Back to Journals » OncoTargets and Therapy » Volume 13
LncRNA XIST Promotes Growth of Human Chordoma Cells by Regulating miR-124-3p/iASPP Pathway
Authors Hai B , Pan X , Du C , Mao T, Jia F, Liu Y , Ma Y , Liu X, Zhu B
Received 2 March 2020
Accepted for publication 30 April 2020
Published 26 May 2020 Volume 2020:13 Pages 4755—4765
DOI https://doi.org/10.2147/OTT.S252195
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Bao Hai,1,* Xiaoyu Pan,1,* Chuanchao Du,1 Tianli Mao,1 Fei Jia,1 Yu Liu,1 Yunlong Ma,2 Xiaoguang Liu,1,2 Bin Zhu2
1Department of Orthopedics, Peking University Third Hospital, Beijing, People’s Republic of China; 2The Center for Pain Medicine, Peking University Third Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoguang Liu
Department of Orthopedics, Peking University Third Hospital, North Garden Street No. 49, Haidian District, Beijing 100191, People’s Republic of China
Tel +86 10 8226 5711
Email [email protected]
Bin Zhu
The Center for Pain Medicine, Peking University Third Hospital, North Garden Street No. 49, Haidian District, Beijing 100191, People’s Republic of China
Tel +86 10 8226 7368
Email [email protected]
Introduction: Chordoma is a malignant primary bone tumor that is found in the spine and skull. X-inactive specific transcript (XIST) is a long non-coding RNA (lncRNA) is known to be involved in the development of various cancers, but its precise function and mechanism in human chordoma have not been elucidated. Here, we investigated the role of lncRNA XIST in chordoma progression.
Methods: Quantitative real time-polymerase chain reaction (qRT-PCR) was performed to determine lncRNA XIST expression in human chordoma tissues and matched-noncancerous tissues. Western blot was used to determine protein expression. Silencing and overexpression of lncRNA XIST were carried out by RNA interference (RNAi) and lentiviral transduction, respectively. Cell Counting Kit-8 (CCK-8) assay and flow cytometry were employed to examine the effects of lncRNA XIST on growth of human chordoma cells. Lastly, the role of lncRNA XIST in vivo was explored using a xenograft model.
Results: We found that lncRNA XIST expression was upregulated in chordoma and strongly correlated with poor patient prognosis. Moreover, lncRNA XIST promoted proliferation and inhibited apoptosis of chordoma cells. Mechanistically, upregulation of lncRNA XIST led to a decrease in miR-124-3p expression, thereby promoting the expression of the miR-124-3p target gene, inhibitor of apoptosis-stimulating protein of p53 (iASPP). Addition of miR-124-3p inhibitor or mimic reversed the effects induced by lncRNA XIST silencing or overexpression on chordoma cell proliferation. Lastly, using a xenograft mouse model, we found that silencing of lncRNA XIST decreased tumorigenicity in vivo, as shown by increased tumor cell apoptosis.
Conclusion: Our findings demonstrate a key role for lncRNA XIST in chordoma progression by regulating miR124-3p/iAPSS pathway.
Keywords: chordoma, lncRNA XIST, miR124-3p, iASPP, proliferation, apoptosis
Introduction
Chordoma is a malignant primary bone tumor that occurs along the spine or the base of the skull and is caused by leftover notochordal cells during fetal development.1 Currently, surgical resection remains the primary means for chordoma treatment. However, this approach causes spinal or brain damage, making chordoma a challenging tumor to treat. In addition, tumor cells are often highly resistant to radiotherapy and chemotherapy.1–3 Hence, understanding the molecular mechanism that governs chordoma progression is crucial for designing novel treatment.
Long non-coding RNAs (lncRNAs) are a type of RNA that lack protein-coding function but can modulate oncogenic and tumor-suppressive pathways in numerous cancers without protein regulation.4–6 X-inactive specific transcript (XIST) is a lncRNA first discovered in X chromosome inactivation.7 Recently, lncRNA XIST has been shown to contribute to the development of various cancers, including non-small cell lung cancer, hepatocellular carcinoma, and pancreatic cancer.8–12 Interestingly, the mechanism through which lncRNA XIST functions in these different types of cancers appear to involve similar regulation, in which lncRNA XIST acts as a sponge that can sequester miRNA and indirectly upregulate oncogene expression in these cancers.8–12 The miRNAs involved are specific and dependent on the cancer type. However, little is known regarding the role and underlying molecular mechanism of lncRNA XIST in the progression of chordoma.
A large body of evidence indicates that lncRNAs can directly bind to miRNAs and attenuate the latter’s function in suppressing gene expression in cancer.13,14 miRNA-124 is one of the most abundant miRNAs in embryonic brain as well as in adult15 and is involved in the differentiation of neuronal stem cells to mature neurons.16 miR-124-3p targets the 3′-UTR of its target gene, inhibitor of apoptosis-stimulating protein of p53 (iASPP), to suppress iASPP expression.17 Previous studies demonstrated that iASPP functions as an oncogene.18–20 Enhanced expression of iASPP confers proliferative, migratory, and invasive capabilities for tumor cells, while inhibition of iASPP expression stimulates pro-apoptotic and growth-suppressive effects on tumor cells.21,22 Furthermore, iAPSS expression is associated with advanced stages of malignancies,23–25 tumor recurrence, and poor prognosis.18,19,26,27 Notably, previous studies showed that miR124-3p/iAPSS regulates proliferation and apoptosis in chordoma cell lines and is associated with clinical outcomes in spinal chordoma.28 However, the molecule network of miR124-3p/iAPSS in chordoma has not been delineated.
In this study, we investigated the role of lncRNA XIST in chordoma and uncovered a functional relationship between lncRNA XIST and miR-124/iAPSS. Specifically, we found that elevated expression of lncRNA XIST in chordoma regulates miR124-3p/iAPSS to promote chordoma cell growth, which was confirmed in vivo. Together, our findings suggest that lncRNA XIST may serve as a novel therapeutic target and prognostic biomarker for chordoma.
Materials and Methods
Human Chordoma Tissue Samples
A total of 38 tumor tissues and 15 normal tissues were surgically resected from chordoma patients. Patients that received chemotherapy or radiotherapy were excluded from this study. All patients provided informed written consent. This study was approved by the independent ethics committee of Peking University Third Hospital, Beijing, China, and was conducted in accordance with the Declaration of Helsinki.
Cell Culture and Chemicals
MUG-Chor1 and U-CH1 cell lines were obtained from the cell bank of Shanghai Biology Institute (Shanghai, China). Chordoma cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Kaukauna, WI, USA), supplemented with 2 mM l-glutamine, 1% penicillin/streptomycin (Solarbio, Beijing, China) and 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA). Cells were cultured in an incubator with 5% CO2 at 37°C. miR-124-3p inhibitor and mimic were dissolved in DMSO solution.
RNA Isolation, Reverse Transcription, and Real-Time Polymerase Chain Reaction (RT-PCR)
Total RNAs were extracted using TRIzol Reagent (Invitrogen, Waltham, MA, USA) following manufacturer’s instructions. Subsequently, 2 ng of purified RNA was reverse transcribed into cDNA using a cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA). Real-time PCR reactions were performed using SYBR Green PCR kit to measure relative gene expression and following the indicated conditions: 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds, 60°C for 45 seconds. Human GAPDH was used as an internal control for mRNA detection. Relative gene expression was calculated by the 2−ΔΔCt method. All experiments were performed in triplicates. Primers used in this study are listed as follows: H19, F: 5ʹ-GCGGGTCTGTTTCTTTACTTCC-3ʹ, R: 5ʹ-CTTTGATGTTGGGCTGATGAGG-3ʹ; HOXA11-AS, F: 5ʹ-AGTTTGAAGCCGTGGATGTG-3ʹ, R. 5ʹ-AGAAGGTGGGATGAAGAGGTAG-3ʹ; MALAT1, F: 5ʹ-TTTCTTCCTGCTCCGGTTC-3ʹ, R: 5ʹ-TTTCAGCTTCCAGGCTCTC-3ʹ; XIST, F: 5ʹ-TTTCATCGCCCATCGGTG-3ʹ, R: 5ʹ-CTGCCTGACCTGCTATCATC-3ʹ; PPP1R13L, F: 5ʹ-AGAAGTGCGACCCTTACC-3ʹ, R: 5ʹ-ACAGCCCGAAGTAGTTCC-3ʹ; GAPDH, F: 5ʹ-AATCCCATCACCATCTTC-3ʹ, R: 5ʹ-AGGCTGTTGTCATACTTC-3ʹ.
Western Blot (WB)
Whole protein lysates were isolated using RIPA lysis buffer (JRDUN, Shanghai, China) containing EDTA-free Protease Inhibitor Cocktail (Roche, Heidelberg, Germany). Equal amounts of total protein (25 μg) were loaded and separated on 10% SDS-PAGE, and subsequently transferred to a nitrocellulose membrane (Millipore, Billerica, MA, USA) overnight. After blocking with 5% non-fat dry milk, membranes were probed with primary antibodies. Protein expression was detected using an enhanced chemiluminescence system (Tanon, Shanghai, China). Primary antibodies used in this study are listed as follows: iASPP (Ab34898, Abcam, UK); GAPDH (#5174, CST, USA); anti-mouse IgG (Beyotime, Shanghai, China).
Overexpression and Knockdown of lncRNA XIST
For overexpression experiments, lentiviral plasmid (pLVX-puro) containing full-length sequence of human XIST (oeXIST) and control plasmid (oeNC) were generated. For knockdown experiments, three siRNAs targeting various regions of human lncRNA XIST (NR_001564.2) and a negative control siRNA (siNC) were synthesized (Major, Shanghai, China). Plasmids and siRNAs were transfected into chordoma cells using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Detailed sequence information for XIST siRNAs are provided as follows: siXIST-1: (5529–5547; GCTTCTAACTAGCCTGAAT) siXIST-2; (6167–6185, GCATGCATCTTGGACATTT) siXIST-3 (8208–8226, CCATGCATCTTGGAAATTT).
Cell Proliferation
Cell Counting Kit-8 (CCK-8) was purchased from Signalway Antibody (Maryland, USA) and used to measure cell proliferation. Briefly, transfected cells were cultured for 0, 24, 48, and 72 hours. Following manufacturer’s protocol, CCK-8 solution (1:10) was added to plates and cells were incubated for 1 hour. To quantify cell proliferation, a microplate reader (Pulangxin, Beijing, P. R. China) was used to measure ODs at a wavelength of 450 nm. Triplicates were performed for each experimental condition and for each time point.
Cell Apoptosis
Forty-eight hours after transfection, MUG-Chor1 and U-CH1 cells were harvested and stained using Annexin V-fluorescein isothiocyanate apoptosis detection kit (Beyotime, Beijing, China) according to manufacturer’s instructions. Cell apoptosis was determined using a flow cytometer (BD, San Diego, CA, USA). Annexin V negative and PI negative populations were healthy cells that were counted as negative staining. Annexin V positive and PI negative cells indicated cells in early apoptosis. Annexin V positive and PI positive staining indicated cells in necrosis (post-apoptotic necrosis or late apoptosis). The above two populations of cells were counted as positive staining. All experiments were performed in triplicates.
Luciferase Activity Assay
Dual-luciferase reporter assay was performed following manufacturer’s instructions to calculate relative luciferase activity. Briefly, the sequence of iAPPS promoter was cloned into the Dual-luciferase vector. U-CH1 cells were co-transfected with mock vector and siRNA. Luciferase activity was measured by a plate reader. Given the relative values, we used a second luciferase reaction to normalize between samples and to correct for transfection efficiency. To normalize the values, each firefly luciferase value was divided by the value for renilla luciferase in the same well.9,29
Tumor Xenograft Model
In vivo study was approved by the ethics committee of the Peking University Third Hospital. All mice were handled according to the Institutional Animal Care and Use Committee (IACUC) guidelines and experiments were conducted following the institute’s guidelines for animal experiments. Nude mice were obtained from Vitalriver company (Beijing, China). Each experimental group included 6 mice. Subcutaneous cell injection was used to establish the tumor model. Equal numbers of siNC- or siXIST-transfected U-CH1 cells (n=2×106) were transplanted into the right flank of 4- to 6-week-old mouse, respectively. Six weeks after injection, mice were sacrificed by cervical dislocation, and tumor tissues were surgically removed and fixed in 4% formalin for further analysis.
TUNEL Staining
TUNEL assays were performed using ApopTag kit (Intergene) according to the supplier’s instructions. Three replicates were conducted for each sample.
Histopathology Assay
All tissues were fixed in 10% formalin for 48 hours. Subsequently, xenografts were embedded in paraffin blocks and cut into slices using a microtome (Leike, China). Slides were deparaffinized and rehydrated in xylene baths and graded alcohols serially. Then, slices were exposed to diaminobenzidine (DAB) substrate for 1 minute followed by hematoxylin and eosin (H & E) staining. Three random fields on each slide were observed and three slides per sample were stained from each xenograft.
Statistical Analysis
Data are shown as mean ± SD for at least three replicates in this study. GraphPad Prism software Version 7.0 (La Jolla CA, USA) was used for statistical analyses. One-way ANOVA was used to determine statistical significance. P-value < 0.05 indicated statistical significance.
Results
LncRNA XIST Expression Negatively Correlated with miR-124-3p Expression in Chordoma and Was Associated with Poor Patient Prognosis
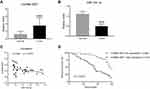
To determine the expression of lncRNA XIST in human chordoma tissues and corresponding adjacent tissues, quantitative RT-PCR was performed. As shown in Figure 1A, lncRNA XIST expression was elevated in chordoma tissues compared to matched adjacent tissues. Moreover, one of the downstream targets of lncRNA XIST, miR-124-3p, was found to be downregulated in chordoma tissues compared to normal tissues (Figure 1B) and showed negative correlation with lncRNA XIST (Figure 1C).
Next, patient survival analysis was conducted to identify prognostic factors for chordoma patients. As shown in a Kaplan–Meier survival plot (Figure 1D), the cumulative total survival rate of patients with high lncRNA XIST expression (n=34) was much shorter than that in patients with low lncRNA XIST expression (n=46). Thus, high lncRNA XIST expression was associated with decreased patient survival rate.
Notably, expression of prognostic cancer biomarkers, including lncRNA H19, HOTAIR, and MALAT1, was upregulated in chordoma tissues (Supplementary Figure 1A–C) though their correlations were not as strong as lncRNA XIST with miR-124-3p (Supplementary Figure 2A–C). Moreover, iASPP, the target gene of miR-124-3p, was found to be upregulated in chordoma tissues (Supplementary Figure 1D). Together, these results demonstrate a strong association between lncRNA XIST and miR-124/iASPP pathway in chordoma.
Knockdown and Overexpression of lncRNA XIST in Human Chordoma Cells
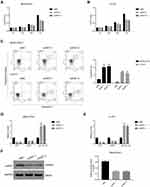
Given the negative correlation between lncRNA XIST and miR-124-3p, we posited that lncRNA XIST was involved in regulating miR-124/iAPSS. To probe the function and molecular mechanism of lncRNA XIST in chordoma, we established knockdown and overexpression models of lncRNA XIST using two chordoma cell lines, MUG-Chor1 and U-CH1. Interestingly, we found that lncRNA XIST expression was upregulated in both cell lines (Figure 2A), as shown by quantitative RT-PCR. To determine the functional relevance, we examined the effects of altering lncRNA XIST expression in these cell lines. Transfection of siRNAs targeting different sequences of lncRNA in MUG-Chor1 and U-CH1 cells resulted in significant downregulation of lncRNA XIST expression (Figure 2B), indicating knockdown efficiency. Similarly, lentiviral infection of lncRNA XIST overexpression vector led to upregulation of lncRNA XIST expression in MUG-Chor1 cells (Figure 2C), demonstrating efficacy of overexpression.
LncRNA XIST Silencing Suppressed Growth of Human Chordoma Cells
To determine the cellular functions of lncRNA XIST, we assessed the effects of lncRNA XIST silencing on cell proliferation and apoptosis. As shown in Figure 3A, the proliferative capacity of MUC-Chor1 cells was significantly repressed after silencing of lncRNA XIST, and this repression was stronger at 72 hours compared to 48 hours. At 24 hours, inhibition of cell proliferation showed a repressive trend but was not statistically significant. Similar results were observed in U-CH1 cells (Figure 3B). Silencing of lncRNA XIST expression by various targeting sequences showed consistent results in both cell lines. Next, apoptosis was assessed in both cell lines by annexin V staining. As shown in Figure 3C, knockdown of lncRNA XIST in cells resulted in increased apoptosis compared with control cells, which was further confirmed by quantification of the data. Consistent with our results, miR-124/IASPP has been reported to play a crucial role in tumor proliferation.18,19,26,27
In light of the negative correlation between lncRNA XIST and miR-124, we examined the effects of lncRNA XIST silencing on miR-124/iAPSS. Interestingly, we found that reduction in lncRNA XIST expression was associated with miR-124-3p induction and iASPP reduction (Figure 3D and E). This association was confirmed in both MUG-Chor1 and U-CH1 cells, indicating that it was not solely cell type-specific. To validate this, we conducted Western blot and found that iASPP protein level was strongly downregulated after silencing of lncRNA XIST (Figure 3F). Taken together, our data demonstrate that lncRNA XIST promoted proliferation and inhibited apoptosis by regulating miRNA-124-3p/iAPSS in chordoma.
LncRNA XIST Overexpression Promoted Growth of Human Chordoma Cells
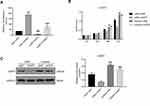
To complement our knockdown experiments and further confirm lncRNA XIST function in chordoma, we examined the effects of lncRNA XIST overexpression. We observed that MUG-Chor1 cell proliferation was significantly inhibited after overexpression of lncRNA XIST at 48 hours and 72 hours, with inhibition stronger at 72 hours compared to 48 hours (Figure 4A). As shown in Figure 4B, overexpression of lncRNA XIST decreased apoptosis. Likewise, induction of lncRNA XIST expression was highly associated with reduction of miR-124-3p expression and iAPSS induction (Figure 4C). Furthermore, lncRNA XIST overexpression significantly increased iAPSS protein level (Figure 4D). Collectively, our results demonstrate that overexpression of lncRNA XIST induced proliferation of chordoma cells.
miR-124-3p Inhibitor Rescued the Effects of lncRNA XIST in U-CH1 Cells
LncRNAs primarily regulate gene expression by acting as miRNA sponges,6,13,30 binding functional miRNAs, and sequestering them from their target genes. We subsequently performed rescue experiments to validate whether lncRNA XIST indeed bound miR-124 to regulate iASPP. Hence, we tested iASPP promoter activity by performing a dual-luciferase reporter assay. Treatment with miR-124 inhibitor in U-CH1 cells resulted in increased iASPP promoter binding and luciferase activity. In addition, iASPP promoter activity was decreased in lncRNA XIST-silenced cells compared with control cells (Figure 5A). Notably, miR124-3p inhibitor rescued promoter activity in cells transfected with XIST siRNA. Moreover, miR-124-3p inhibitor rescued the suppressive effects of lncRNA XIST on cell proliferation (Figure 5B). As shown in Figure 5C, silencing of lncRNA XIST significantly reduced the protein level of iASPP, while miR-124-3p inhibitor increased the protein level of iASPP. miR124-3p inhibitor also elevated the iASPP protein level in cells transfected with XIST siRNA. Taken together, our results demonstrate that restoring lncRNA XIST could reverse the inhibitory effects of miR-124-3p on chordoma cell proliferation and that this rescue was likely mediated by iAPSS.
miR-124-3p Mimic Inhibits Proliferation of MUG-Chor1 Cells Transfected with oeXIST
To further confirm that lncRNA XIST bound to and sequestered miR-124-3p to regulate iASPP expression, cell proliferation assay, and WB analysis were carried out using lncRNA XIST-overexpressing cells. The miR-124 mimic contained an artificial double-stranded miR-124-3p-like RNA fragment. However, the fragment could not bind to iAPSS. As shown in Figure 6A, the miR-124-3p mimic inhibited proliferation of XIST-overexpressing cells and decreased iASPP protein level (Figure 6B). Conversely, miR-124-3p inhibitor displayed the opposite effects in chordoma cells.
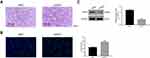
siRNA XIST Significantly Reduced Tumorigenicity of U-CH1 Cells in vivo
To verify lncRNA XIST function in vivo, we used a xenograft tumor model. H&E and TUNEL staining assays were used to assess apoptosis of tumor cells in vivo. Apoptotic cells showed cytoplasmic and nuclear condensation and nuclear fragments. As shown in Figure 7A and B, chordoma cells were more apoptotic after silencing of lncRNA XIST compared with control cells. Furthermore, expression of iAPSS was downregulated in siXIST tumors (Figure 7C).
Discussion
Chordoma is a rare mesenchymal tissue tumor and the molecular mechanism underlying chordoma pathology is not fully understood. Here, our study demonstrates that lncRNA XIST promotes growth of human chordoma cells by regulating miR-124-3p/iASPP pathway. To our knowledge, this is the first report to demonstrate the function of lncRNA XIST in chordoma.
lncRNA XIST is known to regulate cell proliferation and has been shown to play a role in the development of various cancers, including glioma,31 gastric cancer,32 and esophageal cancer.33 Consistent with previous studies, our results indicated that lncRNA XIST promoted proliferation and inhibited apoptosis in chordoma cells, thus serving as an oncogene in chordoma progression.
LncRNA XIST plays a central role in regulating cancer cell growth by sequestering miRNA and indirectly upregulating oncogene expression. Many distinct miRNAs have been identified as downstream targets of IncRNA XIST. For instance, lncRNA XIST has been reported to regulate cell proliferation in non-small cell lung cancer by modulating miR-186-5p and miR-335/SOD2/ROS signaling pathways.8,9 Moreover, lncRNA XIST has been shown to target miR-194-5p and miR-139-5p to regulate growth of hepatocellular carcinoma cells.12,34 Further, lncRNA XIST has been demonstrated to promote proliferation of pancreatic cancer cells by acting as a sponge for miR-429 and miR-133a.10,11 In this study, we identified miR-124-3p/iASPP as the downstream pathway target of lncRNA XIST to regulate chordoma development. iAPSS was highly expressed in chordoma cells and tissues. Interestingly, iAPSS was previously reported to be upregulated in non-small cell lung cancer, leukemia, and human breast carcinomas, which correlated with clinicopathological parameters and patient prognosis. We also found that upregulation of lncRNA XIST led to a decrease in miR-124-3p expression but an increase in the expression of iASPP. Therefore, we speculate that miR-124 negatively regulates iASPP in chordoma cells. Interestingly, iASPP expression has also been associated with metastasis.29 Future studies are needed to explore the clinical value of iASPP in the treatment of chordoma.
Meanwhile, the tight association of miRNAs and lncRNAs with cancer progression and diagnosis suggests their potential use as emerging cancer biomarkers. Detection of circulating cancer-associated lncRNAs can be used to predict, diagnose, and monitor cancer progression. For instance, lncRNAs MALAT1 and PCA3 have been identified as effective prognostic parameters for non-small cell lung cancer and prostate cancer, respectively.35,36 In the present study, we revealed that high lncRNA XIST was closely related to poor prognosis in chordoma patients, indicating that lncRNA has the potential to serve as a biomarker for chordoma.
Conclusion
In summary, the present study uncovered an important role for lncRNA XIST in promoting chordoma development by regulating miR-124-3p/iAPSS pathway. Our results not only illuminated the function of IncRNA XIST in chordoma but also revealed its downstream signaling targets. Moreover, our results suggest the potential use of lncRNA XIST as a therapeutic target in the treatment of chordoma.
Acknowledgment
We sincerely acknowledge the assistance given by the Peking University Third Hospital, 100191, Beijing, China. This research was supported in part by the Youth Program of National Natural Science Foundation of China (81802686)
Disclosure
All authors declared no conflicts of interest.
References
1. Almefty K, Pravdenkova S, Colli BO, Al-Mefty O, Gokden M. Chordoma and chondrosarcoma: similar, but quite different, skull base tumors. Cancer. 2007;110(11):2457–2467. doi:10.1002/cncr.23073
2. Gulluoglu S, Turksoy O, Kuskucu A, Ture U, Bayrak OF. The molecular aspects of chordoma. Neurosurg Rev. 2016;39(2):
3. Patel P, Brooks C, Seneviratne A, Hess DA, Seguin CA. Investigating microenvironmental regulation of human chordoma cell behaviour. PLoS One. 2014;9(12):e115909. doi:10.1371/journal.pone.0115909
4. Tong YS, Wang XW, Zhou XL, et al. Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol Cancer. 2015;14:3. doi:10.1186/1476-4598-14-3
5. Martens-Uzunova ES, Bottcher R, Croce CM, Jenster G, Visakorpi T, Calin GA. Long noncoding RNA in prostate, bladder, and kidney cancer. Eur Urol. 2014;65(6):1140–1151. doi:10.1016/j.eururo.2013.12.003
6. Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi:10.1186/1476-4598-10-38
7. Yang Z, Jiang X, Jiang X, Zhao H. X-inactive-specific transcript: a long noncoding RNA with complex roles in human cancers. Gene. 2018;679:28–35. doi:10.1016/j.gene.2018.08.071
8. Wang H, Shen Q, Zhang X, et al. The long non-coding RNA XIST controls non-small cell lung cancer proliferation and invasion by modulating miR-186-5p. Cell Physiol Biochem. 2017;41(6):2221–2229. doi:10.1159/000475637
9. Liu J, Yao L, Zhang M, Jiang J, Yang M, Wang Y. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging (Albany NY). 2019;11(18):7830–7846. doi:10.18632/aging.102291
10. Shen J, Hong L, Yu D, Cao T, Zhou Z, He S. LncRNA XIST promotes pancreatic cancer migration, invasion and EMT by sponging miR-429 to modulate ZEB1 expression. Int J Biochem Cell Biol. 2019;113:17–26. doi:10.1016/j.biocel.2019.05.021
11. Wei W, Liu Y, Lu Y, Yang B, Tang L. LncRNA XIST promotes pancreatic cancer proliferation through miR-133a/EGFR. J Cell Biochem. 2017;118(10):3349–3358. doi:10.1002/jcb.25988
12. Kong Q, Zhang S, Liang C, et al. LncRNA XIST functions as a molecular sponge of miR-194-5p to regulate MAPK1 expression in hepatocellular carcinoma cell. J Cell Biochem. 2018;119(6):4458–4468. doi:10.1002/jcb.26540
13. Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNA-LncRNA interactions. Methods Mol Biol. 2016;1402:271–286.
14. Ballantyne MD, McDonald RA, Baker AH. lncRNA/MicroRNA interactions in the vasculature. Clin Pharmacol Ther. 2016;99(5):494–501. doi:10.1002/cpt.355
15. Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12(9):735–739. doi:10.1016/S0960-9822(02)00809-6
16. Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12(4):399–408. doi:10.1038/nn.2294
17. Liu X, Kang J, Sun S, et al. iASPP, a microRNA124 target, is aberrantly expressed in astrocytoma and regulates malignant glioma cell migration and viability. Mol Med Rep. 2018;17(1):1970–1978. doi:10.3892/mmr.2017.8097
18. Cao L, Huang Q, He J, Lu J, Xiong Y. Elevated expression of iASPP correlates with poor prognosis and chemoresistance/radioresistance in FIGO Ib1-IIa squamous cell cervical cancer. Cell Tissue Res. 2013;352(2):361–369. doi:10.1007/s00441-013-1569-y
19. Chen J, Xie F, Zhang L, Jiang WG. iASPP is over-expressed in human non-small cell lung cancer and regulates the proliferation of lung cancer cells through a p53 associated pathway. BMC Cancer. 2010;10:694. doi:10.1186/1471-2407-10-694
20. Lu B, Guo H, Zhao J, et al. Increased expression of iASPP, regulated by hepatitis B virus X protein-mediated NF-kappaB activation, in hepatocellular carcinoma. Gastroenterology. 2010;139(6):2183–2194. doi:10.1053/j.gastro.2010.06.049
21. Bell HS, Ryan KM. iASPP inhibition: increased options in targeting the p53 family for cancer therapy. Cancer Res. 2008;68(13):4959–4962. doi:10.1158/0008-5472.CAN-08-0182
22. Liu ZJ, Cai Y, Hou L, et al. Effect of RNA interference of iASPP on the apoptosis in MCF-7 breast cancer cells. Cancer Invest. 2008;26(9):878–882. doi:10.1080/07357900801965042
23. Liu X, Kang J, Liu F, et al. Overexpression of iASPP-SV in glioma is associated with poor prognosis by promoting cell viability and antagonizing apoptosis. Tumour Biol. 2016;37(5):6323–6330. doi:10.1007/s13277-015-4503-y
24. Lu M, Breyssens H, Salter V, et al. Restoring p53 function in human melanoma cells by inhibiting MDM2 and cyclin B1/CDK1-phosphorylated nuclear iASPP. Cancer Cell. 2016;30(5):822–823. doi:10.1016/j.ccell.2016.09.019
25. Morris EV, Cerundolo L, Lu M, et al. Nuclear iASPP may facilitate prostate cancer progression. Cell Death Dis. 2014;5:e1492. doi:10.1038/cddis.2014.442
26. Kong F, Shi X, Li H, et al. Increased expression of iASPP correlates with poor prognosis in FIGO IA2-IIA cervical adenocarcinoma following a curative resection. Am J Cancer Res. 2015;5(3):1217–1224.
27. Jiang L, Siu MK, Wong OG, et al. iASPP and chemoresistance in ovarian cancers: effects on paclitaxel-mediated mitotic catastrophe. Clin Cancer Res. 2011;17(21):6924–6933. doi:10.1158/1078-0432.CCR-11-0588
28. Ma Y, Zhu B, Liu X, et al. iASPP overexpression is associated with clinical outcome in spinal chordoma and influences cellular proliferation, invasion, and sensitivity to cisplatin in vitro. Oncotarget. 2017;8(40):68365–68380. doi:10.18632/oncotarget.20190
29. Fu W, Wu X, Yang Z, Mi H. The effect of miR-124-3p on cell proliferation and apoptosis in bladder cancer by targeting EDNRB. Arch Med Sci. 2019;15(5):1154–1162. doi:10.5114/aoms.2018.78743
30. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–1261. doi:10.1038/nm.3981
31. Xu R, Zhu X, Chen F, et al. LncRNA XIST/miR-200c regulates the stemness properties and tumourigenicity of human bladder cancer stem cell-like cells. Cancer Cell Int. 2018;18:41. doi:10.1186/s12935-018-0540-0
32. Chen DL, Ju HQ, Lu YX, et al. Long non-coding RNA XIST regulates gastric cancer progression by acting as a molecular sponge of miR-101 to modulate EZH2 expression. J Exp Clin Cancer Res. 2016;35(1):142. doi:10.1186/s13046-016-0420-1
33. Chen Z, Hu X, Wu Y, et al. Long non-coding RNA XIST promotes the development of esophageal cancer by sponging miR-494 to regulate CDK6 expression. Biomed Pharmacother. 2019;109:2228–2236. doi:10.1016/j.biopha.2018.11.049
34. Mo Y, Lu Y, Wang P, et al. Long non-coding RNA XIST promotes cell growth by regulating miR-139-5p/PDK1/AKT axis in hepatocellular carcinoma. Tumour Biol. 2017;39(2):1010428317690999. doi:10.1177/1010428317690999
35. Guo F, Yu F, Wang J, et al. Expression of MALAT1 in the peripheral whole blood of patients with lung cancer. Biomed Rep. 2015;3(3):309–312. doi:10.3892/br.2015.422
36. Sartori DA, Chan DW. Biomarkers in prostate cancer: what’s new? Curr Opin Oncol. 2014;26(3):259–264. doi:10.1097/CCO.0000000000000065
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.