Back to Journals » OncoTargets and Therapy » Volume 12
LncRNA MIR210HG promotes proliferation and invasion of non-small cell lung cancer by upregulating methylation of CACNA2D2 promoter via binding to DNMT1
Authors Kang X, Kong F, Huang K, Li L, Li Z, Wang X, Zhang W, Wu X
Received 2 October 2018
Accepted for publication 15 February 2019
Published 16 May 2019 Volume 2019:12 Pages 3779—3790
DOI https://doi.org/10.2147/OTT.S189468
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Xiaowen Kang,1,* Fanwu Kong,2,* Kun Huang,1 Lu Li,1 Zhaoguo Li,1 Xinyan Wang,1 Wei Zhang,3 Xiaomei Wu1
1Department of Pulmonology, The Second Affiliated Hospital, Harbin Medical University, Harbin, People’s Republic of China; 2Department of Nephrology, The Second Affiliated Hospital, Harbin Medical University, Harbin, People’s Republic of China; 3Department of Pulmonology, The First Affiliated Hospital, Harbin Medical University, Harbin, People’s Republic of China
*These authors contributed equally to this work
Background: In recent years, a large number of studies have shown that differentially expressed lncRNAs are capable of promoting the occurrence and development of tumors by regulating cell proliferation and differentiation. However, the biological effects of lncRNAs in non-small cell lung cancer (NSCLC) are still needed to be further investigated.
Methods: The differentially expressed lncRNAs in NSCLC tissues in the downloaded profiles from GEO database were analyzed and further verified in 100 pairs of NSCLC samples collected in our hospital. After identification of the target gene MIR210HG, the relationship between MIR210HG expression and clinical data of NSCLC patients was analyzed. Regulatory effects of MIR210HG on proliferation, migration, and invasion of NSCLC cells were detected by CCK-8, colony formation, and transwell assay, respectively. The binding condition of MIR210HG and DNA methyltransferase 1 (DNMT1) was detected by RNA binding protein immunoprecipitation. Subsequently, chromatin immunoprecipitation assay assessed the promoter binding of DNMT1 to CACNA2D2. Rescue experiments were conducted to assess whether CACNA2D2 can reverse the function of MIR210HG.
Results: MIR210HG was highly expressed in NSCLC tissues not only in GSE30219 dataset but also in our collected NSCLC tissues. MIR210HG expression was correlated to tumor stage and lymph node metastasis of NSCLC patients. Besides, lower disease-free survival (DFS) and overall survival (OS) were found in NSCLC patients with high-level MIR210HG compared with those with low-level MIR210HG. Regression analysis indicated that MIR210HG was the independent risk factor for DFS and OS of NSCLC patients. In vitro experiments demonstrated that MIR210HG knockdown remarkably inhibited proliferation and migration of NSCLC cells. MIR210HG could recruit DNMT1, thereafter promoting methylation of CACNA2D2 promoter region. CACNA2D2 overexpression remarkably inhibited cell proliferation. Moreover, inhibited proliferation induced by MIR210HG knockdown was reversed by CACNA2D2 knockdown.
Conclusion: MIR210HG can promote the tumorigenesis of NSCLC by inhibiting the expression of CACNA2D2. Our findings provide new therapeutic strategies for the future treatment of NSCLC.
Keywords: MIR210HG, DNMT1, CACNA2D2, non-small cell lung cancer, proliferation
Introduction
Lung cancer has become the most common type of malignant tumor in the world. It is reported that the mortality of lung cancer ranks the highest in all cancers.1 In China, the incidence of lung cancer has rapidly increased with the economic development and environment deterioration. More seriously, the mortality rate of lung cancer has increased by 465%, accounting for the first place in urban areas and second in rural areas.2 Non-small cell lung cancer (NSCLC) accounts for 75–80% of lung cancer cases. Despite that many new advances have been made in clinical and experimental oncology, the prognosis of NSCLC remains unsatisfactory, with a 5-year survival rate of only about 15%.3–5 The main cause of the high mortality rate of NSCLC is the invasion and metastasis of tumor cells. Effective treatment measures for NSCLC are still lacking. It is of great importance to explore the mechanisms underlying the occurrence, development, and metastasis of NSCLC, which provide new directions for the early diagnosis and early treatment of NSCLC.6
Genome analysis showed that less than 2% of the genes in the human genome could encode proteins, and more than 90% of the genes are transcribed as non-coding RNAs (ncRNAs). LncRNA is a ncRNA with over 200 nt in length.7,8 LncRNA was initially considered as a “noise” of transcription. However, accumulating evidence have confirmed that lncRNAs are involved in many biological processes, such as transcription, mRNA cleavage, RNA degradation, and translation.9 Numerous studies have shown that lncRNAs exert an important role in the development of cancer via regulating its biological processes, such as the formation, infiltration, and metastasis of cancer.10 Differentially expressed lncRNAs have been found in many types of cancers, including breast cancer, prostate cancer, lung cancer, colon cancer, and liver cancer.11–15 However, only a small amount of lncRNAs have been extensively studied. HOTAIR and MALAT1 are important lncRNAs in lung cancer.11 It is necessary to further explore other differentially expressed lncRNAs in NSCLC and their possible effects.
A cluster of genes in locus 3p21 has been identified as a tumor-suppressor gene. In this cluster lies a CACNA2D2 gene coding region, which is downregulated in NSCLC and able to induce apoptosis. Therefore, it is thought to be a tumor-suppressor gene.16,17
With the development of high-throughput technologies, microarray and sequencing technologies have been applied to explore the differentially expressed lncRNAs in disease progression. Database analyses can help us find important genes related to the disease. In the present study, we analyzed the data of NSCLC in the GEO database. The differentially expressed lncRNAs in NSCLC were compared and we further explored its biological functions.
Methods
Database search
The Affymetrix HGUl33 Plus 2.0 microarray is currently used to study the transcriptome and to re-annotate lncRNA expression profiles. In the GEO database (
Probe annotation
Basic information of lncRNAs were downloaded from the Ensemble database, including transcript type, gene type, transcript ID, gene ID, etc. The probe sequence of the HG U133plus2.0 microarray was downloaded from the Affymerix website. The 54,675 probe sets of the HGU133plus2.0 microarray was compared to the selected lncRNA. When a probe set alignment compared to the same lncRNA length with longer than or equal to 90% of the probe set, it was considered that the probe set matched this lncRNA. Otherwise, the results of the probe set alignment were discarded. Formatted files that were available in the annotation were organized.
Sample collection
A total of 100 NSCLC patients undergoing radical thoracic surgery or palliative resection from December 2012 to December 2016 in the Second Affiliated Hospital of Harbin Medical University were enrolled. Primary and paracancerous tissues (5 cm away from NSCLC tissue) of NSCLC were surgically resected. Samples were immediately frozen in liquid nitrogen. None of the patients received preoperative radiotherapy or chemotherapy. Basic characteristics of patients are listed in Table 1. All resected tissues were pathologically confirmed. This study was approved by the Harbin Medical University Ethics Committee, and patients provided written informed consent. And this study was conducted with the Declaration of Helsinki.
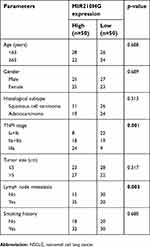 | Table 1 Correlation between MIR210HG expression and clinicopathological characteristics of NSCLC patients |
Isolation of cytoplasmic and nuclear RNA
Separation of the nuclear and cytoplasmic fractions was performed using the PARIS Kit (Life Technologies) according to the protocol. RNA in the cytoplasm and nuclei was subjected to reverse transcription reaction and real-time PCR (SYBR Premix Ex Taq; TaKaRa).
Cell culture
Normal human bronchial epithelial cell line (BEAS-2B) and lung cancer cell lines (A549, H1299, and H1975) were obtained from ATCC. BEAS-2B were cultured in BEGM medium and other cells were cultured in RPMI-1640 (Roswell Park Memorial Institute-1640, Invitrogen, Carlsbad, CA, USA) or DMEM containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin (Hyclone, South Logan, UT, USA). Cells were incubated in a 5% CO2 incubator at 37°C.
RNA extraction and qRT-PCR
Total RNA in treated cells was extracted using TRIzol method for reverse transcription according to the instructions of PrimeScript RT reagent Kit (Takara, Tokyo, Japan). RNA concentration was detected using a spectrometer. qRT-PCR was then performed based on the instructions of SYBR Premix Ex Taq TM (Takara, Tokyo, Japan). The relative gene expression was calculated using 2−ΔCt method. Primers used in the study were as follows: MIR210HG, F: GCTTGGTAGAGTGTCACGCC, R: CATCTGACCGAGCCAGTTTG; CACNA2D2, F: CAACCGGAACCTGTTCGAGG, R: CCTTGGCGTCATAGTACACG; CACNA2D2-CHIP: F: TTCCAGGTGGCATCAGTGTC, R:ACTCTGTGTCGAGGCTTTGG.
Cell transfection
Transfection vectors used in this study were conducted by GenePharma (Shanghai, China). Vector sequences were listed as follows: sh-MIR210HG-1, 5′-GGAGGGAATAATAGAGCAGCGTT-3′, sh-MIR210HG-2, 5′-GCATTAGTACAGGCACCAGCCTA-3′, sh-MIR210HG-3: 5ʹ-UUUAGACCCAUUCUCGUAUGGAGGU-3ʹ. One day prior to cell transfection, cells in good growth condition were seeded into 6-well plates with serum-free medium. Transfection was performed when the confluence was up to 60% following the instructions of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). In brief, Lipofectamine 2000 or plasmid was separately diluted in serum-free medium and mixed together at room temperature for 20 mins. The mixture was then added in each well for incubation. Culture medium was replaced 4–6 hrs later. Plasmids and shRNA used in the study were constructed by GenePharma Co., Ltd., Shanghai, China.
CCK-8 assay
Transfected cells were seeded into 96-well plates at a density of 5×104/μL. A 10 μL of CCK-8 solution (cell counting kit-8, Dojindo, Kumamoto, Japan) was added in each well after cell culture for 0, 1 d, 2 d, and 3 d, respectively. The absorbance at 450 nm of each sample was measured by a microplate reader (Bio-Rad, Hercules, CA, USA).
Colony formation assay
Transfected cells were collected and 100 cells were seeded in each well of a 6-well plate. After cell culture for 2–3 weeks, cells were washed with PBS and fixed in 2 mL of methanol for 20 mins. After PBS wash for three times, colonies were stained with 0.1% crystal violet for 20 mins. Colonies were captured in a light microscope (Olympus, Tokyo, Japan).
Transwell assay
After 48 hrs of transfection, cells were digested and resuspended in serum-free medium. Cell density was adjusted to 2.0×106/mL. Transwell chambers containing Matrigel were placed in 24-well plates. A 200 μL of cell suspension and 500 μL of medium containing 10% FBS were added in the upper and bottom chambers, respectively. After cell culture for 48 hrs, cells were fixed with 4% paraformaldehyde for 15 mins and stained with crystal violet for 15 mins. Inner cells were carefully cleaned. Penetrating cells were captured in 5 randomly selected fields of each sample. Cell invasion procedures were as the same as the above except for Matrigel pre-coating.
Western blot
Cells were lysed with RIPA lysis buffer in the presence of a protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA) to harvest total cellular protein. The protein concentration of each cell lysate was quantified using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). An equal amount of protein sample was loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and then transferred to a polyvinylidene fluoride membrane after being separated. After blocking with skim milk, membranes were incubated with primary antibody (Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C and then incubated with horseradish peroxidase–conjugated secondary antibody for 2–3 hrs at room temperature. Finally, an image of the protein band was captured by the Tanon detection system using ECL reagent (Thermo, Waltham, MA, USA).
RNA binding protein immunoprecipitation (RIP)
Cells were washed and cross-linked with 0.01% formaldehyde for 15 mins. After centrifugation and cell lysis, cell extraction was incubated with RIP buffer containing protein A/G magnetic beads coated with anti-Ago2 or negative control anti-IgG antibody. After overnight incubation at 4°C, cells were incubated with Protein A Agarose for 1 hr at 4°C, followed by the isolation of RNA. DNA methyltransferase 1 (DNMT1) level was then detected by qRT-PCR.
Chromatin immunoprecipitation (ChIP)
The cells were fixed in 1% formaldehyde for 10 mins. Anti-RNA polymerase, normal mouse IgG, and BCL6 antibodies were then immunoprecipitated overnight at 4°C. Protein G agarose (Cell Signaling Technology, Danvers, MA, USA) was added to collect immune complexes. The beads were resuspended in elution buffer and incubated overnight at 65°C before DNA was extracted. The DNA was purified using a spin column and quantified using qRT-PCR.
Statistical analysis
SPSS 21.0 statistical software was used for data analysis. Data were expressed as mean ± SD. Measurement data and classification data were compared using the Student's t-test and chi-square test, respectively. Spearman correlation analysis was introduced for correlation test. Analysis of survival data was performed using Kaplan–Meier method and univariate and multivariate regression analyses. p<0.05 considered the difference was statistically significant.
Results
MIR210HG was highly expressed in NSCLC
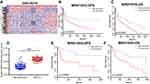
By searching for GEO database, GSE30219 dataset containing 293 NSCLC tissues and 14 non-tumor tissues was selected for analyses. The heatmap of differentially expressed lncRNAs is shown in Figure 1A. In particular, MIR210HG was the most differentially expressed lncRNA, which was selected for the following experiments. By calculating the median expression of MIR210HG, enrolled NSCLC patients were divided into high-level and low-level groups based on their MIR210HG level individually. The results showed that disease-free survival (DFS) (82 in high-level group, p=0.00604, HR=1.251–1.78) and overal survival (OS) (65 in high-level group and 121 in low-level group, p=0.0043, HR=1.32–2.545, 95%CI=0.4017–0.578) in NSCLC patients with high-level MIR210HG were shorter than those with low-level MIR210HG (Figure 1B and C). Similar results were verified in the enrolled NSCLC patients in our study (Figure 1D–F). The data showed that DFS (79 in high-level group, p=0.0023, HR=1.231–2.69) and OS (44 in high-level group and 107 in low-level group, p=1×10−5, HR=1.518–2.785, 95%CI=0.3047–0.555). The relationship between MIR210HG expression and basic characteristics of NSCLC patients was analyzed. We found that MIR210HG expression was correlated to TNM stage and lymph node metastasis, whereas it was not correlated to age, sex, tumor type, tumor size, and smoking history of NSCLC patients (Table 1). Univariate and multivariate regression analyses all indicated that high expression of MIR210HG and tumor stage are the poor prognostic factors for NSCLC (Table 2).
 | Table 2 Univariate and multivariate analysis of survival in NSCLC patients |
Low expression of MIR210HG inhibited proliferation and migration of NSCLC cells
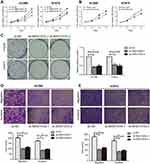
For in vitro experiments, MIR210HG was overexpressed in lung cancer cells compared with that of normal pulmonary epithelial cells. Particularly, H1229 and H1975 cells expressed the highest level of MIR210HG, which were selected for the following experiments (Figure 2A). Subsequently, we constructed sh-MIR210HG-1 and sh-MIR210HG-2 and their transfection efficacies were verified by qRT-PCR (Figure 2B). The MIR210HG expression level was up-regulated when transfected with pcDNA-MIR210HG (Figure 2C). The above results all demonstrated that differentially expressed MIR210HG may participate in NSCLC development. To explore the role of MIR210HG in proliferation and migration of NSCLC cells, we performed CCK-8, colony formation, and Transwell assay. The results indicated that MIR210HG knockdown remarkably inhibited proliferation of NSCLC cells (Figure 3A) and overexpression of MIR210HG promoted proliferation of NSCLC cells (Figure 3B). Similar results were obtained from colony formation assay (Figure 3C). Furthermore, Transwell assay results showed that knockdown of MIR210HG inhibited invasion and migration of NSCLC cells (Figure 3D and E).
MIR210HG inhibited CACNA2D2 expression by binding to DNMT1
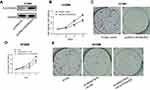
We accessed MIR210HG-related genes in GSE30219 dataset, where CACNA2D2 expression was negatively correlated with MIR210HG (Figure 4A). The same result was also confirmed in collected NSCLC samples (Figure 4B). Besides, protein level of CACNA2D2 was lowly expressed in tumor tissues compared to the normal tissues (Figure S1A). In vitro experiments showed that MIR210HG knockdown upregulated mRNA and protein levels of CACNA2D2, indicating the regulatory effect of MIR210HG on CACNA2D2 (Figure 4C and D). Methylation of the promoter region is closely related to gene expression. Therefore, we detected the methylation level of the CACNA2D2 promoter region. The data showed that the expression level of the CACNA2D2 was negatively correlated to the methylation level of its promoter (Figure 4E). DNMT1 is the most abundant DNA methyltransferase in mammalian cells, that is highly expressed in a variety of tumor tissues.18 Therefore, we speculated whether MIR210HG regulates CACNA2D2 expression through DNMT1. The isolation of cytoplasmic and nuclear RNA assay showed that MIR210HG was mainly located in the nucleus of H1299 and H1975 (Figure S1B and C). The RIP results confirmed that the MIR210HG could bind to DNMT1 (Figure 4F). Furthermore, CACNA2D2 expression was significantly up-regulated after DNMT1 knockdown (Figure 4G). DNMT1 inhibitor treatment also up-regulated CACNA2D2 expression (Figure 4H). We further verified that DNMT1 indeed bound to the CACNA2D2 promoter, validating the regulatory role of DNMT1 on CACNA2D2 (Figure 4I). MIR210HG knockdown remarkably decreased the binding kurtosis of DNMT1 and CACNA2D2 (Figure 4J). The above experiments showed that MIR210HG inhibited CACNA2D2 expression by binding to DNMT1.
 | Figure S1 (A) The protein level of CACNA2D2 in normal and tumor tissues. (B and C) The distribution of MIR210HG in H1975 and H1299. |
Overexpression of CACNA2D2 reversed the effect of MIR210HG on NSCLC
To further verify whether MIR210HG exerts its function through CACNA2D2, we constructed the overexpressed plasmid of CACNA2D2 (Figure 5A). CACNA2D2 overexpression remarkably inhibited proliferation of NSCLC cells (Figure 5B and C). Co-transfection of si-CACNA2D2 and sh-MIR210HG in NSCLC cells remarkably improved the proliferation and colony formation abilities than those transfected with sh-MIR210HG individually (Figure 5D and E). In conclusion, MIR210HG can promote the tumorigenesis of NSCLC by inhibiting the expression of CACNA2D2 (Figure 6).
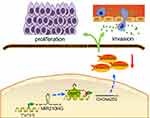 | Figure 6 Proposed model in which MIR210HG mediates the proliferation and invasion progression of NSCLC. Abbreviation: NSCLC, non-small cell lung cancer. |
Discussion
NSCLC accounts for 80–85% of all lung cancer cases, including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. The current treatment of NSCLC mainly relies on surgery and chemotherapy. However, the overall 5-year survival rate of NSCLC remains low due to the untimely diagnosis.19,20 With the increase of cancer-associated lncRNA research, NSCLC-related lncRNAs have been explored. Recent studies have shown that HOTAIR expression is positively associated with lymph node metastasis and clinical grade of NSCLC patients. In addition, NSCLC patients with high expression of HOTAIR have a poor prognosis.21 CCAT2 was found to be highly expressed in NSCLC tissues.22 GAS6-AS1 is lowly expressed in NSCLC tissues, and its expression level is related to lymph node metastasis and clinical grade of NSCLC. GAS6-AS1 is served as an independent prognostic marker in NSCLC patients.23 These studies have demonstrated that lncRNAs participate in the occurrence and development of NSCLC, which may serve as diagnostic and prognostic markers for NSCLC. Invasion and metastasis of tumor cells are the major reasons for the poor outcomes of NSCLC. In this study, we found that high expression of MIR210HG is an independent risk factor for poor prognosis of NSCLC patients. MIR210HG is closely related to tumor stage and lymph node metastasis, suggesting the remarkable role of MIR210HG in the progression of NSCLC.
Previous studies have revealed that many lncRNAs are required for the normal localization of specific protein complexes. The lncRNAs involved in dose compensation and imprinting (Xist, Kcnq1ot1, Air) regulate gene silencing as a guide in an allele-specific manner. For example, HOTAIR serves as a guide to localize PRC2 in development and cancer-related gene expressions.10,24 LncRNA-p21 is directly induced by p53 after DNA damage, which in turn physically binds to nuclear factor hnRNP-K to relocate to a specific promoter.25 Previous studies on MIR210HG suggested that it can regulate the proliferation and migration of osteosarcoma cells by adsorbing miR-530.26 Epithelial–mesenchymal transition (EMT) is the process that the epithelial cells with polarity lose their original characteristics and morphology and transform to mesenchymal cells under the external stimuli or internal factors. EMT was firstly proposed by Greenburg et al in 1982. During the process of EMT, pseudopods are formed in the culture of three-dimensional collagen gel of epithelial cells. Gradually, intracellular connections become loose and eventually transformed to mesenchymal cells. It is found that E-cadherin is re-distributed on the cell membrane and its activity decreases. Meanwhile, β-catenin aggregates in the cytoplasm, leading to the loss of polarity of epithelial cells and affecting E-cadherin expression. Downregulated E-cadherin transforms to N-cadherin, which in turn increases the content of free β-catenin. Finally, the aggravated nuclear β-catenin activates EMT-relevant signaling pathway.27 In this study, we found that MIR210HG could affect the capacity of migration. The potential mechanism may regulate the expression level of EMT-related proteins.
DNA methyltransferases (DNMTs) are important enzymes that catalyze and maintain DNA methylation in epigenetics. In mammals, DNMTs are divided into three families, namely, DNMT1, DNMT2, and DNMT3.28 DNA methylation is a common epigenetic modification mediated by DNA methyltransferases (DNMT, including DNMT1, DNMT3A, and DNMT3B). In the process of DNA methylation, the methyl group of S-adenosylmethionine is transferred to the pyrimidine. Hypermethylation of CpG islands inhibits the binding of transcription factors, thereby inhibiting gene transcription. DNMT1 was the first DNMT extracted from Escherchia coli by Gold and Hurwit in 1964.29 DNMT1 is a key enzyme in DNA methylation. Numerous studies have found that DNMT1 is associated with abnormal methylation of DNA, and both of them are closely related to the occurrence and development of tumors. Studies have shown that DNMT1 is specifically involved in the regulation of cell growth.30 DNMT1 consumption inhibits cell transcription but does not induce the invasion of MCF-7 and ZR-75-1 cells.31 Overexpression of DNMT1 can also transcribe those cells without transcriptional function.32 Knockdown of DNMT1 can reduce the risk of colorectal tumors in mice.33 In this study, correlation analysis was performed to find the potential target gene of MIR210HG and CACNA2D2 was screened out. In order to explore the specific role of CACNA2D2, we analyzed the methylation level of its promoter region. The results suggested the presence of aberrant methylation of CACNA2D2 in NSCLC tissues. Subsequently, the regulatory relationship between DNMT1 and CACNA2D2 was detected. ChIP results demonstrated that DNMT1 can bind to the promoter region of CACNA2D2, thereby inhibiting the expression of CACNA2D2. RIP results further confirmed that the binding condition between DNMT1 and CACNA2D2 was regulated by MIR210HG.
However, there are still some limitations in this study. In the present study, we found that MIR210HG had a significant role in the invasion and migration of NSCLC cells. A large number of studies have shown that EMT promotes the distant metastasis of tumor cells.34 However, we did not investigate whether MIR210HG could regulate expressions of EMT-related genes. Meanwhile, previous studies have already proved the role of MIR210HG as a ceRNA. MIR210HG is mainly expressed in the cytoplasm in osteosarcoma cells. Further studies need to be conducted to quantify the cytoplasmic and nuclear expressions of MIR210HG, thereafter clarifying the temporal and spatial specificity of lncRNA. RNA pull-down is also needed to confirm whether the protein binding of MIR210HG is dependent on the methylation level.
In conclusion, MIR210HG was found to be highly expressed in NSCLC by database search, which promoted proliferation and migration of NSCLC cells by inhibiting CACNA2D2 through binding to DNMT1. Our results provide a theoretical basis for NSCLC treatment.
Acknowledgment
This work was supported by Heilongjiang Postdoctoral Science Fund (LBH-Z16110).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Poston GJ. Global cancer surgery: the Lancet oncology review. Eur J Surg Oncol. 2015;41:1559–1561.
2. Zhao P, Dai M, Chen W, Li N. Cancer trends in China. Jpn J Clin Oncol. 2010;40:281–285. doi:10.1093/jjco/hyp187
3. Wistuba II. Genetics of preneoplasia: lessons from lung cancer. Curr Mol Med. 2007;7. doi:10.2174/156652407779940468
4. Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: a 2000-02 period analysis of Eurocare-4 data. Lancet Oncol. 2007;8:784–796. doi:10.1016/S1470-2045(07)70246-2
5. Klebe S, Henderson DW. Facts and fiction: premalignant lesions of lung tissues. Pathology. 2013;45:305. doi:10.1097/PAT.0b013e32835f45fd
6. Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi:10.1038/nature11233
7. Prensner JR, Chinnaiyan AM. The emergence of lncrnas in cancer biology. Cancer Discov. 2011;1:391. doi:10.1158/2159-8290.CD-11-0209
8. Sallam T, Sandhu J, Tontonoz P. Long noncoding RNA discovery in cardiovascular disease. Circul Res. 2018;122:155–166. doi:10.1161/CIRCRESAHA.117.311802
9. Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding rnas and cancer: a new frontier of translational research? Oncogene. 2012;31:4577–4587. doi:10.1038/onc.2011.621
10. Gupta RA, Shah N, Wang KC, et al. Long non-coding rna hotair reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi:10.1038/nature08975
11. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi:10.1038/nrg3074
12. Takayama KI, Horie Inoue K, Katayama S, et al. Androgen‐responsive long noncoding RNA ctbp1‐as promotes prostate cancer. Embo J. 2013;32:1665–1680. doi:10.1038/emboj.2013.99
13. Ji P, Diederichs S, Wang W, et al. Malat-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031. doi:10.1038/sj.onc.1206928
14. Kogo R, Shimamura T, Mimori K, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi:10.1158/0008-5472.CAN-11-1021
15. Yang F, Zhang L, Huo XS, et al. Long noncoding rna high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54:1679–1689. doi:10.1002/hep.24563
16. Lerman MI, Minna JD. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. Cancer Res. 2000;60:6116–6133.
17. Carboni GL, Gao B, Nishizaki M. CACNA2D2-mediated apoptosis in NSCLC cells is associated with alterations of the intracellular calcium signaling and disruption of mitochondria membrane integrity. Oncogene. 2003;22:615–626. doi:10.1038/sj.onc.1206134
18. Robert M, Morin S, Beaulieu N, et al. DNMT1 is required to maintain cpg methylation and aberrant gene silencing in human cancer cells. Nat Genet. 2003;33:61. doi:10.1038/ng1068
19. Yang J, Lin J, Liu T, et al. Analysis of lncRNA expression profiles in non-small cell lung cancers (NSCLC) and their clinical subtypes ☆. Lung Cancer. 2014;85:110–115. doi:10.1016/j.lungcan.2014.05.011
20. Tang Q, Ni Z, Cheng Z, Xu J, Yu H, Yin P. Three circulating long non-coding rnas act as biomarkers for predicting NSCLC. Cell Physiol Biochem. 2015;37:1002–1009. doi:10.1159/000430226
21. Liu X, Liu Z, Sun M, Liu J, Wang Z, Wei D. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. Bmc Cancer. 2013;13:464. doi:10.1186/1471-2407-13-464
22. Qiu M, Xu Y, Yang X, et al. Ccat2 is a lung adenocarcinoma-specific long non-coding RNA and promotes invasion of non-small cell lung cancer. Tumor Biol. 2014;35:5375–5380. doi:10.1007/s13277-014-1700-z
23. Han L, Kong R, Zhang E-B, De W, Yin -D-D. Low expression of long noncoding RNA gas6-as1 predicts a poor prognosis;in patients with NSCLC. Med Oncol. 2013;30:1–7. doi:10.1007/s12032-013-0694-5
24. Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human hox loci by noncoding rnas. Cell. 2007;129:1311–1323. doi:10.1016/j.cell.2007.05.022
25. Huarte M, Guttman M, Feldser D, et al. A large intergenic noncoding rna induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi:10.1016/j.cell.2010.06.040
26. Li J, Wu QM, Wang XQ, Zhang CQ. Long noncoding rna mir210hg sponges mir-503 to facilitate osteosarcoma cell invasion and metastasis. Dna Cell Biol. 2017;36. doi:10.1089/dna.2017.3888
27. Yang Y, Shao N, Luo G, Li L, Nilsson Ehle P, Xu N. Relationship between PTEN gene expression and differentiation of human glioma. Scand J Clin Lab Invest. 2006;66:469–475. doi:10.1080/00365510600763285
28. Smith ZD, Meissner A. Dna methylation: roles in mammalian development. Nat Rev Genet. 2013;14:204–220. doi:10.1038/nrg3354
29. Svedružić M. Dnmt1 structure and function. Prog Mol Biol Transl Sci. 2011;101:221–254. doi:10.1016/B978-0-12-387685-0.00006-8
30. Szyf M. The role of dna methyltransferase 1 in growth control. Front Biosci. 2001;6:D599–D609. doi:10.2741/A630
31. Chik F, Szyf M. Effects of specific dnmt gene depletion on cancer cell transformation and breast cancer cell invasion; Toward selective dnmt inhibitors. Carcinogenesis. 2011;32:224. doi:10.1093/carcin/bgq221
32. Wu J, Issa JP, Herman J, Bassett DE, Nelkin BD, Baylin SB. Expression of an exogenous eukaryotic dna methyltransferase gene induces transformation of nih 3t3 cells. Proc Natl Acad Sci U S A. 1993;90:8891–8895. doi:10.1073/pnas.90.19.8891
33. Zhang L, Li J, Li L, et al. Il-23 selectively promotes the metastasis of colorectal carcinoma cells with impaired socs3 expression via the stat5 pathway. Carcinogenesis. 2014;35:1330–1340. doi:10.1093/carcin/bgu017
34. Singh A, Settleman J. Emt, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi:10.1038/onc.2010.215
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.