Back to Journals » Cancer Management and Research » Volume 12
LncRNA NR2F2-AS1 Silencing Induces Cell Cycle Arrest in G0/G1 Phase via Downregulating Cyclin D1 in Colorectal Cancer
Authors Liu J, Qian J, Mo Q, Tang L, Xu Q
Received 4 July 2019
Accepted for publication 27 January 2020
Published 11 March 2020 Volume 2020:12 Pages 1835—1843
DOI https://doi.org/10.2147/CMAR.S221996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Jianfeng Liu, Jun Qian, Qi Mo, Liming Tang, Qiang Xu
Department of Gastrointestinal Surgery, Changzhou No.2 People’s Hospital of Nanjing Medical University, Changzhou City, Jiangsu Province 213003, People’s Republic of China
Correspondence: Qiang Xu
Department of Gastrointestinal Surgery, Changzhou No.2 People’s Hospital of Nanjing Medical University, No. 29 Xinglong Road, Changzhou City, Jiangsu Province 213003, People’s Republic of China
Tel +86-519-88104931
Email [email protected]
Purpose: LncRNA NR2F2-AS1 has been characterized as an oncogenic lncRNA in non-small cell lung cancer. This study aims to explore the role of NR2F2-AS1 in colorectal cancer (CRC).
Methods: All CRC patients (n=60, 35 males and 25 females, 40 to 68 years old) in this study were enrolled in Changzhou No.2 People’s Hospital of Nanjing Medical University during the time period between July 2011 and December 2013. Tumor samples, CRC cells, vectors, transient transfections, RT-qPCR, western-blotting, as well as measurements of cell cycle, apoptosis and cell proliferation assay were carried out during the research.
Results: Our data showed that NR2F2-AS1 was upregulated in CRC and CRC patients with high levels of NR2F2-AS1 showed a low overall survival rate. Cyclin D1 was also upregulated in CRC and a positive correlation was found between Cyclin D1 and NR2F2-AS1. In CRC cells, NR2F2-AS1 siRNA silencing mediated the downregulation of Cyclin D1 and G0/G1 arrest, while Cyclin D1 overexpression rescued G0/G1 arrest caused by NR2F2-AS1 siRNA silencing.
Conclusion: Our results indicated that NR2F2-AS1 silencing mediates the downregulation of Cyclin D1 to induce G0/G1 arrest in colorectal cancer.
Keywords: colorectal cancer, lncRNA NR2F2-AS1, Cyclin D1, G0/G1 arrest
Introduction
According to the latest cancer statistics data (2019), colorectal cancer (CRC) is the third most common type of malignancy among all cancers.1 Annually, CRC affect more than 1.2 million new cases worldwide.2 The mortality of CRC in China is on the rise dramatically in recent years, with an incidence of 370,000 new cases and 180,000 deaths in 2014 alone.3 Most CRC patients are not candidate for surgical resection, which is the only radical treatment, due to the late diagnosis.4 The existence of tumor metastasis by the time of initial diagnosis is the major cause of the high mortality rate.5 Moreover, early diagnosis of CRC is still challengeable due to the lack of effective markers. Therefore, novel therapeutic targets are still needed to improve the survival of CRC patients, especially the ones diagnosed at advanced stages.
Although initially thought to be transcriptional noise,6 long (>200 nt) non-coding RNAs (LncRNAs) are identified that participate in diverse biological processes by regulating gene expression at translational levels, post-transcriptional levels or through epigenetic pathways recently.7 The aberrant expression of certain lncRNAs may confer the dysregulation of certain tumor suppressors or oncogenes.8,9 Therefore, regulation of lncRNA expression may contribute to the treatment and prevention of cancer.10 LncRNA NR2F2-AS1, also named COUP-TFII, is located at the chromosome locus 15q26.2, transcribes a 1161nt transcript, and has been reported to be involved in the occurrence and development of tumors. Recently lncRNA NR2F2-AS1 had been found to be mediated by the downregulation of tumor-suppressive miR-320b in non-small cell lung cancer.11 So far, the function of lncRNA NR2F2-AS1 in CRC has not been explored. This study therefore explored the role of NR2F2-AS1 and relevant mechanisms of its regulatory network in CRC.
Materials and Methods
Research Subjects
Changzhou No.2 People’s Hospital of Nanjing Medical University admitted 102 patients with CRC during the time period between July 2011 and December 2013. This study selected 60 cases from those patients. Inclusion criteria: 1) new CRC cases; 2) patients willing to join and completed a 5-year follow-up. Exclusion criteria: 1) recurrent CRC; 2) any other clinical disorders were observed; 3) previous history of malignancies; 4) any therapies were initiated before admission; 5) patients died of other causes or were lost during follow-up. Based on the staging criteria proposed by AJCC, the 60 patients were classified into 12, 14, 17 and 17 cases at stages I–IV, respectively. Written informed consent was obtained from each patient. Ethics Committee of Changzhou No.2 People’s Hospital of Nanjing Medical University approved this study. Experiments using human materials were performed in strict accordance with declaration of Helsinki.
Tissues
All the 60 CRC patients were diagnosed through histopathological biopsy and CRC (cancer) and non-cancer tissues (0.06–0.09g) were obtained from each patient. At least 3 experienced pathologists confirmed all tissue specimens.
Follow-Up
The 60 CRC patients were followed up for 5 years after admission. Follow-up was administrated through telephone or by outpatient visit. Patients who were lost during follow-up were excluded. Their survival conditions were recorded in detail.
Cells and Transient Transfections
Human CRC cell lines RKO and CR4 were used in this study. RKO cells were from ATCC (CRL-2577, USA) and CR4 cells were from Sigma-Aldrich (USA). Eagle’s Minimum Essential Medium (10% FBS) was used as cell culture medium. Cell culture conditions were 37°C and 5% CO2.
NR2F2-AS1 siRNA and negative control siRNA were from GenePharma (Shanghai, China). Cyclin D1 expression vector was constructed using pcDNA3 vector by Sangon (Shanghai, China). RKO and CR4 cells were collected at confluence of 70–90%. Lipofectamine 2000 (Thermo Fisher Scientific) was used to transfect 10 nM NR2F2-AS1 siRNA, 10 nM negative control siRNA (negative control, NC), or 35 nM Cyclin D1 expression vector, or 35 nM empty pcDNA3 vector (NC) into 105 cells. Control (C) group included cells without transfections. All subsequent experiments were carried out using cells collected at 24 hrs after transfections.
RT-qPCR
RKO and CR4 cells were collected at 24 hrs after transfections. The tissues were collected in frozen and fresh conditions and transferred by nitrogen tank from surgery room to the laboratory directly. Liquid nitrogen was added and the samples were ground separately before RNA extraction. RNAzol reagent (Sigma-Aldrich, USA) was used to extract total RNAs from tissues (0.05g tissue per 1 mL RNAzol reagent) and cells (105 cells per 1 mL RNAzol reagent). All RNA samples were digested with DNase I at 37°C for 2 h. After that, AMV Reverse Transcriptase (Promega Corporation, USA) and SYBR® Green master mix (Bio-Rad, USA) were used to carry out reverse transcriptions and prepare qPCR mixtures. With 18S rRNA and GAPDH as endogenous controls, NR2F2-AS1 and Cyclin D1 mRNA were detected. The following were used for LncRNA NR2F2-AS1 primer: Forward primer: 5ʹ TCAGCCGGAAAACTACAAGCTC 3ʹ and Reverse primer: 5ʹ TCTTCGTGTA -GCTGTTCCACC3ʹ; Cyclin D1 primer: Forward primer: 5ʹ AGGAACAGAAG-TGCGAGGAGG 3ʹ and Reverse primer: 5ʹ GGATGGAGTTGTCGGTGTAGATG 3ʹ; GAPDH primer: Forward primer: 5ʹ GCACCGTCAAGGCTGAGAAC 3ʹ and Reverse primer: 5ʹ TGGTGAAGACGCCA GTGGA 3ʹ.
Analysis of Cell Cycle
RKO and CR4 cells (collected at 24 hrs post-transcriptions) were subjected to trypsinization. Following washing with pre-cold PBS, 75% ethanol was used to incubate with the cells at 4°C for 4 hrs. Cells were then washed with pre-cold PBS, followed by BD Pharmingen™ PI/RNase staining at 25°C in dark for 40 mins. Finally, cells were separated using FACSCantoTM Flow Cytometer (BD Biosciences, USA). 105 events were counted for each sample.
Cell Proliferation Assay
RKO and CR4 cells (collected at 24 hrs post-transcriptions) were mixed with Eagle’s Minimum Essential Medium (10% FBS) with a ratio of 3×104 cells per 1 mL cell culture medium to prepare single-cell suspensions. Under the conditions of 37°C and 5% CO2, cells were cultivated in 96-well plates (100 µL cell suspension per well) and 10 µL CCK-8 solution (Sigma,-Aldrich, USA) was added into each well at 2 hrs before the end of cell culture. Cells were collected every 24 hrs for 4 times. Following the addition of 10 µL DMSO, OD values (450 nm) were measured.
Western Blot
RKO and CR4 cells (collected at 24 hrs post-transcriptions) were mixed with RIPA solution (Thermo Fisher Scientific) with a ratio of 105 cells per 1 mL RIPA solution to extract total protein. Following denaturing, SDS-PAGE gel (10%) was used to perform electrophoresis. Following gel transfer (PVDF membranes) and blocking (25 mins at 25°C in 5% non-fat milk), membranes were incubated with Cyclin D1 antibody (ab226977, 1:800, Abcam) and GAPDH (ab9485, 1:800, Abcam) rabbit polyclonal primary antibodies (4°C overnight), followed by incubation with IgG-HRP (1:1500, MBS435036, MyBioSource) goat anti rabbit secondary antibody (2 hrs at 25°C). Signals were developed using ECL (Sigma-Aldrich, USA) and processed using Image J v1.46 software.
Statistical Analysis
All data presented in this paper were mean values from at least 3 biological replicates. Differences between two types of tissues from 60 CRC patients were explored using paired t-test. Differences among different cell transfection groups were analyzed by performing one-way ANOVA and Tukey’s test. Correlations were analyzed by performing linear regression. The 60 CRC patients were divided into low (n=28) and high (n=32) NR2F2-AS1 level groups. Based on survival data, survival curves were plotted using K-M method and were compared by Log rank test. P<0.05 was statistically significant. The Cox regression model was used to perform multivariate analysis.
Results
NR2F2-AS1 Was Upregulated and Correlated with the Clinicopathological Features in CRC
In order to explore the role of lncRNA NR2F2-AS1 in CRC carcinogenesis, the expression of NR2F2-AS1 in two types of tissues collected from CRC patients was detected by RT-qPCR. Expression data were compared between two types of tissues by performing paired t-test. The data showed that NR2F2-AS1 expression levels were significantly higher in CRC tissues comparing to non-cancer tissues (Figure 1A, p<0.05). Based on this finding, we carried out the statistical analyses in the field of age, gender, tumor stage (according to the 7th TNM staging system24) to further investigate the relationship between lncRNA NR2F2-AS1 expression and clinicopathological features in patients suffering from CRC. The results obviously demonstrated that the level of lncRNA NR2F2-AS1 was up-regulated in patients with advanced TNM stage and positive lymphatic metastasis (P < 0.05, Table 1).
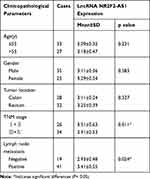 |
Table 1 Clinicopathological Characteristics and LncRNA NR2F2-AS1 Expression Levels in Colorectal Cancer (CRC) Patients |
LncRNA NR2F2-AS1 Was a Poor Prognostic Factor for Patients with CRC
Kaplan-Meier survival analysis revealed a close correlation between lncRNA NR2F2-AS1 expression and overall survival (OS). The data showed that patients in high (n=32) NR2F2-AS1 level group had significantly lower overall survival rate compared to patients in low (n=28) NR2F2-AS1 level group (Figure 1B; P= 0.0237). The univariate analysis of prognostic markers of CRC was summarized in Table 2: lymph node metastasis (P=0.009, hazards ratio (HR): 3.931, 95% confidence interval (CI): 0.981–6.881). NR2F2-AS1 expression (P=0.015, HR: 2.758, 95% CI: 0.764–7.099) and were prognostic factors for OS by univariate analysis. Factors of univariate analysis were adopted in multivariate Cox proportional hazards analysis. The data revealed that NR2F2-AS1 expression and lymph node status were independent prognostic factors that could affect the OS of CRC patients (Table 2).
 |
Table 2 Univariate and Multivariate Analysis for OS |
Cyclin D1 Was Positively Correlated with NR2F2-AS1 in CRC
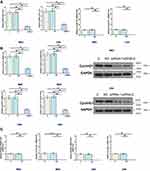
Cyclin D1 mRNA in two types of tissues collected from CRC patients was also detected by RT-qPCR. Expression data were compared between two types of tissues by performing paired t-test. It was observed that expression levels of Cyclin D1 mRNA were significantly higher in CRC tissues comparing to non-cancer tissues (Figure 2A, p<0.05). Correlations between Cyclin D1 mRNA and NR2F2-AS1 were analyzed by performing linear regression. It was found that Cyclin D1 mRNA and NR2F2-AS1 were positively correlated in CRC tissues (Figure 2B).
NR2F2-AS1 Positively Regulated Cyclin D1 in CRC Cells
RKO and CR4 cells were transfected with NR2F2-AS1 siRNA, NR2F2-AS1 and Cyclin D1 expression vector. Transfections were confirmed by qPCR at 24 hrs post-transfection. Comparing to two control groups, the expression levels of NR2F2-AS1 and Cyclin D1 mRNA were significantly changed (Figure 3A, p<0.05), indicating successful transfections. In addition, NR2F2-AS1 siRNA silencing led to downregulated Cyclin D1 (Figure 3B, p<0.05), while NR2F2-AS1 overexpression led to upregulated Cyclin D1 (Figure 3C, p<0.05). NR2F2-AS1 positively regulated Cyclin D1 in CRC cells.
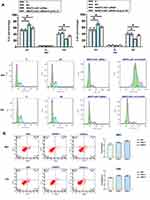
NR2F2-AS1 Regulated Cell Cycle Progression Through Cyclin D1
In consideration of that Cyclin D1 is a key cell cycle regulator, we detected the effect of NR2F2-AS1 on cell cycle progression. Comparing to two controls, percentage of cells at G0/G1 phase were significantly increased, while percentage of cells at G2 phase was significantly decreased after NR2F2-AS1 siRNA silencing, and Cyclin D1 overexpression rescued G0/G1 arrest caused by NR2F2-AS1 siRNA silencing (Figure 4A, p<0.05). As we all know, Cyclin D1 regulates the cell-cycle during G1/S transition, the results indicated that NR2F2-AS1 siRNA induced cell cycle arrest in G0/G1 phase through downregulating Cyclin D1. In condition, NR2F2-AS1 siRNA had no significant effect on apoptosis (Figure 4B).
NR2F2-AS1 Regulated Cell Proliferation Through Cyclin D1
Cell proliferation data were compared among different groups by performing ANOVA (one-way) and Tukey’s test. It was found that compared to two controls (C and NC), NR2F2-AS1 siRNA silencing resulted in a significantly decreased cell proliferation rate of both RKO and CR4 cells (Figure 5, p<0.05). In addition, Cyclin D1 overexpression attenuated the effects of NR2F2-AS1 siRNA silencing (Figure 5, p<0.05).
Discussion
This study mainly investigated the expression pattern and function of NR2F2-AS1 in CRC, and explored the clinical values of NR2F2-AS1. The results indicated that NR2F2-AS1 acts as an oncogenic lncRNA in CRC and regulates cancer cell progression through Cyclin D1, and NR2F2-AS1 can be used to predict the survival of CRC patients.
Accurate prognosis is critical for survival of cancer patients.12,13 Accurate prognosis assessment not only assists the selection of treatment approaches but also provides guidance for the development of individualized postoperative care.12,13 Certain lncRNAs have shown prognostic values for CRC.14,15 For instance, lncRNA ATB and AFAP1-AS1 were upregulated in CRC and the increased expression levels of ATB and AFAP1-AS1 were closely correlated with the poor survival of CRC patients.14,15 In this study, we found that the CRC patients with high levels of NR2F2-AS1 had significantly worse overall survival conditions, and its high expression closely correlated with advanced tumor stage and lymph node metastases, suggesting that NR2F2-AS1 was involved in CRC development. Multivariate analysis indicated that NR2F2-AS1 served as an independent prognostic marker for OS in patients with CRC. Therefore, NR2F2-AS1 may serve as a potential prognostic biomarker for CRC. However, this conclusion remains to be further confirmed by future studies with bigger sample size.
Accelerated cell cycle progression is the basis of tumor growth, and cell cycle control is a promising target for cancer treatment.16 Cyclin D1 plays key roles in cell cycle progression by mediating G1-S transition.17 Cyclin D1 is overexpressed in many types of cancer and overexpression of Cyclin D1 is closely correlated with tumor growth and progression.18 Consistently, we also observed the upregulated expression of Cyclin D1 in CRC. Interestingly, we found that NR2F2-AS1 siRNA silencing resulted in the downregulated expression of Cyclin D1 in CRC cells, and the interaction between NR2F2-AS1 and Cyclin D1 is involved in the regulation of CRC cell progression. Therefore, NR2F2-AS1 knockdown may serve as a potential therapeutic target for CRC by inhibiting cancer cell cycle progression through the downregulation of Cyclin D1. However, clinical studies and animal model studies are needed to further test this hypothesis.
Conclusion
In conclusion, NR2F2-AS1 is upregulated in CRC and NR2F2-AS1 knockdown inhibits CRC cell cycle progression via downregulating Cyclin D1.
Disclosure
The authors report no funding and no conflicts of interest for this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.v69.1
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
3. Chen WQ, Li H, Sun KX, et al. Report of cancer incidence and mortality in China, 2014. Zhonghua Zhong Liu Za Zhi. 2018;40(1):5–13. doi:10.3760/cma.j.issn.0253-3766.2018.01.002
4. Edwards BK, Noone AM, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120(9):1290–1314. doi:10.1002/cncr.28509
5. Riihimaki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. doi:10.1038/srep29765
6. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi:10.1038/nrg2521
7. Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21. doi:10.1038/nrg3606
8. Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9(6):703–719. doi:10.4161/rna.20481
9. Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31(43):4577–4587. doi:10.1038/onc.2011.621
10. Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26(2):155–165. doi:10.1038/modpathol.2012.160
11. Zhang S, Zhang X, Sun Q, et al. LncRNA NR2F2-AS1 promotes tumourigenesis through modulating BMI1 expression by targeting miR-320b in non-small cell lung cancer. J Cell Mol Med. 2019;23(3):2001–2011. doi:10.1111/jcmm.2019.23.issue-3
12. Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5(11):845–856. doi:10.1038/nrc1739
13. Klingbiel D, Tejpar S. Microsatellite instability and BRAF and KRAS mutations in stage III colon cancer: requirements for accurate prognosis assessment. JAMA Oncol. 2016;2:653. doi:10.1001/jamaoncol.2015.5226
14. Yue B, Qiu S, Zhao S, et al. LncRNA-ATB mediated E-cadherin repression promotes the progression of colon cancer and predicts poor prognosis. J Gastroenterol Hepatol. 2016;31(3):595–603. doi:10.1111/jgh.2016.31.issue-3
15. Wang F, Ni H, Sun F, Li M, Chen L. Overexpression of lncRNA AFAP1-AS1 correlates with poor prognosis and promotes tumorigenesis in colorectal cancer. Biomed Pharmacother. 2016;81:152–159. doi:10.1016/j.biopha.2016.04.009
16. Williams GH, Stoeber K. The cell cycle and cancer. J Pathol. 2012;226(2):352–364. doi:10.1002/path.v226.2
17. Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11(8):558–572. doi:10.1038/nrc3090
18. Diehl JA. Cycling to cancer with cyclin D1. Cancer Biol Ther. 2002;1(3):226–231. doi:10.4161/cbt.72
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.