Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
LncRNA SOX2OT is Upregulated in Gestational Diabetes Mellitus (GDM) and Correlated with Multiple Adverse Events
Received 11 May 2021
Accepted for publication 29 July 2021
Published 10 September 2021 Volume 2021:14 Pages 3989—3995
DOI https://doi.org/10.2147/DMSO.S319739
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Guangqin Ran, Xiaofan Zhu, Yan Qin
Department of Obstetrics and Gynecology, Chongqing General Hospital, University of Chinese Academy of Sciences, Chongqing, 401147, People’s Republic of China
Correspondence: Yan Qin
Department of Obstetrics and Gynecology, Chongqing General Hospital, University of Chinese Academy of Sciences, No. 118, Xingguang Avenue, Liangjiang New Area, Chongqing, 401147, People’s Republic of China
Tel +86 23-63390545
Email [email protected]
Purpose: LncRNA SOX2OT plays protective roles in high glucose-induced injuries, suggesting its potential involvement in diabetes. Therefore, we analyzed the role of SOX2OT in gestational diabetes mellitus (GDM).
Methods: A total of 216 pregnant women with a gestational age of about 2 months were enrolled in this study. The 216 pregnant women were monitored until delivery to record the occurrence of GDM. Adverse events, including miscarriage, premature delivery, intrauterine distress, intrauterine death, intrauterine infection, fetal malformation, macrosomia, and hypertension, were recorded.
Results: Two hundred sixteen pregnant women were divided into high and low SOX2OT level groups (n=108), with the median plasma SOX2OT level on the day of admission as the cutoff value. It was observed that the incidence of GDM was higher in the high SOX2OT level group (40/108) than in the low SOX2OT level group (12/108). Moreover, the SOX2OT expression level was higher in GDM patients than in non-GDM participants, and ROC curve analysis showed that plasma SOX2OT levels on the day of admission could separate potential GDM patients from the rest participants. Importantly, higher incidences of miscarriage, premature delivery, intrauterine distress, intrauterine death, intrauterine infection, fetal malformation, macrosomia, and hypertension were observed in the high SOX2OT group compared to the low SOX2OT group.
Conclusion: SOX2OT is highly expressed in GDM and is closely correlated with multiple adverse events.
Keywords: gestational diabetes mellitus, SOX2OT, adverse events
Introduction
Gestational diabetes mellitus (GDM) refers to the development of any type of glucose intolerance during pregnancy in women who have no history of diabetes before.1 During pregnancy, the placenta stimulates blood glucose accumulation by producing hormones. GDM will develop in cases when the pancreas fails to secrete sufficient insulin to handle the increased blood glucose.2,3 It is estimated that about 13.9% of pregnant women will develop GBM,4 and GDM is closely correlated with a series of adverse events in both mother and infants, such as preterm birth, excessive birth weight, breathing difficulties, obesity, and increased risk of type 2 diabetes later in life.5 Insulin injection is the most common treatment for GDM, while in many cases, blood glucose cannot be effectively controlled, and adverse events are inevitable.6,7
At present, early diagnosis of GDM is still the key to treat GDM.8 Pregnancy outcomes in GDM patients diagnosed at early stages are much better than that of GDM patients diagnosed at advanced stages.9 In addition, some pathological changes, such as villous fibrinoid necrosis, villous immaturity, increased angiogenesis, and charangoists, cannot be fully reversed in patients with advanced GDM.9 To this end, many biomarkers have been developed to detect GDM at early stages.10,11 However, these biomarkers are either limited by the low accuracy or challenged by the detection techniques.10,11 Specifically, most biomarkers are affected by gestational age, different sample sizes and types, and the methods of analysis used. Therefore, more early diagnostic biomarkers are needed to increase the accuracy. Although no protein-coding capacity has been reported in studies of lncRNAs, these non-coding RNA transcripts may affect protein synthesis to participate in human diseases, including GDM.12,13 Therefore, lncRNAs represent a gold mine to explore diagnostic biomarkers for GDM. LncRNA SOX2OT plays a protective role in high glucose-induced injuries,14 suggesting its potential involvement in GDM. We then analyzed the role of SOX2OT in GDM, focusing on its predictive values for GDM and adverse events.
Materials and Methods
Study Population
The study population included 216 pregnant females with a gestational age of about 2 months (0.9 to 2.8). All participants were enrolled at Chongqing General Hospital, University of Chinese Academy of Sciences from January 2019 to March 2020 after the Ethics Committee of this hospital approved this study. Our study complies with the Declaration of Helsinki. Patients with a previous history of diabetes were excluded from this study. Table 1 showed the baseline clinical data of the 216 participants.
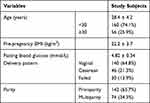 |
Table 1 The Clinical Data of the Baseline Data of 216 Participants |
Follow-Up Analysis and the Diagnosis of GDM
All 216 participants were followed up weekly for a total of 32 weeks to monitor the occurrence of GDM. GDM was diagnosed by determining plasma glucose levels. The diagnostic criteria were 1) 1h post glucose load higher than 180 mg/dl, 2) 2 h post glucose load higher than 153 mg/dl, and 3) fasting plasma glucose higher than 92 mg/dl. All 216 participants completed the follow-up. During the follow-up, adverse events, including miscarriage, premature delivery, intrauterine distress, intrauterine death, intrauterine infection, fetal malformation, macrosomia, and hypertension, were also recorded.
Blood Extraction and the Preparation of Plasma Samples
About 3 to 5 mL of blood was extracted from elbow veins of all participants after overnight fasting and prior to breakfast, placed into BDVacutainer PPT Plasma Preparation Tubes, and centrifuged at 2000 RPM for 20 min to separate plasma samples. About 1.2 to 2 mL of plasma sample was separated from each blood sample and directly subjected to total RNA isolations.
RNA Preparations and Process
RNA samples were isolated from plasma samples using miRNeasy Serum/Plasma Kits (QIAGEN) and digested with DNase I (Invitrogen) to remove DNA. RNA concentration and integrity were analyzed using Bioanalyzer. RNA samples with an integrity number (RIN) higher than 8 were used in subsequent reverse transcriptions.
RT-qPCRs
A total of 4 μg RNA were reverse transcribed (RTs) into cDNA samples using SuperScript™ III First-Strand Synthesis System (Thermo Fisher). After diluted 10 times, cDNA samples were subjected to qPCR using Bio-Rad SYBR Green Master Mix to determine SOX2OT expression with 18S rRNA as the internal control using primers 5ʹ-GCTCGTGGCTTAGGAGATTG-3ʹ (forward) and 5ʹ-CTGGCAAAGCATGAGGAACT-3ʹ (reverse) for SOX2OT and 5ʹ CTACCACATCCAAGGA AGCA-3ʹ (forward) and 5ʹ-TTTTTCGTCACTACCTCCCCG-3ʹ for 18S rRNA. Ct values of SOX2OT were normalized to 18S rRNA using the 2−ΔΔCt method: ΔCt = Ct (target) - Ct (18S rRNA). The sample with the biggest ΔCt value was set to value “1”, and other samples were normalized to this sample.
Statistical Analysis
SOX2OT expression levels in GDM and non-GDM groups were compared with unpaired t-test. SOX2OT expression levels at two different time points in the same group were compared using the paired t-test. The diagnostic value of plasma SOX2OT on the day of admission for GDM was analyzed with ROC curve analysis. The 216 participants were divided into high and low SOX2OT level groups (n=108), with the median SOX2OT level on the day of admission as the cutoff value. GDM-free curves were plotted based on the 32 weeks follow-up data. Associations between plasma SOX2OT levels in GDM patients and the incidence of adverse effects were analyzed using the Chi-squared test or Fisher exact test (for value 0). In this analysis, the 52 GDM patients were divided into high and low SOX2OT level groups (n=26), with the median level of plasma SOX2OT expression on the day of admission as the cutoff value. P<0.05 was statistically significant.
Results
Follow-Up Outcomes and Comparison of SOX2OT Expression Between GDM and Non-GDM Groups on the Day of Admission and the Day of Diagnosis
During the 32 weeks follow-up, a total of 52 GDM cases were diagnosed, and the incidence rate of GDB is 24.1%, which is within the normal range. SOX2OT expression levels in GDM and non-GDM groups on the day of admission and the day of diagnosis were compared by unpaired t-test. On the day of admission, the SOX2OT expression level was higher in the GDM group (n=52) than in the non-GDM group (n=164), suggesting the involvement of SOX2OT in GDM (Figure 1A, p<0.05). On the day of diagnosis of GDM, SOX2OT expression level was also higher in the GDM group (n=52) than in the non-GDM group (n=164), and the difference was further enlarged (Figure 1B, p<0.01). SOX2OT expression levels at two different time points within the same group were compared using paired t-test. In the GDM group, SOX2OT expression levels were significantly higher on the day of diagnosis than that on the day of admission (Figure 1C, p<0.05). No significant differences in SOX2OT expression level were observed between two different time points in the non-GDM group (Figure 1D).
SOX2OT Expression on the Day of Admission Was Sufficient to Separate GDM Patients from Non-GDM Participants
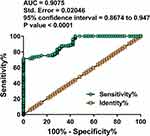
The diagnostic value of plasma SOX2OT on the day of admission for GDM was analyzed with ROC curve analysis, which was performed with GDM patients as the true positive cases and non-GDM participants as the true negative cases. As shown in Figure 2, the area under the curve (AUC) was 0.9075, with Std. Error of 0.02046 and 95% confidence interval of 0.8674 to 0.9476 (P< 0.0001). Therefore, SOX2OT expression on the day of admission was sufficient to separate GDM patients from non-GDM participants.
Analysis of the Prognostic Value of SOX2OT Expression on the Day of Admission for GDM
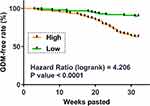
The 216 participants were divided into high and low SOX2OT level groups (n=108), with the median SOX2OT level on the day of admission as the cutoff value. The high SOX2OT level group included 40 GDM patients, and the low SOX2OT level group included 12 GDM patients. GDM-free curve analysis showed that the incidence of GDM was higher in the high SOX2OT level group (40/108) than in the low SOX2OT level group (12/108). Therefore, high plasma SOX2OT level predicted GDM (Figure 3).
Chi-Squared Test or Fisher Exact Test Analysis of the Associations of SOX2OT Expression on the Day of Admission and Adverse Events
In this analysis, the 52 GDM patients were divided into high and low SOX2OT level groups (n=26) with the median plasma SOX2OT expression level on the day of admission as the cutoff value. The incidence of adverse events, including miscarriage, premature delivery, intrauterine distress, intrauterine death, intrauterine infection, fetal malformation, macrosomia, and hypertension, in the high and low SOX2OT groups was compared. The results revealed that the incidences of miscarriage, premature delivery, intrauterine distress, intrauterine death, intrauterine infection, fetal malformation, macrosomia, and hypertension were significantly higher in the high SOX2OT group than in the low SOX2OT group (Table 2).
 |
Table 2 Chi-Squared Test or Fisher Exact Test Analysis of the Associations of SOX2OT Expression on the Day of Admission and Adverse Events |
Discussion
The present study explored the involvement of SOX2OT in GDM. We found that the altered plasma SOX2OT in GDM may serve as a promising prognostic and diagnostic biomarker for GDM.
The role of SOX2OT has been reported in multiple human diseases, especially cancers. For instance, SOX2OT regulates SOX2 expression to increase the stemness of bladder cancer cells.15 In gastric cancer, SOX2OT was reported to upregulate AKT2 by sponging miR-194-5p to promote gastric cancer progression.16 In a recent study, SOX2OT was reported to prevent podocytes from high glucose-induced injury by regulating the axis of miR-9/SIRT1 to induce autophagy.14 However, the role of SOX2OT in diabetes and its complications has not been reported. In the present study, we showed that SOX2OT expression level on the day of admission was significantly higher in pregnant women who developed GDM during the follow-up. High SOX2OT expression level on the day of admission effectively separated GDM patients from the healthy controls. Therefore, detecting plasma SOX2OT levels may predict the occurrence of GDM. Based on the detection outcomes, therapeutic approaches could be applied to prevent the occurrence of GDM and its adverse events.
In this study, altered SOX2OT expression not only predicted the occurrence of GDM but also showed prediction values in the occurrence of adverse events in patients who developed GDM. Therefore, in clinical practices, more attention should be paid to patients with high SOX2OT levels to present consequent adverse events. This study only explored the clinical values of SOX2OT for GDM. The role of SOX2OT in the initiation and progression of GDM remains to be further analyzed.
Previous studies have developed different biomarkers for GDM, such as circulating RNAs, DNA methylation, and single-nucleotide polymorphisms. However, these biomarkers are frequently affected by sample type, sample size, detection method, and gestational age.17 The predictive value of SOX2OT for GDM should be tested in different populations with sample sizes and gestational ages. In addition, future studies may consider the combination of different biomarkers to improve the prediction accuracy. Moreover, this study is limited by the lack of the functional characterization of SOX2OT in GDM. Future studies are needed to explore the role of SOX2OT in the initiation and progression of GDM.
In conclusion, SOX2OT was upregulated in GDM. SOX2OT upregulation predicted the occurrence of GDM and its adverse events.
Abbreviation
GDM, Gestational diabetes mellitus.
Data Sharing Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Chongqing General Hospital, University of Chinese Academy of Sciences. All procedures were conducted following the guidelines of this hospital. This study complies with the Declaration of Helsinki. Written consent for participation was obtained from each patient.
Acknowledgments
The authors would like to express our gratitude to those who have critically reviewed this manuscript and those who give us help during this experiment.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47. doi:10.1038/s41572-019-0098-8
2. Chiefari E, Arcidiacono B, Foti D, Brunetti A. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest. 2017;40(9):899–909. doi:10.1007/s40618-016-0607-5
3. Ruiz-Palacios M, Ruiz-Alcaraz AJ, Sanchez-Campillo M, Larqué E. Role of insulin in placental transport of nutrients in gestational diabetes mellitus. Ann Nutr Metab. 2017;70(1):16–25. doi:10.1159/000455904
4. He Z, Xie H, Liang S, et al. Influence of different diagnostic criteria on gestational diabetes mellitus incidence and medical expenditures in China. J Diabetes Investig. 2019;10(5):1347–1357. doi:10.1111/jdi.13008
5. Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018;29(11):743–754. doi:10.1016/j.tem.2018.09.004
6. Immanuel J, Simmons D. Screening and treatment for early-onset gestational diabetes mellitus: a systematic review and meta-analysis. Curr Diab Rep. 2017;17(11):115. doi:10.1007/s11892-017-0943-7
7. Egan AM, Bogdanet D, Griffin TP, et al. A core outcome set for studies of gestational diabetes mellitus prevention and treatment. Diabetologia. 2020;63(6):1120–1127. doi:10.1007/s00125-020-05123-6
8. Yoffe L, Polsky A, Gilam A, et al. Early diagnosis of gestational diabetes mellitus using circulating microRNAs. Eur J Endocrinol. 2019;181(5):565–577. doi:10.1530/EJE-19-0206
9. Feghali MN, Abebe KZ, Comer DM, Caritis S, Catov JM, Scifres CM. Pregnancy outcomes in women with an early diagnosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2018;138:177–186. doi:10.1016/j.diabres.2018.02.004
10. Rodrigo N, Glastras SJ. The emerging role of biomarkers in the diagnosis of gestational diabetes mellitus. J Clin Med. 2018;7(6):120. doi:10.3390/jcm7060120
11. Alyas S, Roohi N, Ashraf S, Ilyas S, Ilyas A. Early pregnancy biochemical markers of placentation for screening of gestational diabetes mellitus (GDM). Diabetes Metab Syndr. 2019;13(4):2353–2356. doi:10.1016/j.dsx.2019.06.006
12. Lu J, Wu J, Zhao Z, Wang J, Chen Z. Circulating LncRNA serve as fingerprint for gestational diabetes mellitus associated with risk of macrosomia. Cell Physiol Biochem. 2018;48(3):1012–1018. doi:10.1159/000491969
13. Ge J, Huang M, Zhou Y, et al. Effects of different temperatures on seawater acclimation in rainbow trout Oncorhynchus mykiss: osmoregulation and branchial phospholipid fatty acid composition. J Comp Physiol B. 2021;191:669–679. doi:10.1007/s00360-021-01363-z
14. Zhang Y, Chang B, Zhang J, Wu X. LncRNA SOX2OT alleviates the high glucose-induced podocytes injury through autophagy induction by the miR-9/SIRT1 axis. Exp Mol Pathol. 2019;110:104283. doi:10.1016/j.yexmp.2019.104283
15. Zhan Y, Chen Z, He S, et al. Long non-coding RNA SOX2OT promotes the stemness phenotype of bladder cancer cells by modulating SOX2. Mol Cancer. 2020;19(1):25. doi:10.1186/s12943-020-1143-7
16. Qu F, Cao P. Long noncoding RNA SOX2OT contributes to gastric cancer progression by sponging miR-194-5p from AKT2. Exp Cell Res. 2018;369(2):187–196. doi:10.1016/j.yexcr.2018.05.017
17. Dias S, Pheiffer C, Abrahams Y, et al. Molecular biomarkers for gestational diabetes mellitus. Int J Mol Sci. 2018;19(10):2926. doi:10.3390/ijms19102926
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.