratch assay and Transwell assay were carried out for the detection of migration and invasion, and migration distance (Figure 6C and D) and invasion rates (Figure 7A and B) were obviously reduced in the cells treated by miR-135a-5p mimic as compared with MC group (P<0.05). However, compared with IC group, the cell migration distance (Figure 6C and D) and invasion rates (Figure 7A and B) of the cells treated by miR-135a-5p inhibitor were up-regulated, while siZFAS1 partially reversed the effects of miR-135a-5p inhibitor on the migration and invasion (P<0.05).
Expressions of EMT-related marks were detected by Western blotting in PC3 and DU145 cells, and we found increased E-cad expression (Figure 7C and D) and decreased N-Cad (Figure 7C and D) and Snail (Figure 7C and D) expressions in the cells transfected with miR-135a-5p mimic (P<0.05). Moreover, the results showed that the E-cad level was down-regulated and the levels of N-Cad and Snail were up-regulated in I group compared with IC group (P<0.05), moreover, knocking down ZFAS1 expression significantly reversed the role of miR-135a-5p inhibitor in the expressions of EMT-related marks.
It could be concluded that miR-135a-5p mimic inhibited proliferation, invasion and EMT transformation of PCa cancer cells and promoted apoptosis, and the effects of miR-135a-5p mimic were similar to those of siZFAS1. Moreover, the effect of miR-135a-5p inhibitor on PCa cancer cells was the opposite from those of miR-135a-5p mimic. It was also predicted that miR-135a-5p was the target gene of ZFAS1, and siZFAS1 partially reversed the role of miR-135a-5p inhibitor in PCa cells.
Discussion
PCa is one of the most common malignant tumors in the male reproductive and urinary system.26 A recent study demonstrated that lncRNAs are also involved in the occurrence of PCa, and Zhu et al27 found that lncRNA H19 inhibits the metastasis of PCa through targeting miR-675 and TGFBI. Moreover, Shi et al suggested that lncRNA APP promotes PCa development through competitively binding to miR-218.28 A study has reported that ZFAS1 and GAS5 are the prognostic markers involved in translation targeted by miR-940 in PCa.29 In the current study, we detected ZFAS1 expression in clinical PCa samples, and found that the ZFAS1 expression level was increased in PCa tissues and its level was also increased in PCa cell lines, including in 22RV1, DU145, LNCAP and PC3, suggesting that ZFAS1 functioned in PCa.
Cell growth and apoptosis are important biological processes for tissues and cells to maintain stability and balance of internal environment.30,31 However, once the stability and balance are disrupted, the proliferation ability and growth rate of tumor cells will be greatly increased, leading to the occurrence of malignant tumors.32,33 For in vitro experiment, the present study successfully established the PC3 and DU145 cell lines with ZFAS1 knockdown using siRNA, and among the four PCa cell lines, ZFAS1 had the highest level in PC3 and DU145 cells. On such a basis, experiments on tumor biological phenotypes confirmed that the down-regulation of ZFAS1 had significant effects on DU145 and PC3 cell lines, as it inhibited the proliferation and invasion of PCa cells and promoted cell apoptosis. Such findings suggest that high expression of ZFAS1 in PCa cells may promote the growth of PCa cells.
Malignant progression and distant metastasis of tumor are a complex process during which multiple factors and biological changes are involved. Epithelial-mesenchymal transformation (EMT) is considered as a prerequisite for distant metastasis of tumor,34 and through EMT, cancer cells become more aggressive and gain greater metastasis ability.35–37 Previous studies suggested that ZFAS1 is involved in EMT and plays a critical role during distant metastasis of cancer.38,39 Here, we found that silencing of ZFAS1 up-regulated the E-cad level and down-regulated the levels of N-cad and Snail. E-cad is a marker related to epithelial cells, and its reduction and loss indicate changes in EMT. Meanwhile, Snail could suppress the E-cad level, and high expressions of Snail and N-cad are often predictive of the occurrence of EMT.40,41 This study further verified the effect of high-expressed ZFAS1 on promoting malignant progression and distant metastasis of PCa.
Though the specific mechanisms through which lncRNAs realize their functions in cells and tissues are different, it involves the interaction with their target proteins, DNA, and sometimes other lncRNAs.42 In addition, lncRNAs can act as cRNAs to function as miRNA sponges and inhibit the degradation effect of miRNAs on their target gene RNAs.43 In rheumatoid arthritis-fibroblast-like synoviocytes, research found that ZFAS1 could directly interact with miR-27a and reduce the expression of miR-27a, thereby regulating cell migration and invasion.44 ZFAS1 is considered as an oncogene in colorectal cancer occurrence, and it interacts with miR-484 as a sponge.24 Cui et al reported that ZFAS1 promoted the tumorigenesis of PCa by regulating c-Myc expression via a regulatory network of competing endogenous RNAs.45 To further explore the mechanism of ZFAS1 in PCa cells, we found that there were binding sites between ZFAS1 and miR-135a-5p, and ZFAS1 directly bound to miR-135a-5p and acted as sponge for miR-135a-5p in PCa cells to inhibit miR-135a-5p expression. The interaction of ZFAS1 with miR-135a-5p has already been shown in other studies, for example, Wang et al found that silencing ZFAS1 could inhibit proliferation, migration and invasion by sponging miR-135a in nasopharyngeal carcinoma cells;23 Cui et al also revealed that miR-27a/15a/16 are targeted by ZFAS1 in PCa. Moreover, our results are similar with those of Cui et al’s.45
miRNAs are a class of non-coding small RNAs with 17–25 nucleotides.46 miR-135a-5p is involved in a series of cell biological processes.47 Zhou et al48 showed that miR-135a-5p inhibits the proliferation of gallbladder cancer cells in vitro and in vivo. Muller et al49 demonstrated that miR-135a-5p is down-regulated in renal clear cell carcinoma tissue, and it acts as an inhibitory factor. In this study, the results showed that miR-135a-5p overexpression inhibited the PCa cell migration and invasion, promoted cell apoptosis and regulated the level of EMT-related markers, thereby inhibiting the occurrence of EMT; however, low-expressed miR-135a-5p in PCa cells showed completely opposite results. In addition, it was found that down-regulating ZFAS1 could partially reverse the increase of PCa cell invasion and metastasis induced by miR-135a-5p inhibitor, suggesting that ZFAS1 may promote malignant progression and distant metastasis of PCa cells by competitively binding to miR-135a-5p and inhibiting activity of PCa cells. Previous studies have shown that miR-135a-5p can regulate the proliferation and apoptosis of cancer cells through targeted mRNA, for example, miR-135a-5p could repress head and neck squamous cell carcinoma cell proliferation and enhance apoptosis by targeting HOXA10;50 miR-135a-5p could inhibit malignancy of glioblastoma by targeting syndecan binding protein.51 Thus, the mRNA targeted by miR-135a-5p in regulating the proliferation, invasion and migration of PCa cells remains to be determined.
Conclusions
In conclusion, we preliminarily found that ZFAS1 was high-expressed in PCa tissues and cells, and ZFAS1 directly bound to miR-135a-5p. Through overexpression of ZFAS1 in PCa cells, the results showed that miR-135a-5p expression was increased, while PCa cell proliferation, invasion and metastasis were suppressed, suggesting that the up-regulation of ZFAS1 could promote the proliferation and metastasis of PCa cells by competitively binding to miR-135a-5p, thereby contributing to the development of PCa.
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. No animals are involved in this research.
Data Sharing Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Author Contributions
Substantial contributions to conception and design: JP, XX. Data acquisition, data analysis and interpretation: JP, XX. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved: GW. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
2. Stuchbery R, Kurganovs NJ, McCoy PJ, et al. Target acquired: progress and promise of targeted therapeutics in the treatment of prostate cancer. Curr Cancer Drug Targets. 2015;15(5):394–405. doi:10.2174/1568009615666150416113453
3. Jones C, Fam MM, Davies BJ. Expanded criteria for active surveillance in prostate cancer: a review of the current data. Transl Androl Urol. 2018;7(2):221–227. doi:10.21037/tau
4. Thorstenson A, Garmo H, Adolfsson J, Bratt O. Cancer specific mortality in men diagnosed with prostate cancer before age 50 years: a nationwide population based study. J Urol. 2017;197(1):61–66. doi:10.1016/j.juro.2016.06.080
5. Feldman AS, Meyer CP, Sanchez A, et al. Morbidity and mortality of locally advanced prostate cancer: a population based analysis comparing radical prostatectomy versus external beam radiation. J Urol. 2017;198(5):1061–1068. doi:10.1016/j.juro.2017.05.073
6. Barry MJ, Simmons LH. Prevention of prostate cancer morbidity and mortality: primary prevention and early detection. Med Clin North Am. 2017;101(4):787–806. doi:10.1016/j.mcna.2017.03.009
7. Essink-Bot ML, Korfage IJ, De Koning HJ. Including the quality-of-life effects in the evaluation of prostate cancer screening: expert opinions revisited? BJU Int. 2003;92(Suppl 2):101–105. doi:10.1111/j.1464-410X.2003.04409.x
8. Kornblith AB, Herr HW, Ofman US, Scher HI, Holland JC. Quality of life of patients with prostate cancer and their spouses. The value of a data base in clinical care. Cancer. 1994;73(11):2791–2802. doi:10.1002/1097-0142(19940601)73:11<2791::AID-CNCR2820731123>3.0.CO;2-9
9. Yeku O, Slovin SF. Metabolism and pharmacokinetics of radium-223 in prostate cancer. Expert Opin Drug Metab Toxicol. 2015;11(5):843–849. doi:10.1517/17425255.2015.1021332
10. Abeshouse A, Ahn J, Akbani R, et al. The molecular taxonomy of primary prostate cancer. Cell. 2015;163(4):1011–1025. doi:10.1016/j.cell.2015.10.025
11. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi:10.1016/j.cell.2015.05.001
12. Hauptman N, Glavac D. Long non-coding RNA in cancer. Int J Mol Sci. 2013;14(3):4655–4669. doi:10.3390/ijms14034655
13. Zhang H, Chen Z, Wang X, Huang Z, He Z, Chen Y. Long non-coding RNA: a new player in cancer. J Hematol Oncol. 2013;6:37. doi:10.1186/1756-8722-6-37
14. Qi P, Zhou X-Y, Du X. Circulating long non-coding RNAs in cancer: current status and future perspectives. Mol Cancer. 2016;15(1):39. doi:10.1186/s12943-016-0524-4
15. Tano K, Akimitsu N. Long non-coding RNAs in cancer progression. Front Genet. 2012;3:219. doi:10.3389/fgene.2012.00219
16. Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21. doi:10.1038/nrg3606
17. Mehra R, Udager AM, Ahearn TU, et al. Overexpression of the long non-coding RNA SChLAP1 independently predicts lethal prostate cancer. Eur Urol. 2016;70(4):549–552. doi:10.1016/j.eururo.2015.12.003
18. Tian X, Zhang G, Zhao H, Li Y, Zhu C. Long non-coding RNA NEAT1 contributes to docetaxel resistance of prostate cancer through inducing RET expression by sponging miR-34a. RSC Adv. 2017;7:42986–42996. doi:10.1039/C7RA06107B
19. Zhang SR, Yang JK, Xie JK, Zhao LC. Long noncoding RNA HOTTIP contributes to the progression of prostate cancer by regulating HOXA13. Cell Mol Biol (Noisy-Le-Grand). 2016;62(3):84–88.
20. Li J, Li Z, Leng K, et al. ZEB1-AS1: a crucial cancer-related long non-coding RNA. Cell Prolif. 2018;51:1. doi:10.1111/cpr.2018.51.issue-1
21. Nie F, Yu X, Huang M, et al. Long noncoding RNA ZFAS1 promotes gastric cancer cells proliferation by epigenetically repressing KLF2 and NKD2 expression. Oncotarget. 2017;8(24):38227–38238. doi:10.18632/oncotarget.v8i24
22. Lv QL, Chen SH, Zhang X, et al. Upregulation of long noncoding RNA zinc finger antisense 1 enhances epithelial-mesenchymal transition in vitro and predicts poor prognosis in glioma. Tumour Biol. 2017;39(3):1010428317695022. doi:10.1177/1010428317695022
23. Wang M, Ji YQ, Song ZB, Ma XX, Zou YY, Li XS. Knockdown of lncRNA ZFAS1 inhibits progression of nasopharyngeal carcinoma by sponging miR-135a. Neoplasma. 2019;66(6):939–945. doi:10.4149/neo_2018_181213N963
24. Xie S, Ge Q, Wang X, Sun X, Kang Y. Long non-coding RNA ZFAS1 sponges miR-484 to promote cell proliferation and invasion in colorectal cancer. Cell Cycle (Georgetown, Tex). 2018;17(2):154–161. doi:10.1080/15384101.2017.1407895
25. Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013;3(3):71–85.
26. Liu Y. The context of prostate cancer genomics in personalized medicine. Oncol Lett. 2017;13(5):3347–3353. doi:10.3892/ol.2017.5911
27. Zhu M, Chen Q, Liu X, et al. lncRNA H19/miR-675 axis represses prostate cancer metastasis by targeting TGFBI. FEBS J. 2014;281(16):3766–3775. doi:10.1111/febs.2014.281.issue-16
28. Shi X, Zhang W, Nian X, et al. The previously uncharacterized lncRNA APP promotes prostate cancer progression by acting as a competing endogenous RNA. Int J Cancer. 2020;146(2):475–486. doi:10.1002/ijc.32422
29. Chen X, Yang C, Xie S, Cheung E. Long non-coding RNA GAS5 and ZFAS1 are prognostic markers involved in translation targeted by miR-940 in prostate cancer. Oncotarget. 2018;9(1):1048–1062. doi:10.18632/oncotarget.23254
30. Ponizovskiy MR. Biophysical and biochemical models of mechanisms of cellular development via the cellular cycle in normal tissue, cancerous tissue, and inflammatory processes. Crit Rev Eukaryot Gene Expr. 2013;23(2):171–193. doi:10.1615/CritRevEukaryotGeneExpr.v23.i2
31. Ponizovskiy M. The central regulation of all biophysical and biochemical processes as the mechanism of maintenance stability of internal energy and internal medium both in a human organism and in cells of an organism. Modern Chem Appl. 2013;1.
32. Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14:48. doi:10.1186/s12943-015-0321-5
33. Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833(12):3448–3459. doi:10.1016/j.bbamcr.2013.06.001
34. Heerboth S, Housman G, Leary M, et al. EMT and tumor metastasis. Clin Transl Med. 2015;4:6. doi:10.1186/s40169-015-0048-3
35. De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. doi:10.1038/nrc3447
36. Tiwari N, Gheldof A, Tatari M, Christofori G. EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol. 2012;22(3):194–207. doi:10.1016/j.semcancer.2012.02.013
37. Brabletz T. EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell. 2012;22(6):699–701. doi:10.1016/j.ccr.2012.11.009
38. Zhou H, Wang F, Chen H, et al. Increased expression of long-noncoding RNA ZFAS1 is associated with epithelial-mesenchymal transition of gastric cancer. Aging (Albany NY). 2016;8(9):2023–2038. doi:10.18632/aging.v8i9
39. Pan L, Liang W, Fu M, et al. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143(6):991–1004. doi:10.1007/s00432-017-2361-2
40. Strobl-Mazzulla PH, Bronner ME. Epithelial to mesenchymal transition: new and old insights from the classical neural crest model. Semin Cancer Biol. 2012;22(5–6):411–416. doi:10.1016/j.semcancer.2012.04.008
41. Zhao R, Sun F, Bei X, et al. Upregulation of the long non-coding RNA FALEC promotes proliferation and migration of prostate cancer cell lines and predicts prognosis of PCa patients. Prostate. 2017;77(10):1107–1117. doi:10.1002/pros.v77.10
42. Kim ED, Sung S. Long noncoding RNA: unveiling hidden layer of gene regulatory networks. Trends Plant Sci. 2012;17(1):16–21. doi:10.1016/j.tplants.2011.10.008
43. Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing miRNA-lncRNA interactions. Meth Mol Biol (Clifton, NJ). 2016;1402:271–286.
44. Ye Y, Gao X, Yang N. LncRNA ZFAS1 promotes cell migration and invasion of fibroblast-like synoviocytes by suppression of miR-27a in rheumatoid arthritis. Hum Cell. 2018;31(1):14–21. doi:10.1007/s13577-017-0179-5
45. Cui X, Piao C, Lv C, Lin X, Zhang Z, Liu X. ZNFX1 anti-sense RNA 1 promotes the tumorigenesis of prostate cancer by regulating c-Myc expression via a regulatory network of competing endogenous RNAs. Cell Mol Life Sci. 2019. doi:10.1007/s00018-019-03226-x
46. Liu B, Li J, Cairns MJ. Identifying miRNAs, targets and functions. Brief Bioinform. 2014;15(1):1–19. doi:10.1093/bib/bbs075
47. Wang Q, Zhang H, Shen X, Ju S. Serum microRNA-135a-5p as an auxiliary diagnostic biomarker for colorectal cancer. Ann Clin Biochem. 2017;54(1):76–85. doi:10.1177/0004563216638108
48. Zhou H, Guo W, Zhao Y, et al. MicroRNA-135a acts as a putative tumor suppressor by directly targeting very low density lipoprotein receptor in human gallbladder cancer. Cancer Sci. 2014;105(8):956–965. doi:10.1111/cas.2014.105.issue-8
49. Müller S, Nowak K. Exploring the miRNA-mRNA regulatory network in clear cell renal cell carcinomas by next-generation sequencing expression profiles. Biomed Res Int. 2014;2014:948408. doi:10.1155/2014/948408
50. Guo LM, Ding GF, Xu W, et al. MiR-135a-5p represses proliferation of HNSCC by targeting HOXA10. Cancer Biol Ther. 2018;19(11):973–983. doi:10.1080/15384047.2018.1450112
51. Lin J, Wen X, Zhang X, et al. miR-135a-5p and miR-124-3p inhibit malignancy of glioblastoma by downregulation of syndecan binding protein. J Biomed Nanotechnol. 2018;14(7):1317–1329. doi:10.1166/jbn.2018.2579
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.