Back to Journals » Cancer Management and Research » Volume 11
LncRNA PCAT-1 Promoted ESCC Progression via Regulating ANXA10 Expression by Sponging miR-508-3p
Authors Zang B, Zhao J, Chen C
Received 9 October 2019
Accepted for publication 5 December 2019
Published 30 December 2019 Volume 2019:11 Pages 10841—10849
DOI https://doi.org/10.2147/CMAR.S233983
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Bao Zang, Jianqiang Zhao, Chen Chen
Department of Thoracic Surgery, The Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University, Huai’an 223300, Jiangsu, People’s Republic of China
Correspondence: Bao Zang
Department of Thoracic Surgery, The Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University, Huai’an 223300, Jiangsu, People’s Republic of China
Tel +86-13861554118
Email [email protected]
Background: Given the poor prognosis of metastatic esophageal squamous cell carcinoma (ESCC) patients, molecular mechanisms underlying the progression and metastasis of ESCC are highly desired in the scientific community. Prostate cancer associated transcript-1 (PCAT-1) is a lncRNA up-regulated in major types of cancers and is associated with the poor prognosis of cancer patients. This study aimed to understand the expression and role of PCAT-1 in the progression and metastasis of ESCC and to identify the potential lncRNA-miRNA interactions and signaling pathways underlying the mechanisms of action of PCAT-1 in ESCC.
Materials and Methods: Gene expression levels were determined by qRT-PCR; protein levels were determined by Western blot assay; cell proliferation, invasion and migration were determined by CCK-8, Transwell invasion and wound healing assays, respectively; in vivo tumor growth was evaluated by xenograft nude mice model.
Results: Our data showed the up-regulation of PCAT-1 in different human ESCC cell lines and in clinical ESCC tissues. Knockdown of PCAT-1 in ESCC cells significantly inhibited the proliferation, invasion and migration of the cancer cells. Moreover, we showed the interactions between PCAT-1 and miR-508-3p and demonstrated that PCAT-1 was able to repress miR-508-3p expression in ESCC cells via acting as a competing endogenous RNA. Besides, Annexin A10 (ANXA10) was identified to be the downstream target of the PCAT-1 and miR-508-3p interactions.
Conclusion: This study demonstrated the functional role of PCAT-1 in promoting the proliferation, invasion and migration of ESCC cells. We also identified a PCAT-1/miR-508-3p/ANXA10 axis in mediating the promoting role of PCAT-1 in the progression of ESCC. The findings provide experimental evidence to support lncRNA PCAT-1 as a potential therapeutic target of ESCC.
Keywords: esophageal squamous cell carcinoma, PCAT-1, ANXA10, miR-508-3p, progression, metastasis
Introduction
Esophageal squamous cell carcinoma (ESCC) occurs most often in the upper and middle portions of the esophagus and is one of the two main types of esophageal cancer.1 Based on the cancer statistics in China, the new cases of esophageal cancer were around 477,900 and 375,000 esophageal cancer-related deaths were estimated in 2015.1 The main treatment options for ESCC include surgery, chemotherapy and radiation therapy. However, an obstacle to the successful therapy of ESCC in clinics is the metastasis of the cancer. As a matter of fact, approximately 50% of ESCC patients present with metastases to distant lymph nodes or organs at initial diagnosis.2 ESCC often spreads to the liver, lung, bone and brain, and the five-year survival rate of metastatic ESCC patients is less than 5%.3 Therefore, molecular mechanisms underlying the progression and metastasis of ESCC are worthy of investigation in the scientific community.
Recently, long non-coding RNAs (lncRNAs), a group of non-protein coding transcripts longer than 200 nucleotides, have gained widespread attention as potentially new and crucial regulators of various biological processes.4 Accumulating evidences have showed that lncRNAs play active and functional roles in the development and progression of ESCC, via their regulatory effects on the proliferation, apoptosis, stemness, chemoresistance, invasion and metastasis of the cancer cells.5 For example, knockdown of AK001796 led to an increase expression levels of p53 and p21, suggesting the role of AK001796 in regulating cell proliferation and cell cycle in ESCC cells.6 Prostate cancer associated transcript-1 (PCAT-1), a lncRNA with a length of ~1900 nt, was originally identified in prostate cancer by large-scale RNA-Seq analyses in 2011.7 It has been reported that PCAT-1 is up-regulated in major types of cancers and is able to promote the proliferation of cancer cells, such as hepatocellular cancer, bladder cancer, non-small cell lung cancer, breast cancer, gastric cancer, colorectal cancer and glioblastoma.8–11 In ESCC, PCAT-1 was also found to be up-regulated by Shi et al,12 however, the functional role of PCAT-1 in ESCC progression and its underlying mechanisms have not been fully understood yet.
In this regard, the current study aims to investigate the expression and functional role of PCAT-1 in the progression and metastasis of ESCC and to identify the potential PCAT-1-miRNA interactions with miR-508-3p.
Materials and Methods
Cell Culture and Clinical Samples
Human esophageal squamous epithelial cells HET1A and three ESCC cell lines including EC109, KYSE150 and KYSE450 were obtained from the type Culture Collection of Chinese Academy of Sciences (Shanghai, China). The cells were maintained in BEBM (HET1A), Ham’s F12 (KYSE150) or RPMI-1640 (EC109 and KYSE450) medium supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, USA) at 37°C.
Fifty pairs of human ESCC samples and normal adjacent tissues were collected at The Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University between July 2016 and June 2018. Immediately after collection, the tissues were frozen stored in liquid nitrogen. Both ESCC samples and normal adjacent tissues were confirmed by pathological examination. This study was approved by the Institutional Ethics Committee of the hospital, and the written informed consent was collected before sample collection.
MiRNAs, Small Interfering RNAs (siRNAs), Plasmids and Cell Transfections
The miRNAs including miR-508 mimics (miR mimics), inhibitors (miR inhibitors) and the corresponding negative controls (NCs; mimics NC and inhibitors NC), the siRNAs for PCAT1 and scrambled siRNA were purchased from Ribobio (Guangzhou, China). The vectors for overexpressing PCAT-1 (pcDNA3.1-PCAT-1) or annexin A10 (ANXA10; pcDNA3.1-ANXA10) were purchased from GenePharma (Shanghai, China). Cells transfections with miRNAs, siRNAs or plasmids were performed using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, USA).
Cell Proliferation Assay
Cell Counting Kit-8 (CCK-8) assay (Sigma-Aldrich) was used to measure the proliferation of the ESCC cells. Briefly, 100 μL cell suspensions (5 x 103 cells/mL) were added into a 96-well plate and incubated for 24, 48, 72 and 96 h respectively. Afterwards, 10 μL CCK-8 reagent was added to each well and incubated at 37°C for 2 h. Optical density was measured at 450 nm using a microplate spectrophotometer (Thermo Fisher Scientific).
Cell Migration and Invasion Assay
Cell invasion assay was performed using transwell inserts from Corning (Corning, Corning, USA). The membrane (8 μm pore size) was coated with 50 μL Matrigel before the assay (30 μg/well; BD Biosciences, San Jose, USA). To start the assay, ESCC cells (1 x 106 cells/mL) were resuspended in 100 μL FBS-free medium and seeded into the upper chambers of transwell inserts in a 24-well plate. The lower chambers were filled with 600 μL medium with 10% FBS. After incubation at 37°C for 24 h, invaded cells on the lower surface of the transwell membrane were fixed and stained with crystal violet. The images of the cells were recorded using a Nikon microscope with a digital camera.
Cell migration was carried out using wound healing assay. Briefly, cells at density of 1 x 106 cells/mL were seeded into a 6-well plate and allowed to grow until 80% confluence as a monolayer. A 200 μL pipette tip was used to scratch the cell monolayer across the center of the well, and detached cells were removed by gently washing the wells twice with culture medium. After incubation for additional 48 h, the cells were washed and fixed. The images of the cells were recorded using a Nikon microscope equipped with a digital camera.
Luciferase Reporter Assay
The binding site in the PCAT-1 gene and ANXA10 3ʹ untranslated region (3ʹUTR) was amplified and cloned downstream of the luciferase gene in the pGL3 vector (Promega, Madison, WI, USA), yielding the wide-type plasmid (PCAT-1-WT and ANXA10 3ʹUTR-WT). The plasmid containing the mutant seed region (PCAT-1-MUT and ANXA10 3ʹUTR-MUT) was obtained through site-specific mutagenesis on the base of the plasmid PCAT-1-WT. KYSE150 cells were co-transfected with respective miRNAs and luciferase reporter vectors using Lipofectamine 2000. Luciferase assay was performed using the Dual-Luciferase Reporter Assay System according to the manufacturer’s instructions (Promega).
Reverse Transcription and Quantitative Real-Time PCR
The total RNA was isolated using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. The same amount of total RNA (1 μg) was used to synthesize cDNA by reverse transcription using the PrimeScript RT Master Mix Kit (TaKaRa-Bio, Dalian, China). Real-time PCR was performed using an ABI 7900 system (ABI) at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The 2−ΔΔCt method was used for relative quantification of the data and all the values were normalized to U6 or GAPDH, as appropriate.
Western Blot Analysis
To relatively quantify the expression of ANXA10 protein, equal amounts (20 μg) of proteins were resolved by 10% SDS-PAGE and transferred onto PVDF membranes (Bio-Rad, CA, USA). After blocking in 5% non-fat milk for 1 hr, the PVDF membranes were incubated with primary antibody of ANXA10 (Cell Signaling Technology, Danvers, USA) diluted in 5% non-fat milk at 4°C overnight. Afterwards, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology) at room temperature for 2 h. The chemiluminescent signals were developed and detected by the ChemiDoc XRS gel documentation system (Bio-Rad). GAPDH was used as loading control.
Tumor Xenograft Model in Nude Mice
SCID mice (6-week old) were obtained from Beijing Vital River Laboratory Animal Technology Co.,Ltd (Beijing, China). Briefly, xenograft tumors were established in male SCID mice by injection of sh-control and sh-PCAT-1 KYSE150 cells into the right flank of the mice. Tumor volume was monitored every week for 5 weeks and calculated by length×width2/2. By the time of sacrifice, tumors were removed, weighted and stored at −80 °C for further analysis. This study was approved by the Institutional Animal Care and Use Committee of The Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University. The experiments were performed in accordance with the National Guidelines for Experimental Animal Welfare (the Ministry of Science and Technology, China).
Statistical Analysis
Data analysis was performed by using GraphPad Prism 6.0 (GraphPad Software, La Jolla, USA). The significance of difference between groups was estimated by t test or one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. P < 0.05 indicated statistically significant difference. All the assays were performed in three independent experiments.
Results
LncRNA PCAT-1 Promoted ESCC Cell Proliferation, Invasion and Migration
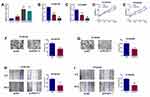
As shown in Figure 1A, all three ESCC cells lines exhibited significantly higher PCAT-1 expression when compared with the normal esophageal squamous epithelial cells. Knockdown of PCAT-1 in KYSE150 and KYSE450 cells was carried out using RNA interference. Figure 1B and C demonstrated the successful knockdown of PCAT-1 in KYSE150 and KYSE450 cells by two different PCAT-1 siRNAs (si-1 and si-2). As si-2 was more effective to down-regulated PCAT-1 expression, si-2 was selected for further in vitro functional assays and was named as si-PCAT1. As shown in Figure 1D and E, after PCAT-1 knockdown, the proliferative rates of these two cells lines were significantly decreased when compared with the siRNA control cells. In addition, transwell invasion assay and wound healing assay showed that both invasion and migration of KYSE150 and KYSE450 cells were inhibited after PCAT-1 knockdown (Figure 1F–I).
PCAT-1 Repressed miR-503-3p Expression via Acting as a ceRNA
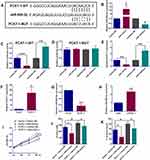
Using StarBase online analysis tool, miR-508-3p was found to potentially bind to PCAT-1 with putative binding sites indicated in Figure 2A. To study the interactions between miR-508-3p and PCAT-1, miR-508-3p mimics and inhibitors were used to manipulate its expression in ESCC cells. In KYSE150 cells, as shown in Figure 2B, miR-508-3p mimics and inhibitors successfully increased and decreased the relative expression level of miR-508-3p, respectively. Dual-luciferase reporter assay demonstrated that the luciferase activity of the reporter containing PCAT-1-WT, rather than PCAT-1-MUT, was negatively correlated with the expression of miR-508-3p in KYSE150 cells (Figure 2C and D). Accordingly, the relative PCAT-1 expression in KYSE150 cells was also found to be negatively correlated with the expression of miR-508-3p (Figure 2E). On the other hand, the relative expression levels of miR-508-3p was down-regulated and up-regulated by overexpression and knockdown of PCAT-1, respectively (Figure 2F–H). Moreover, as shown in Figure 2I, overexpression of PCAT-1 also led to an increase in the proliferation of KYSE150 cells. In the presence of miR-508-3p mimics, the increase in KYSE150 cell proliferation was decreased. Similarly, both invasion and migration of KYSE150 cells were increased by overexpression of PCAT-1, such increases were reversed by miR-508-3p mimics (Figure 2J and K).
PCAT-1 Regulated ESCC Progression via Modulating ANXA10 Expression by Acting as a miR-508-3p Sponge
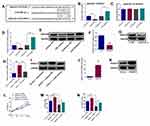
Furthermore, using StarBase online analysis tool, miR-508-3p was also found to potentially bind to ANXA10 3ʹUTR with putative binding sites indicated in Figure 3A. As shown in Figure 3B and C, dual-luciferase reporter assay indicated that the luciferase activity of the reporter containing ANXA10 3ʹUTR-WT, rather than ANXA10 3ʹUTR-MUT, was negatively correlated with the expression of miR-508-3p in KYSE150 cells. Besides, both mRNA and protein expression of ANXA10 were down-regulated and up-regulated by miR-508-3p mimics and inhibitor in KYSE150 cells, respectively (Figure 3D and E). Given the direct relationship between lncRNA PCAT-1 and miR-508-3p, the regulation of ANXA10 by PCAT-1 was also explored. As shown in Figure 3F and G, after knockdown of PCAT-1 in KYSE150 cells, both mRNA and protein expression of ANXA10 were down-regulated. Moreover, overexpression of PCAT-1 led to an increase in the mRNA and protein expression of ANXA10, such increase was also reversed in the presence of miR-508-3p mimics (Figure 3 3H and I).
After transfection with pcDNA3.1-ANXA10, both mRNA and protein expression levels of ANXA10 were up-regulated (Figure 3J and K). More importantly, as shown in Figure 3L, overexpression of ANXA10 also led to an increase in the proliferation of KYSE150 cells, such increase was reversed by PCAT-1 knockdown. In addition, the invasion and migration of KYSE150 cells were also stimulated by ANXA10 overexpression, knockdown of PCAT-1 reversed such effects of ANXA10.
PCAT-1 Promoted in vivo Tumor Growth and Its Up-Regulation in Clinical ESCC Samples
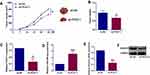
As indicated by the tumor growth curve in Figure 4A, the volume of PCAT-1 knockdown KYSE150 cells xenograft was much lower than that of control KYSE150 cells xenograft. Accordingly, the tumor weight in PCAT-1 knockdown group was also significantly lower than that of control group (Figure 4B). RT-PCR analysis of the tumor tissues confirmed the down-regulation of PCAT-1 in PCAT-1 knockdown xenograft group, in which the relative expression of miR-508-3p was increased (Figure 4C and D). Besides, both mRNA and protein expression levels of ANXA10 were down-regulated in PCAT-1 knockdown KYSE150 cells xenograft, when compared with the control group (Figure 4E and F).
In ESCC tissues, when compared with adjacent normal tissues, the relative expression levels of PCAT-1 and ANXA10 were up-regulated and the expression of miR-508-3p was down-regulated (Figure 5A–C).
Discussion
In this study, we have reported the up-regulation of PCAT-1 in different human ESCC cell lines and, more importantly, in clinical ESCC tissues. PCAT-1 was found to promote the proliferation, invasion and migration of ESCC cells. We also demonstrated that regulation of ANXA10 by PCAT-1 via sponging miR-508-3p represents one important mechanism by which PCAT-1 exhibits its promoting role in the progression of ESCC.
Like the majority of human cancer types, the expression of PCAT-1 was also up-regulated in ESCC. Our findings were consistent with a previous report by Shi et al, who also found an up-regulation of PCAT-1 in ESCC clinical samples.12 However, the underlying mechanisms regarding the promoting effects of PCAT-1 remain elusive. In this study, we provided the experimental evidence showing that PCAT-1 promoted the proliferation, invasion and migration of ESCC cells. As a matter of fact, PCAT-1 has also been reported to promote the cell proliferation, invasion and migration of prostate cancer, liver cancer, colorectal cancer and gastric cancer, etc.13 Interestingly, knockdown of PCAT-1 led to G0/G1 phase cell cycle arrest in colorectal cancer cells HT-29 and Caco-2.11 The similar findings were also reported in osteosarcoma cells, where knockdown of PCAT-1 in U2OS cells caused an increase in the cell population at G0/G1 phase and a decrease in cell population at S phase.14 In this regard, whether the cell cycle distribution in ESCC cells is changed or not after knockdown of PCAT-1 is also worthy of investigation.
Since sponging miRNA is one of the mechanisms by which lncRNAs exhibit their regulatory effects on the expression of target genes, we also identified the interactions between PCAT-1 and miR-508-3p and showed that PCAT-1 repressed miR-508-3p expression via acting as a ceRNA. There are a number of studies showing that the ceRNA regulatory network represents a major mechanism of PCAT in regulating the development and progression of cancer. In prostate cancer, for example, PCAT-1 was found to function as a ceRNA of miR-145-5p to modulate the expression of fascin homolog 1, such PCAT-1/miR-145-5p/fascin homolog 1 regulatory axis contributes to the functional role of PCAT-1 in the progression of prostate cancer.15 Besides, PCAT-1 also acted as a ceRNA of miR-122 in extrahepatic cholangiocarcinoma, by regulating miR-122, PCAT-1 activated the Wnt/β-catenin-signaling pathways and promoted the proliferation of extrahepatic cholangiocarcinoma.16 In a different study in hepatocellular carcinoma, PCAT-1 was found to promote the invasion and metastasis by binding to miR-129-5p and subsequent inhibition on the expression of high mobility group box1.17 Our findings, together with others mentioned above, demonstrated the interactions between PCAT-1 and a number of miRNAs and the involvement of such interactions in mediating the promoting role of PCAT-1 in the development and progression of cancer.
Additionally, we also identified ANXA10 as a downstream target of the PCAT-1 and miR-508-3p interactions and demonstrated its promoting role in mediating the proliferation, invasion and migration of ESCC cells. ANXA10 was firstly reported in hepatocellular carcinoma and its down-regulation was found to correlate with the vascular invasion, early recurrence and poor prognosis of this type of cancer.18 The down-regulation of ANXA10 also correlated with the poor prognosis of prostate cancer, bladder cancer and gastric cancer.19 Based on our findings, ANXA10 exhibits the similar functions in ESCC and colorectal cancer in terms of the promoting role in cancer progression. A number of studies also suggested that the alteration in the expression of ANXA10 is also related to the development of drug resistance in cancer cells.19 Drug resistance in cancer cells leads to a decreased therapeutic effects of anticancer drugs and is also an obstacle to the successful therapy of cancer.20 The mechanisms of drug resistance include the overexpression of drug efflux pumps, evasion of apoptosis, altered drug targets, etc.21,22 Given the up-regulation of ANXA10 in ESCC cells and clinical samples, it would be interesting to examine the responses of ESCC cells to chemotherapeutic agents and changes in the regulatory mechanisms of drug resistance. Such studies may establish the possible relationship between PCAT-1/miR-508-3p/ANXA10 axis and the development of drug resistance in cancer cells.
In summary, this study identified a PCAT-1/miR-508-3p/ANXA10 axis in mediating the promoting role of PCAT-1 in the progression of ESCC. The findings provide experimental evidence to support lncRNA PCAT-1 as a potential therapeutic target of ESCC.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgements
This study was supported by The Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–2252. doi:10.1056/NEJMra035010
3. Wu SG, Zhang WW, He ZY, Sun JY, Chen YX, Guo L. Sites of metastasis and overall survival in esophageal cancer: a population-based study. Cancer Manag Res. 2017;9:781–788. doi:10.2147/CMAR.S150350
4. Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193(3):651–669. doi:10.1534/genetics.112.146704
5. Su M, Xiao Y, Ma J, et al. Long non-coding RNAs in esophageal cancer: molecular mechanisms, functions, and potential applications. J Hematol Oncol. 2018;11(1):118. doi:10.1186/s13045-018-0663-8
6. Liu B, Pan CF, Yao GL, Wei K, Xia Y, Chen YJ. The long non-coding RNA AK001796 contributes to tumor growth via regulating expression of p53 in esophageal squamous cell carcinoma. Cancer Cell Int. 2018;18:38. doi:10.1186/s12935-018-0537-8
7. Prensner JR, Iyer MK, Balbin OA, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29(8):742–749. doi:10.1038/nbt.1914
8. Liu L, Liu Y, Zhuang C, et al. Inducing cell growth arrest and apoptosis by silencing long non-coding RNA PCAT-1 in human bladder cancer. Tumour Biol. 2015;36(10):7685–7689. doi:10.1007/s13277-015-3490-3
9. Yan TH, Yang H, Jiang JH, et al. Prognostic significance of long non-coding RNA PCAT-1 expression in human hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8(4):4126–4131.
10. Zhao B, Hou X, Zhan H. Long non-coding RNA PCAT-1 over-expression promotes proliferation and metastasis in non-small cell lung cancer cells. Int J Clin Exp Med. 2015;8(10):18482–18487.
11. Qiao L, Liu X, Tang Y, Zhao Z, Zhang J, Feng Y. Down regulation of the long non-coding RNA PCAT-1 induced growth arrest and apoptosis of colorectal cancer cells. Life Sci. 2017;188:37–44. doi:10.1016/j.lfs.2017.08.024
12. Shi WH, Wu QQ, Li SQ, et al. Upregulation of the long noncoding RNA PCAT-1 correlates with advanced clinical stage and poor prognosis in esophageal squamous carcinoma. Tumour Biol. 2015;36(4):2501–2507. doi:10.1007/s13277-014-2863-3
13. Yang Z, Zhao S, Zhou X, Zhao H, Jiang X. PCAT-1: a pivotal oncogenic long non-coding RNA in human cancers. Biomed Pharmacother. 2019;110:493–499. doi:10.1016/j.biopha.2018.12.014
14. Zhang X, Zhang Y, Mao Y, Ma X. The lncRNA PCAT1 is correlated with poor prognosis and promotes cell proliferation, invasion, migration and EMT in osteosarcoma. Onco Targets Ther. 2018;11:629–638. doi:10.2147/OTT.S152063
15. Xu W, Chang J, Du X, Hou J. Long non-coding RNA PCAT-1 contributes to tumorigenesis by regulating FSCN1 via miR-145-5p in prostate cancer. Biomed Pharmacother. 2017;95:1112–1118. doi:10.1016/j.biopha.2017.09.019
16. Zhang F, Wan M, Xu Y, et al. Long noncoding RNA PCAT1 regulates extrahepatic cholangiocarcinoma progression via the Wnt/beta-catenin-signaling pathway. Biomed Pharmacother. 2017;94:55–62. doi:10.1016/j.biopha.2017.07.025
17. Zhang D, Cao J, Zhong Q, et al. Long noncoding RNA PCAT-1 promotes invasion and metastasis via the miR-129-5p-HMGB1 signaling pathway in hepatocellular carcinoma. Biomed Pharmacother. 2017;95:1187–1193. doi:10.1016/j.biopha.2017.09.045
18. Liu SH, Lin CY, Peng SY, et al. Down-regulation of annexin A10 in hepatocellular carcinoma is associated with vascular invasion, early recurrence, and poor prognosis in synergy with p53 mutation. Am J Pathol. 2002;160(5):1831–1837. doi:10.1016/S0002-9440(10)61129-7
19. Munksgaard PP, Mansilla F, Brems Eskildsen AS, et al. Low ANXA10 expression is associated with disease aggressiveness in bladder cancer. Br J Cancer. 2011;105(9):1379–1387. doi:10.1038/bjc.2011.404
20. Hu T, Li Z, Gao CY, Cho CH. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J Gastroenterol. 2016;22(30):6876–6889. doi:10.3748/wjg.v22.i30.6876
21. Hu T, To KK, Wang L, et al. Reversal of P-glycoprotein (P-gp) mediated multidrug resistance in colon cancer cells by cryptotanshinone and dihydrotanshinone of Salvia miltiorrhiza. Phytomedicine. 2014;21(11):1264–1272. doi:10.1016/j.phymed.2014.06.013
22. Hu T, Wang L, Zhang L, et al. Sensitivity of apoptosis-resistant colon cancer cells to tanshinones is mediated by autophagic cell death and p53-independent cytotoxicity. Phytomedicine. 2015;22(5):536–544. doi:10.1016/j.phymed.2015.03.010
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.