Back to Journals » OncoTargets and Therapy » Volume 13
lncRNA NEAT1 Facilitates Cell Proliferation, Invasion and Migration by Regulating CBX7 and RTCB in Breast Cancer
Authors Yan L, Zhang Z, Yin X, Li Y
Received 2 December 2019
Accepted for publication 25 February 2020
Published 24 March 2020 Volume 2020:13 Pages 2449—2458
DOI https://doi.org/10.2147/OTT.S240769
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
This paper has been retracted.
Lixia Yan, 1,* Ze Zhang, 1,* Xingmei Yin, 1 Yongxia Li 2
1Department of Breast and Thyroid Surgery, Dongying People’s Hospital, Dongying, Shandong 257091, People’s Republic of China; 2Department of Stomatology and Eye, Dongying People’s Hospital, Dongying, Shandong 257091, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xingmei Yin
Department of Breast and Thyroid Surgery, Dongying People’s Hospital, Dongying People’s Hospital, No. 317, Nanyi Road, Dongying, Shandong 257091, People’s Republic of China
Tel +86-15505462059
Fax +86-546-8901211
Email [email protected]
Purpose: To investigate the association between the lncRNA NEAT1 and breast cancer, and to determine the influence of NEAT1 on regulation of other signaling molecules in breast cancer.
Methods: In the present study, we measured levels of the lncRNA NEAT1 in 106 breast cancer patients and in a human breast cancer cell line by qRT-PCR. The correlation between NEAT1 expression and patients’ clinical characteristics was analyzed with in-house and TCGA data. We used cellular functioning assays and cell immunofluorescence assay to evaluate the role of NEAT1 and its target molecules in proliferation, invasion and migration in breast cancer. We used Western blotting to explore possible targets of NEAT1 and a subcellular fractionation assay to locate NEAT1 expression.
Results: NEAT1 was overexpressed in breast cancer tissue and also closely related to advanced clinical stages and positive lymph node metastases. NEAT1 levels were also tightly correlated to prognosis for breast cancer patients in survival analyses. Cellular function assays revealed that downregulation of NEAT1 could inhibit breast cancer cell viability, invasion and migration. Western blotting revealed down-regulation of CBX7 and up-regulation of RTCB following NEAT1 inhibition. Based on the cytoplasmic and nuclear expression of NEAT1, we investigated the possible regulation of CBX7 and RTCB by NEAT1. Results showed that NEAT1 regulated the expression of CBX7 and RTCB, possibly by binding of NEAT1 to DNA in the nucleus, which facilitates cell proliferation, invasion and migration.
Conclusion: The current results suggest that the lncRNA NEAT1 is upregulated in breast cancer and facilitates tumor cell viability, invasion and migration via CBX7 and RTCB.
Keywords: breast cancer, clinical biomarkers, NEAT1, CBX7, RTCB
Introduction
Breast cancer is the most prevalent malignant tumor in females, holding a high incidence and mortality rate.1 At least 1 million women are diagnosed with breast cancer annually, and worse, about four hundred thousand people lose their lives due to breast cancer each year.2 Although considerable progress has been made in the detection of breast cancer and therapeutic approach to breast cancer and survival rate has improved, long-term survival is still a challenge. Overall prognosis remains poor, particularly in patients at advanced stages or with metastases.3 Breast cancer pathogenesis has not been fully clarified and there are currently no clinical biomarkers that are accurate and sensitive enough to predict breast cancer prognosis. There is an urgent need to identify biomarkers that are both closely related to the pathogenesis of breast cancer and useful for predicting prognosis of patients with breast cancer.
Long non-coding RNAs (lncRNAs) are a set of non-coding transcripts more than 200 nucleotides in length that are often related to proliferation and migration in multiple types of tumours.4 lncRNAs act as signaling molecules, scaffolds, and instructors, and exert their influence at several levels including transcription, post-transcription, and translation.5 In recent years, abundant evidence has revealed that anomalous expression of lncRNAs such as HOXD-AS1, H19, CASC9, and NORAD6–9 may drive the pathogenesis of breast cancer. In addition, anomalous expression of lncRNAs may serve as a biomarker for breast cancer.10–12
The lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1), a potential cancer-related lncRNA, is abnormally expressed in various types of tumours including colorectal cancer,13 lung cancer, hepatocellular carcinoma14 and, breast cancer.15,16 Moreover, upregulation of NEAT1 is related to poorer prognosis.14 Previous work has shown that NEAT1 is expressed in breast cancer and associated with cell proliferation and chemoresistance,17,18 but the role and mechanisms of NEAT1 in breast cancer are far from fully elucidated. Here, we provide evidence for NEAT1’s function in cell nuclei, revealed a new carcinogenic role for NEAT1, in addition to its inclusion among the competing endogenous RNAs (ceRNA) that regulate gene expression, and observed its impact on the proliferation and migration capacity of breast cancer cells. This study is the first to find a link between NEAT1 and CBX7 or RTCB, which could ultimately shed light on novel mechanisms for targeting multiple cancers, including breast cancer.
Materials and Methods
Ethics Statement
All work was conducted with the formal approval of the Ethics Committees at Dongying People’s Hospital. Every patient understood and signed the informed consent paperwork.
Specimen Collection
106 breast cancer tissues and para-carcinoma tissue were collected from patient volunteers who had their tumor removed at Dongying People’s Hospital. Those patients that had received chemotherapy prior to the operation or had other tumor types were excluded from the study. The para-carcinoma tissues were taken from approximately 2.5 cm beyond from the tumor margin. As the fresh tissues were collected, they were immediately snap frozen in liquid nitrogen and stored at −80°C.
TCGA Database Analysis
Existing TCGA data for lncRNA NEAT1 expression in breast cancer tissues and clinicopathological data from the associated patients were downloaded from https://gdc.cancer.gov/. We selected for inclusion those patients whose histologic diagnosis was breast cancer and who had a follow up history longer than one month. And for the exclusion criteria those patients who suffered from other malignant neoplasms except breast cancer and whose overall survival was more than five years. A total of 410 normal tissue samples and 228 breast cancer tissue samples were obtained from the TCGA database of the included patients. We analyzed lncRNA NEAT1 expression in breast cancer and its correlations with clinical parameters of breast cancer. A statistical cut-off value—the highest Youden index (specificity+sensitivity-1) from receiver operating characteristic (ROC) curves—was used to define high and low lncRNA NEAT1 expression. Using this, mRNA expression values >20 defined high lncRNA NEAT1 expression, and mRNA expression values ≤4 defined low lncRNA NEAT1 expression.
Cell Culture and Transfection
For in vitro experiments, we used human breast cancer cell lines and normal breast MCF10A cells. We obtained all cell lines from the American Type Culture Collection. Cells were incubated at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) obtained from Invitrogen with added fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO), penicillin (100 μL/mL), streptomycin (100 mg/mL) and glutamine. MCF-7 and SUM-159PT cells were transfected with NEAT1 silencing siRNAs (si-NEAT1#1 and si-NEAT1#2, 100 nmol/L, C02004) or negative control siRNA (si-NC, 100 nmol/L, C03002) obtained from Shanghai GenePharma company. Besides, shRNAs to RTCB (#1 and #2) and a pcDNA3.1 vector targeting CBX7#1, CBX7#2 and NEAT1 constructed by Genechem (Shanghai, China) was added when MCF-7, SUM-159PT or MCF10A cells reached 40% confluence. Lipofectamine 3000 (Invitrogen) was used for all cell transfections. After transfection for 36 h, we performed reverse transcription quantitative polymerase chain reaction (qRT-PCR) for verification of transfection efficiency and further functional assays.
RNA Extraction and qRT-PCR
Homogenized tissues and cell lines underwent RNA extraction and purification performed with TRIzol reagent and RNase-Free DNase, respectively, both obtained from Invitrogen. RNA concentration and purity were assayed with a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Inc). cDNA was then synthesized from 100 µg RNA with the Primer-Script 1st Strand cDNA Synthesis Kit (Invitrogen). lncRNA NEAT1 levels (in tissues and cell lines) were determined by qRT-PCR in the Roche LightCycler 480 qRT-PCR System with SYBR-Green PCR Master mix (Roche, Mannheim, Germany). Relative lncRNA NEAT1 levels were compared between groups via the 2–ΔΔCt method after normalization to GAPDH. Primer sequences are as follows: NEAT1 (sense) CAGTTAGTTTATCAGTTCTCCCATCCA; NEAT1 (antisense): GTTGTTGTCGTCACCTTTCAACTCT; GAPDH (sense): GATATTGTTGCCATCAATGAC; GAPDH (antisense): TTGATTTTGGAGGGATCTCG; U1 (sense): 5ʹ-CCGAATTCATGGCAGGAAGAAGCGGA-3ʹ; U1 (antisense): 5ʹ-AAGGATCCGTTCACTAATCGAATGGA-3ʹ; CBX7 (sense): 5ʹ- CATGGAGCTGTCAGCCATC-3ʹ; CBX7 (antisense): 5ʹ- CTGTACTTTGGGGGCCATC-3ʹ; RTCB (sense): 5ʹ-GGAAGTCGAGGACTTGGACA-3ʹ; RTCB (antisense): 5ʹ- GTTAACCCAGGCGAAGTTTG-3ʹ
Cell Counting Kit-8 (CCK-8) Assay
CCK-8 solution (Everbright lnc) was used to assess the viability of SUM-159PT, MCF-7, and MCF10A cells. Briefly, 3×103 transfected cells (si-NEAT1- or si-NC- translation for SUM-159PT, MCF-7 cells; pcDNA-NEAT1- and Empty Vector- translation for MCF10A cells) were planked in 96-well plates and cultivated for 8 h. CCK-8 (10 μL) solution was added to each well and incubated for 90 min. Optical density at 450 nm was then recorded every 24 h (total 72 h) using an ELISA microplate reader.
Cell Invasion Assay
Transwell assays were used to assess tumor cells’ invasion capacity. In short, an equal number (50,000/well) of transfected cells with were loaded into a transwell chamber (BD Biosciences, New York, NJ, USA) with Matrigel. 450 μL DMEM and 50 μL FBS were added to each well as attractors. The breast cancer and MCF10A transfected cells were allowed to invade for 36 h. After 36 h, the cells that did not invade through the membrane were washed away and the cells that invaded through the membrane were stained with crystal violet (1%). The number of invaded breast cancer cells was calculated in triplicate (three random, nonoverlapping places) using a light microscope (Leica Microsystems GmbH) under white light at a magnification of ×100.
Wound Healing Assay
Transfected cells were added into 6-well plates. Artificial wounds for live cells analysis were made on the cell monolayer by culture-inserts. Migratory cells as well as wound healing were monitored at 0 and 24 h. Three artificial wounds were at least immediately photographed for each group at indicated time points following the wound formation. Through measuring the difference of wound areas, cell migration was assessed.
Bioinformatics Analysis
StarBase v3.0 is an open-source platform for studying the miRNA-ncRNA, miRNA-mRNA, ncRNA-RNA, RNA-RNA, RBP-ncRNA and RBP-mRNA interactions from CLIP-seq, degradome-seq and RNA-RNA interactome data. In the current study, the RBP-mRNA section of StarBase v3.0 (http://starbase.sysu.edu.cn/index.php) was used to predict targets of NEAT1.
Western Blot Assay
Si-NEAT1- or si-NC-transfected cell lysates were harvested and washed after 36 h transfection. Ice-cold radioimmunoprecipitation assay (RIPA) buffer was utilized for cell disruption. Homogenization centrifugation was carried out and the supernatant collected. Homogenous protein separation and transfer was conducted using 12% sodiumdodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and polyvinylidene difluoride (PVDF) membranes. Membranes were saturated with 5% skim milk for 2 h then incubated with primary rabbit polyclonal anti-CBX7 (1:1000), anti-RTCB (1:1000), or anti- GAPDH (1:1000; Wuhan Fine Biotech Co., Ltd., Wuhan, China) at 4°C for 24 h. The membranes were then incubated with HRP-conjugated goat anti-rabbit antibody (1:1000, Sigma) for 120 min and exposed to enhanced chemiluminescence substrate (Millipore, Rockford, USA). Bands were visualized with the Bio-Rad Gel Doc XR + system (Bio-Rad, Hercules, CA, USA).
Subcellular Fractionation Assay
RNA was isolated from either the nuclear or cytoplasmic fraction using the Nuclear/Cytosol Fractionation Kit (Biovision, San Francisco Bay, CA, USA) and was measured with qRT-PCR. U1 or GAPDH served as the identifiers for the nuclear and cytoplasmic fractions, respectively.
Cell Immunofluorescence
Cells were briefly fixed in paraformaldehyde and permeabilized with 0.1% Triton X‐100 (Sigma‐Aldrich) followed by incubation in normal goat serum for 30 min, then incubation with anti‐human Ki-67 antibody (1:100) overnight. Cells were then washed and incubated with secondary anti‐Rabbit IgG (H+L) antibody conjugated to Alexa Fluor 488 (A21442, Invitrogen, ThermoFisher) followed by additional washing and counterstaining with DAPI. Slides were mounted and coverslipped with FluorSave (Calbiochem). Fluorescence was imaged using an Axio Observer Z1 immunofluorescence microscope (Carl Zeiss Inc).
Statistical Analysis
Data analysis was conducted using SPSS 21.0 and Graphpad prism 7. The variance between groups was assessed by student’s t-test. Chi-square test or Fisher’s exact test were used to evaluate associations between NEAT1 expression levels and clinicopathological features. The Kaplan-Meier method was applied to evaluate the prognostic value of NEAT1 in breast cancer. P<0.05 was considered the cutoff value for a significant difference.
Results
lncRNA NEAT1 Is Up-Regulated in Breast Cancer Tissues and Cell Lines
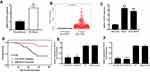
NEAT1 levels were measured in 106 cases of breast cancer and normal tissues. We found that NEAT1 levels were remarkably elevated in breast cancer tissues compared with corresponding normal tissues (p<0.05, Figure 1A). We found similar results in the TCGA database (p<0.05, Figure 1B), and in cell lines, which revealed that NEAT1 expression was 4.35 and 3.76 times higher in MCF-7 (p< 0.01) and SUM-159PT (p< 0.01) cells compared to MCF10A cells, respectively (Figure 1C); The relationship between NEAT1 expression and clinicopathological parameters of breast cancer patients was also investigated, which uncovered a noteworthy correlation between NEAT1 level and clinical stage (p=0.003) and lymph node metastasis (p=0.001; Table S1). Specifically, breast cancer patients with low NEAT1 expression were more likely to sustain advanced clinical stage and positive lymph node metastasis.
The Prognostic Value of lncRNA NEAT1 in Breast Cancer
The prognostic value of NEAT1 as a biomarker for breast cancer was estimated by Cox proportional hazard regression analyses (univariate analysis and multivariate analysis, Table S2) and Kaplan-Meier survival curves. In the univariate analysis, clinical stage (HR=6.723, 95% CI=3.136–14.145, p=0.001), lymph node metastasis (HR=8.731, 95% CI=3.742–20.371, p=0.001), and NEAT1 expression (HR=6.357, 95% CI=2.431–16.718, p=0.001) were independent causes impacting overall survival of breast cancer patients. The multivariate analysis suggested that lymph node metastasis (HR=3.990, 95% CI=1.316–12.091, p=0.014) and NEAT1 expression (HR=3.042, 95% CI=1.034–8.910, p=0.043) were independent causes impacting overall survival of patients. Furthermore, the Kaplan-Meier curves demonstrated a relatively poor survival in breast cancer individuals with high NEAT1 expression levels (p<0.001, Figure 1D).
lncRNA NEAT1 Promoted Cell Viability, Invasion and Migration
Changes in NEAT1 expression in breast cancer cells (MCF-7 and SUM-159PT) transfected with si-NEAT1#1 and si-NEAT1#2 or Negative Control (NC) were measured via qRT-PCR. MCF-7 cells transfected with si-NEAT1#1 and si-NEAT1#2 presented about 0.3–0.4 times higher NEAT1 levels compared to the si-NC group (p<0.05, Figure 1E). We found similar results in SUM-159PT cells, where cells transfected with si-NEAT1#1 and si-NEAT1#2 presented about 0.3 times higher NEAT1 expression than the si-NC group (p<0.01, Figure 1F).
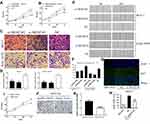
CCK-8 assays demonstrated that the viability of MCF-7 cells and SUM-159PT cell could be remarkably suppressed by down-regulation of NEAT1 (Figure 2A and B, respectively). Transwell assay results demonstrated that the invasive capacity of MCF-7 and SUM-159PT cells can also be remarkably suppressed by down-regulation of NEAT1 (p<0.05, Figure 2C and D). Similarly, the migration of breast cancer cells can be remarkably suppressed by down-regulation of NEAT1 (p<0.01, Figure 2E and F). In addition, immunofluorescent staining showed that compared with control cells, MCF-7 cells with si-NEAT1#1 had lower levels of Ki-67, a marker of cellular proliferation (Figure 2G and H). We also analyzed the role of overexpression of NEAT1 on proliferation and invasive behavior of normal MCF10A cells. As expected, CCK-8 assays demonstrated that MCF10A cell viability was remarkably enhanced by up-regulation of NEAT1 (all p < 0.05, Figure 2I). Moreover, the invasive ability of MCF10A cells was remarkably accelerated by up-regulation of NEAT1 (p < 0.001, Figure 2J and K). Taken together, our results suggest that higher NEAT1 expression promotes breast cancer cell viability, invasion and migration.
NEAT1 Regulates CBX7 and RTCB Target Gene Expression
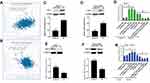
In the current study, StarBase 3.0 interaction target analyses revealed that CBX7 is positively co-expressed with NEAT1 in breast cancer (Figure 3A) while RTCB is negatively co-expressed with NEAT1 in breast cancer (Figure 3B). Western blot was carried out for target gene validation, and we found that protein levels of CBX7 in MCF-7 and SUM-159PT cells were remarkably down-regulated following down-regulation of NEAT1 (p<0.01, Figure 3C and D). The opposite effect was observed in these two cell lines for protein levels of RTCB (p<0.05, Figure 3E and F). qRT-PCR also showed that the changes of CBX7 and RTCB expression transfected with si-NEAT1#1 and si-NEAT1#2. Moreover, a pcDNA3.1 vector targeting CBX7#1 and CBX7#2 and shRNAs to RTCB (#1 and #2) can change the effect of NEAT1 on their expression after simultaneous transfection (Figure 3G and H). These findings demonstrate that CBX7 and RTCB may be two target genes regulated by NEAT1 in breast cancer.
NEAT1 Regulates the Expression of CBX7 and RTCB to Facilitate Cellular Proliferation, Invasion and Migration
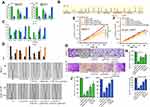
To investigate the mechanism by which the lncRNA NEAT1 exerts its effects in breast cancer, we first determined its cellular location. A subcellular fractionation assay revealed that NEAT1 is predominantly located in the cytoplasm but can also be found in the nucleus (Figure 4A). In addition, the two previously identified proteins (CBX7 and RTCB), which may be regulated by NEAT1, are very close to each other on the long arm of chromosome 22 (Figure 4B). Based on these findings, we considered that lncRNA NEAT1 may play a regulatory role in chromatin state, DNA binding, or the fate of newly-transcribed mRNA in the nucleus to regulate the expression of CBX7 and RTCB. To test this hypothesis, a subcellular fractionation along with qRT-PCR was used to determine mRNA levels of CBX7 and RTCB in the nucleus and cytoplasm in two cell lines. The results indicated that si-NEAT1 can effectively regulate mRNA expression of CBX7 and RTCB in the nucleus but not in the cytoplasm (Figure 4C and D). Rescue assays were conducted in which MCF-7 cells were separately transfected with either si-NC, si-NEAT1#2, siNEAT1#2+CBX7#1, si-NEAT1#2+sh-RTCB#1 or siNEAT1#2+CBX7#1+sh-RTCB#1 and SUM-159PT cells were separately transfected with either si-NC, si-NEAT1#1, siNEAT1#1+CBX7#2, si-NEAT1#1+sh-RTCB#2 or siNEAT1#1+CBX7#2+sh-RTCB#2. CCK-8 assays also demonstrated that the decrease in cellular proliferation observed after silencing NEAT1 was rescued by direct promotion of CBX7 or inhibition of RTCB (Figure 4E and F). In addition, the inhibition of cellular invasion and migration after silencing NEAT1 was recovered with CBX7 addition or RTCB repression, as observed from transwell and wound healing experiments (Figure 4G–J). Overall, these results support the idea that NEAT1 regulates cellular proliferation and migration through regulation of CBX7 and RTCB expression in breast cancer.
Discussion
Breast cancer holds the second highest rate of cancer-related death among women worldwide.19 Accurate postoperative prediction of breast cancer patient prognosis is essential for cancer treatment and control. If accurate prognosis prediction can be achieved, individualized postoperative treatment and follow-up will become more manageable, which will ultimately reduce the economic burden of patients, reduce drug side effects, improve the patients’ quality of life, and extend patient survival time. A major hindrance to accurate prognosis prediction is that the exact mechanism of breast cancer pathogenesis is not yet clear. In recent years, the discovery of lncRNA and elucidation of its features has opened powerful new avenues of cancer research. LncRNAs perform gene regulation at transcriptional and post-transcriptional levels, affecting numerous biological processes of tumors—including breast cancer tumors—such as initiation, growth, and metastasis.18,20
NEAT1, located near nuclear paraspeckles, can act as an oncogene in various solid cancers such as osteosarcoma, gastric cancer, cervical cancer, and prostate cancer.21–24 NEAT1 is also supposed to serve as a potential biomarker in various cancers.25 Previous studies have demonstrated that NEAT1 is upregulated in breast cancer and contributes to breast cancer progression,15,26 however, the roles and mechanisms of NEAT1 in breast cancer still remains unknown.
The lncRNA NEAT1 also regulates cell functions in various cancers. NEAT1 is upregulated in retinoblastoma, where decreased NEAT1 expression remarkably inhibited cell proliferation and migration and facilitated cell apoptosis via effects on miR-204 and CXCR4.27 In osteosarcoma, NEAT1 is upregulated compared to normal tissues and knockdown of NEAT1 inhibited cell proliferation, migration, and invasion.21 Overexpression of NEAT1 has also been found in cervical cancer tissues where it facilitates cell proliferation and migration, and exerts stimulative functions by binding to miR-9-5p.23 In lung cancer, NEAT1 knock-down inhibited cell invasion and migration via binding to let-7a.28 NEAT1 can also promote tumor cell function in osteosarcoma, myeloma, prostate cancer and gastric cancer.22,24,29,30 In all of the aforementioned studies, NEAT1 functions as a member of ceRNA in the cytoplasm. The current study, however, in addition to finding that NEAT1 is overexpressed in breast cancer tissue and that downregulation of NEAT1 can inhibit breast cancer cell viability and invasion, is also the first to find evidence that NEAT1 plays a role in the nucleus.
Chromobox homolog 7 (CBX7) protein, a significant component of the Polycomb Repressive Complex (PRC) 1, plays a significant role in tumorigenesis and the development of various cancer types.31,32 In the current study, StarBase 3.0 (http://starbase.sysu.edu.cn/index.php) was utilized to predict the targets of NEAT1. We found that target CBX7 expression was positively correlated with NEAT1 in breast cancer. Previous studies have revealed that CBX7 is related to cancer cell proliferation, migration, and invasion.33,34 Thus, CBX7 was selected as a target of interest in this study. We verified CBX7 as a NEAT1 target with Western blotting and found that protein levels of CBX7 were positively correlated with NEAT1 in breast cancer cells, demonstrating that CBX7 is indeed a target gene for regulation by NEAT1.
The full name of RTCB is RNA 2ʹ,3ʹ-cyclic phosphate and 5ʹ-OH ligase. RTCB is an essential human tRNA ligase required for ligating cleaved tRNA halves during tRNA splicing and XBP1 fragments during endoplasmic reticulum stress. Activation of XBP1 has been implicated in various human tumors including breast cancer.35 The current results demonstrated that protein levels of RTCB were negatively correlated with NEAT1 in breast cancer cells, which was in line with the StarBase prediction, and demonstrated that RTCB is a target gene regulated by NEAT1 with an important regulatory role in breast cancer.
The lncRNA NEAT1 can serve as a prognostic biomarker in various cancers. NEAT1 expression is elevated in nasopharyngeal carcinoma tissues and is related to clinical stage. NEAT1 expression levels are also negatively correlated with overall survival in nasopharyngeal carcinoma, where upregulation of NEAT1 was shown to be an undesirable prognostic factor. Similarly in renal cancer, NEAT1 expression is related to specific clinicopathologic features (tumour size, lymph node metastasis, and fuhrman grade) and downregulation of NEAT1 predicts a better prognosis for patients with renal cancer.36 In gastric cancer, NEAT1 is elevated in tumour tissue and related to specific clinicopathologic features including clinical stage and distant metastasis. Elevated expression of NEAT1 is also an undesirable prognostic factor and predicts poorer survival in gastric cancer.37 In the current study, NEAT1 expression was closely related to clinical stage and positive lymph node metastasis and higher NEAT1 expression predicted poorer prognosis for breast cancer patients in survival analyses, demonstrating the potential use of NEAT1 as a prognostic biomarker in breast cancer.
Data Sharing Statement
Data that support the findings of this study are available from the corresponding author Xingmei Yin, upon reasonable request.
Author Contributions
Lixia Yan performed experimental work and assisted in writing the manuscript. Ze Zhang and Yongxia Li collected tissue samples, designed the study, and assisted in writing the manuscript. Xingmei Yin designed the study, observed the patients, and examined controls. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yu X, Liang J, Xu J, et al. Identification and validation of circulating microRNA signatures for breast cancer early detection based on large scale tissue-derived data. J Breast Cancer. 2018;21(4):363–370. doi:10.4048/jbc.2018.21.e56
2. Hagrass HA, Sharaf S, Pasha HF, Tantawy EA, Mohamed RH, Kassem R. Circulating microRNAs - a new horizon in molecular diagnosis of breast cancer. Genes Cancer. 2015;6(5–6):281–287.
3. Li J, Song ZJ, Wang YY, Yin Y, Liu Y, Nan X. Low levels of serum miR-99a is a predictor of poor prognosis in breast cancer. Genet Mol Res. 2016;15(3):gmr8338.
4. Dai Y, Wan Y, Qiu M, et al. lncRNA MEG3 suppresses the tumorigenesis of hemangioma by sponging miR-494 and regulating PTEN/PI3K/AKT pathway. Cell Physiol Biochem. 2018;51(6):2872–2886. doi:10.1159/000496040
5. Liu Z, Wu C, Xie N, Wang P. Long non-coding RNA MEG3 inhibits the proliferation and metastasis of oral squamous cell carcinoma by regulating the WNT/beta-catenin signaling pathway. Oncol Lett. 2017;14(4):4053–4058. doi:10.3892/ol.2017.6682
6. Li Y, Han X, Li Q, Wang C, Lou Z, Wang X. Long noncoding RNA HOXD-AS1 induces epithelial-mesenchymal transition in breast cancer by acting as a competing endogenous RNA of miR-421. J Cell Biochem. 2019;120(6):10633–10642. doi:10.1002/jcb.v120.6
7. Shao G, Wang M, Fan X, et al. lncRNA CASC9 positively regulates CHK1 to promote breast cancer cell proliferation and survival through sponging the miR195/497 cluster. Int J Oncol. 2019;54(5):1665–1675. doi:10.3892/ijo.2019.4734
8. Qi C, Xiaofeng C, Dongen L, et al. Long non-coding RNA MACC1-AS1 promoted pancreatic carcinoma progression through activation of PAX8/NOTCH1 signaling pathway. J Exp Clin Cancer Res. 2019;38(1):344. doi:10.1186/s13046-019-1332-7
9. Zhou K, Ou Q, Wang G, Zhang W, Hao Y, Li W. High long non-coding RNA NORAD expression predicts poor prognosis and promotes breast cancer progression by regulating TGF-beta pathway. Cancer Cell Int. 2019;19:63. doi:10.1186/s12935-019-0781-6
10. Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem. 2017;17(2):152–163. doi:10.2174/1871520616666160502122724
11. Yang Y, Qian J, Xiang Y, Chen Y, Qu J. The prognostic value of long noncoding RNA HOTTIP on clinical outcomes in breast cancer. Oncotarget. 2017;8(4):6833–6844. doi:10.18632/oncotarget.14304
12. Yao Y, Li J, Wang L. Large intervening non-coding RNA HOTAIR is an indicator of poor prognosis and a therapeutic target in human cancers. Int J Mol Sci. 2014;15(10):18985–18999. doi:10.3390/ijms151018985
13. Peng W, Wang Z, Fan H. LncRNA NEAT1 impacts cell proliferation and apoptosis of colorectal cancer via regulation of akt signaling. Pathol Oncol Res. 2017;23(3):651–656. doi:10.1007/s12253-016-0172-4
14. Yu X, Li Z, Zheng H, Chan MT, Wu WK. NEAT1: a novel cancer-related long non-coding RNA. Cell Prolif. 2017;50(2). doi:10.1111/cpr.12368
15. Li X, Wang S, Li Z, et al. The lncRNA NEAT1 facilitates cell growth and invasion via the miR-211/HMGA2 axis in breast cancer. Int J Biol Macromol. 2017;105(Pt 1):346–353. doi:10.1016/j.ijbiomac.2017.07.053
16. Zhao WY, Geng DH, Li SQ, Chen ZF, Sun M. LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer Med. 2018;7(3):842–855. doi:10.1002/cam4.2018.7.issue-3
17. Li W, Zhang Z, Liu X, et al. The FOXN3-NEAT1-SIN3A repressor complex promotes progression of hormonally responsive breast cancer. J Clin Invest. 2017;127(9):3421–3440. doi:10.1172/JCI94233
18. Shin VY, Chen J, Cheuk IW, et al. Long non-coding RNA NEAT1 confers oncogenic role in triple-negative breast cancer through modulating chemoresistance and cancer stemness. Cell Death Dis. 2019;10(4):270. doi:10.1038/s41419-019-1513-5
19. Ding X, Zhang Y, Yang H, et al. Long non-coding RNAs may serve as biomarkers in breast cancer combined with primary lung cancer. Oncotarget. 2017;8(35):58210–58221. doi:10.18632/oncotarget.17356
20. Yang J, Ye Z, Mei D, Gu H, Zhang J. Long noncoding RNA DLX6-AS1 promotes tumorigenesis by modulating miR-497-5p/FZD4/FZD6/Wnt/beta-catenin pathway in pancreatic cancer. Cancer Manag Res. 2019;11:4209–4221. doi:10.2147/CMAR.S194453
21. Li P, Huang R, Huang T, Cheng S, Chen Y, Wang Z. Long non-coding RNA NEAT1 promotes proliferation, migration and invasion of human osteosarcoma cells. Int J Med Sci. 2018;15(11):1227–1234. doi:10.7150/ijms.25662
22. Wang H, Zhang M, Sun G. Long non-coding RNA NEAT1 regulates the proliferation, migration and invasion of gastric cancer cells via targeting miR-335-5p/ROCK1 axis. Pharmazie. 2018;73(3):150–155. doi:10.1691/ph.2018.7877
23. Xie Q, Lin S, Zheng M, Cai Q, Tu Y. Long noncoding RNA NEAT1 promotes the growth of cervical cancer cells via sponging miR-9-5p. Biochem Cell Biol. 2019;97(2):100–108. doi:10.1139/bcb-2018-0111
24. Xiong W, Huang C, Deng H, et al. Oncogenic non-coding RNA NEAT1 promotes the prostate cancer cell growth through the SRC3/IGF1R/AKT pathway. Int J Biochem Cell Biol. 2018;94:125–132. doi:10.1016/j.biocel.2017.12.005
25. Dong P, Xiong Y, Yue J, et al. Long non-coding RNA NEAT1: a novel target for diagnosis and therapy in human tumors. Front Genet. 2018;9:471. doi:10.3389/fgene.2018.00471
26. Zhao D, Zhang Y, Wang N, Yu N. NEAT1 negatively regulates miR-218 expression and promotes breast cancer progression. Cancer Biomarkers. 2017;20(3):247–254. doi:10.3233/CBM-170027
27. Fu JW, Kong Y, Sun X. Long noncoding RNA NEAT1 is an unfavorable prognostic factor and regulates migration and invasion in gastric cancer. J Cancer Res Clin Oncol. 2016;142(7):1571–1579. doi:10.1007/s00432-016-2152-1
28. Qi L, Liu F, Zhang F, et al. lncRNA NEAT1 competes against let-7a to contribute to non-small cell lung cancer proliferation and metastasis. Biomed Pharmacother. 2018;103:1507–1515. doi:10.1016/j.biopha.2018.04.053
29. Yang G, Li J, Cai Y, Yang Z, Li R, Fu W. Glycyrrhizic acid alleviates 6-hydroxydopamine and corticosterone-induced neurotoxicity in SH-SY5Y cells through modulating autophagy. Neurochem Res. 2018;43(10):1914–1926. doi:10.1007/s11064-018-2609-5
30. Wu Y, Wang H. LncRNA NEAT1 promotes dexamethasone resistance in multiple myeloma by targeting miR-193a/MCL1 pathway. J Biochem Mol Toxicol. 2018;32(1). doi:10.1002/jbt.22008
31. Sepe R, Formisano U, Federico A, et al. CBX7 and HMGA1b proteins act in opposite way on the regulation of the SPP1 gene expression. Oncotarget. 2015;6(5):2680–2692. doi:10.18632/oncotarget.v6i5
32. Ni SJ, Zhao LQ, Wang XF, et al. CBX7 regulates stem cell-like properties of gastric cancer cells via p16 and AKT-NF-kappaB-miR-21 pathways. J Hematol Oncol. 2018;11(1):17. doi:10.1186/s13045-018-0562-z
33. Ni S, Wang H, Zhu X, et al. CBX7 suppresses cell proliferation, migration, and invasion through the inhibition of PTEN/Akt signaling in pancreatic cancer. Oncotarget. 2017;8(5):8010–8021. doi:10.18632/oncotarget.v8i5
34. Xie D, Shang C, Zhang H, Guo Y, Tong X. Up-regulation of miR-9 target CBX7 to regulate invasion ability of bladder transitional cell carcinoma. Med Sci Monit. 2015;21:225–230. doi:10.12659/MSM.893232
35. Nandy A, Saenz-mendez P, Gorman AM, Samali A, Eriksson LA. Homology model of the human tRNA splicing ligase RtcB. Proteins. 2017;85(11):1983–1993. doi:10.1002/prot.v85.11
36. Liu Z, Wu K, Wu J, et al. NEAT1 is a potential prognostic biomarker for patients with nasopharyngeal carcinoma. J Cell Biochem. 2019;120(6):9831–9838. doi:10.1002/jcb.v120.6
37. Ning L, Li Z, Wei D, Chen H, LncRNA YC. NEAT1 is a prognosis biomarker and regulates cancer progression via epithelial-mesenchymal transition in clear cell renal cell carcinoma. Cancer Biomarkers. 2017;19(1):75–83. doi:10.3233/CBM-160376
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.