Back to Journals » OncoTargets and Therapy » Volume 12
lncRNA MALAT1 potentiates the progression of tongue squamous cell carcinoma through regulating miR-140-5p-PAK1 pathway
Authors Zhu M , Zhang C, Chen D, Chen S, Zheng H
Received 24 October 2018
Accepted for publication 20 January 2019
Published 19 February 2019 Volume 2019:12 Pages 1365—1377
DOI https://doi.org/10.2147/OTT.S192069
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr XuYu Yang
Minhui Zhu,* Caiyun Zhang,* Donghui Chen, Shicai Chen, Hongliang Zheng
Department of Otorhinolaryngology-Head and Neck Surgery, Changhai Hospital, Second Military Medical University, Shanghai 200433, China
*These authors contributed equally to this work
Background: Tongue squamous cell carcinoma (TSCC) is the second most common malignancy in oral carcinoma. lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was regarded as an oncogenic factor in various carcinomas. However, its underlying molecular mechanisms in the development and progression of TSCC have not been well featured till now.
Methods: The expressions of MALAT1, miR-140-5p and p21 (RAC1)-activated kinase 1 (PAK1) mRNA were measured by RT-qPCR assay. The protein level of PAK1 was determined by western blot analysis. Cell viability was detected by Cell Counting Kit-8 assay. Transwell chamber was used to detect cell migratory and invasive capability. Luciferase reporter assay, RNA-binding protein immunoprecipitation (RIP) assay and biotin pull-down assay were applied to evaluate the relationship between MALAT1, miR-140-5p and PAK1. Xenograft experiments were performed to assess the effect and mechanism of MALAT1 in TSCC tumor growth.
Results: The expression of MALAT1 and p21 (RAC1)-activated kinase 1 (PAK1) was upregulated and microRNA-140-5p (miR-140-5p) expression was downregulated in TSCC tissues and cells. MALAT1 knockdown induced miR-140-5p expression by direct interaction. Moreover, MALAT1 knockdown inhibited proliferation, migration, and invasion by upregulating miR-140-5p expression in TSCC cells. Additionally, PAK1 was identified as a direct target of miR-140-5p. Also, MALAT1 knockdown inhibited PAK1 expression by upregulating miR-140-5p in TSCC cells. Furthermore, miR-140-5p overexpression curbed the proliferation, migration, and invasion of TSCC cells by targeting PAK1. Finally, MALAT1 knockdown inhibited tumor growth by upregulating miR-140-5p and downregulating PAK1 in mouse xenograft models of TSCC.
Conclusion: MALAT1 contributed to TSCC progression via miR-140-5p-PAK1 regulatory axis, highlighting a potential target for TSCC management.
Keywords: tongue squamous cell carcinoma, lncRNA, MALAT1, miRNA-140-5p, PAK1
Introduction
Tongue squamous cell carcinoma (TSCC), the second most common oral malignancy, was responsible for ~30% of all newly diagnosed oral carcinomas in the USA in 2018.1 In China, the incidence rate of oral carcinoma is ~4.81% and the mortality rate is about 2.21% in all carcinomas.2 Moreover, the 5-year survival rate of TSCC patients has shown no obvious improvement over the past decades, while the incidence is gradually increasing in young people.3 Despite the great advances in diagnosis and therapy of TSCC,4,5 it is still necessary to clarify the molecular mechanisms underlying TSCC pathogenesis in order to identify more effective interventions.
lncRNAs, a group of transcripts longer than 200 nucleotides (nt) without protein-coding potential, have been well documented as critical players in the development and progression of carcinomas including TSCC.6,7 Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), an lncRNA located at chromosome 11q13, was first identified as a prognostic marker in non-small-cell lung carcinoma.8 Recently, MALAT1 has been found to be implicated in tumorigenesis and progression of a great variety of carcinomas.9,10 Moreover, some researchers have provided evidence of MALAT1 as a biomarker and prognostic indicator in human carcinomas.11,12 For instance, MALAT1 facilitated cell epithelial–mesenchymal transition (EMT) and suppressed apoptosis by regulating Wnt/β-catenin signaling in TSCC.13 Knockdown of MALAT1 resulted in the upregulation of small proline-rich proteins, thereby impairing the proliferative and migratory capacities of TSCC cells.14 MALAT1 promoted EMT-mediated metastasis in oral squamous cell carcinoma (OSCC) through activating β-catenin and NF-κB pathways.15 However, the molecular mechanisms of MALAT1 in the development and progression of TSCC have not been thoroughly elucidated.
miRNAs, a class of endogenous small non-coding RNAs about 22 nt long, can regulate the translation and stability of mRNAs at post-transcriptional levels.16 Emerging evidence shows that miRNAs can act as potential oncogenic factors or tumor suppressors in human carcinomas by regulating the processes associated with tumorigenesis, such as inflammation, cell cycle, stress response, differentiation, apoptosis, and invasion.17 miR-140-5p has been reported as an antitumor factor in multiple carcinomas, such as gastric carcinoma,18 hepatocellular carcinoma,19 lung carcinoma,20 and ovarian carcinoma.21 Moreover, Kai et al pointed out that miR-140-5p could suppress cell migration and invasion in TSCC.22
In the present study, it is demonstrated that MALAT1 expression was upregulated and miR-140-5p expression was downregulated in TSCC tissues and cells. Further functional and mechanism analysis demonstrated that MALAT1 promoted the development of TSCC by miR-140-5p/p21 (RAC1)-activated kinase 1 (PAK1) regulatory pathway in vitro and in vivo.
Materials and methods
Tissue samples
TSCC tissues and adjacent normal tissues were obtained from 40 patients diagnosed with TSCC between January 2014 and December 2016 at our hospital. All tissues were immediately frozen in liquid nitrogen during the surgical procedure. The study was approved by the Ethics Committee of Changhai Hospital, Second Military Medical University. All patients signed the written informed consents prior to enrolling in this study. This study was conducted in accordance with the Declaration of Helsinki.
Cell culture
Primary normal human oral keratinocytes (NHOKs) were isolated from keratinized oral epithelial tissues of patients who suffered from flap operation to remove impacted wisdom teeth. This experiment was carried out with the written informed consents from the patients and the approval of Ethics Committee of Changhai Hospital. NHOKs were cultured in Defined Keratinocyte-serum-free medium (Thermo Fisher Scientific, Waltham, MA, USA). Human TSCC cell lines (Tca8113, SCC-2, SCC-4, SCC-9, and Cal-27) were purchased from BeNa Culture Collection (Beijing, China) and incubated in Roswell Park Memorial Institute (RPMI) 1640 medium (Thermo Fisher Scientific) supplemented with 10% FBS (Thermo Fisher Scientific). All cells were grown in a humidified incubator containing 5% CO2 at 37°C.
Cell transfection
siRNA targeting MALAT1 (si-MALAT1), scrambled control siRNA (si-NC), miR-140-5p mimics, miRNA control (miR-NC), miR-140-5p inhibitor (anti-miR-140-5p) and its control anti-miR-NC were synthesized by GenePharma Co., Ltd (Shanghai, China). To construct MALAT1 or PAK1 overexpression plasmid, the full-length sequences of MALAT1 or PAK1 were amplified by PCR and then subcloned into pcDNA3.1 vector (Thermo Fisher Scientific), named as pcDNA3.1-MALAT1 (MALAT1) and pcDNA3.1-PAK1 (PAK1). All these plasmids and oligonucleotides were transfected into TSCC cells by Lipofectamine 2000 reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Quantitative reverse transcriptase PCR (RT-qPCR) assay
Total RNA was extracted from TSCC tissues and cells by TRIzol reagent (Thermo Fisher Scientific) following the manufacturer’s protocols. For the expression analysis of MALAT1, an equal amount of RNA (1 μg) was reverse transcribed into cDNA first strand using M-MLV reverse transcriptase (Promega, Madison, WI, USA), followed by quantitative analysis using FastStart Universal SYBR Green Master (Roche Diagnostics, Mannheim, Germany) with GAPDH as the internal reference. The expression analysis of miR-140-5p was performed by the S-Poly(T) method. Briefly, RNA was first polyadenylated using Poly(A) Polymerase Tailing Kit (Epicenter, Madison, WI, USA), followed by the reverse transcription with M-MLV High-Performance Reverse Transcriptase (Epicenter) via miR-140-5p-RT primer. At last, the expression level of miR-140-5p was detected by miR-140-5p primers (forward and reverse) and Taqman probe with small nucleolar RNA SNORD47 as an endogenous control.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was detected by CCK-8 (MedChemExpress, Monmouth Junction, NJ, USA), referring to the manufacturer’s instructions. Briefly, Tca8113 and SCC-9 cells (104–105 cells/well) were inoculated into 96-well plates and transfected with corresponding oligonucleotides or plasmids. Then, CCK-8 solution (10 μL) was added into each well of 96-well plates at the indicated time points (24, 48, 72, and 96 hours) after transfection and then incubated for another 3 hours. At last, the absorbance was measured by a microplate reader at the wavelength of 450 nm.
Cell migration and invasion assay
Cell migration assay was performed using the Transwell chamber (8 μm pore size; BD Biosciences, Franklin Lakes, NJ, USA) to detect cell migratory capability. Briefly, Tca8113 and SCC-9 cells (5×104) in serum-free RPMI 1640 medium were inoculated into the upper chambers, while medium with 10% FBS was added to the lower chambers. After 48 hours of incubation at 37°C, cells on the upper side of the membranes were removed using a cotton swab. Cells adhering to the lower surface were photographed and counted after fixing with 100% pre-cold methanol and staining using 0.1% crystal violet solution. For cell invasion assay, the same experimental procedures were performed, except that the Transwell chambers were precoated with Matrigel (BD Biosciences).
Western blot assay
Total protein was obtained from TSCC Cells using pre-cold RIPA lysis buffer (Sigma-Aldrich, St Louis, MO, USA) containing protease inhibitor cocktail (Roche Diagnostics). An equal amount of protein (40 μg per lane) was separated by 10% SDS-PAGE gel and transferred to nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). After blocking with 5% skimmed milk for 1 hour at room temperature, the membranes were incubated with primary antibody against PAK1 or β-actin (Abcam, Cambridge, UK) overnight at 4°C. Subsequently, the membranes were further probed with appropriate HRP-conjugated secondary antibody for 1 hour at room temperature. Finally, the protein signals were measured by BeyoECL Plus (Beyotime, Shanghai, China) and quantified with ImageJ software. The experiment was repeated three times.
Bioinformatics analysis
The potential miRNAs that have a chance to interact with MALAT1 were predicted by miRcode database (http://www.mircode.org/). The candidate target mRNAs of miR-140-5p were predicted using the miRcode (http://www.mircode.org/) and miRwalk (http://mirwalk.umm.uni-heidelberg.de/) databases.
Luciferase reporter assay
The fragments of MALAT1 and PAK1-3′UTR region containing putative miR-140-5p binding sites were amplified by PCR and constructed into pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega), named as MALAT1-WT and PAK1-3′UTR-WT reporter, respectively. QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) was used to generate MALAT1-MUT and PAK1-3′UTR-MUT reporters containing mutant miR-140-5p binding sites. Then, the constructed luciferase reporters were, respectively, co-transfected with miR-NC or miR-140-5p mimic into TSCC cells. At 48 hours after transfection, luciferase activity was detected with Dual-Luciferase Reporter Assay System (Promega) referring to the manufacturer’s instructions.
RNA-binding protein immunoprecipitation (RIP) assay
The RIP assay was performed using the EZ-Magna RIP Kit (EMD Millipore), referring to the instructions of the manufacturer. Briefly, TSCC cells were lysed by RIP lysis buffer supplemented with cocktail (Roche Diagnostics). Then, cell supernatants were incubated overnight at 4°C with primary antibody against Ago2 or mouse IgG (EMD Millipore) and protein A/G magnetic beads. Following this, RNase-free DNase I (Promega) and Proteinase K were used to digest extra DNAs and proteins. At last, purified RNA was analyzed by the RT-qPCR assay to measure the expression of MALAT1 and miR-140-5p.
Biotin pull-down assay
The pull-down assay was performed according to a previous report.23 Briefly, Tca8113 cells were transfected with biotinylated MALAT1 probe (Bio-MALAT1-probe) or a negative control probe (Bio-NC-probe). At 48 hours after transfection, cells were harvested for biotin-based pull-down assay, followed by RT-qPCR assay to determine miR-140-5p level.
Animal experiments
The animal experiments were performed with the approval of the Ethics Committee of Changhai Hospital, Second Military Medical University. Animal experiments were carried out according to the National Institutes of Health guidelines to the Care and Use of Laboratory Animals. Female Nu/Nu nude mice (6–8 weeks old, n=12) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). Lentiviruses carrying MALAT1 shRNA (sh-MALAT1) or control shRNA (sh-NC) were purchased from Genomeditech Co., Ltd (Shanghai, China) and then added into Tca8113 cells. Next, Tca8113 cells (5×106) infected with lentiviruses carrying sh-NC or sh-MALAT1 were injected subcutaneously into the right flanks of sh-NC or sh-MALAT group mice with six mice in each group. Tumor volume was monitored using a caliper every week with the formula: volume = (long diameter × short diameter2)/2. Tumors were resected, photographed, and weighed at 5 weeks after injection. The levels of MALAT1 and miR-140-5p were measured by RT-qPCR assay in resected xenograft tumors at the end of experiments. PAK1 protein level in xenograft tumors was determined by Western blot assay at the end of experiments.
Statistical analysis
Results were presented as mean ± SD from at least three independent experiments. Student’s t-test/Wilcoxon test (for two-group data) or one-way ANOVA together with Tukey’s post hoc test (for more than two-group data) was used for the analysis of differences. The correlation between miR-140-5p and MALAT1 or PAK1 was evaluated by Pearson correlation analyses. P<0.05 represented the difference was statistically significant.
Results
MALAT1 expression was upregulated and miR-140-5p expression was downregulated in TSCC tissues and cells
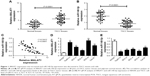
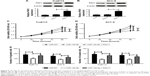
First, the expression patterns of MALAT1 and miR-140-5p were investigated in TSCC tissue specimens and cell lines by RT-qPCR assay. Results showed that MALAT1 expression was markedly increased (Figure 1A) and miR-140-5p expression was strikingly decreased (Figure 1B) in TSCC tissues compared with adjacent non-carcinomatous tissues. Moreover, MALAT1 expression was found to be associated with tumor size, lymph node metastasis (LNM), and TNM stage in TSCC (Table 1). Correlation coefficient analysis also showed that miR-140-5p level was inversely associated with MALAT1 level in TSCC tissues (Figure 1C). Moreover, RT-qPCR assay showed that MALAT1 expression was remarkably upregulated (Figure 1D) and miR-140-5p expression was notably downregulated (Figure 1E) in TSCC cell lines (Tca8113, SCC-2, SCC-4, SCC-9, and Cal-27) compared with NHOK cell line. In summary, these data indicated that MALAT1 and miR-140-5p might be implicated in the development of TSCC.
MALAT1 inhibited miR-140-5p expression by direct interaction
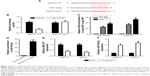
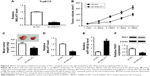
Accumulating evidence reveals that lncRNAs can serve as miRNA sponges or decoys to modulate endogenous miRNAs, thus leading to derepression of target mRNAs.24 Thus, whether MALAT1 could act as a miR-140-5p sponge to exert its oncogenic effects was further explored in TSCC. Bioinformatics analysis by miRcode website showed that there were several complementary sites between MALAT1 and miR-140-5p (Figure 2A). Dual-luciferase reporter assay proved that the overexpression of miR-140-5p markedly attenuated luciferase activity of MALAT1-WT reporter, but not that of MALAT1-MUT reporter in Tca8113 cells (Figure 2B). RIP assay further revealed that both MALAT1 and miR-140-5p were remarkably enriched by Ago2 antibody in Tca8113 cells compared with anti-IgG negative control group (Figure 2C). Subsequent pull-down assay demonstrated that biotin-labeled MALAT1 probe (Bio-MALAT1-probe) could substantially enrich miR-140-5p in Tca8113 cells compared with the control group (Figure 2D). Moreover, MALAT1 expression was reduced (Figure 2E) and miR-140-5p expression was enhanced (Figure 2F) in Tca8113 and SCC-9 cells transfected with si-MALAT1. That is to say, MALAT1 knockdown promoted miR-140-5p expression via direct interaction in TSCC cells.
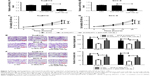
MALAT1 knockdown suppressed the proliferation, migration, and invasion by regulating miR-140-5p in TSCC cells
First, the transfection efficiency of miR-140-5p inhibitor in TSCC cells was confirmed. As presented in Figure 3A and B, a notable decrease in miR-140-5p expression was observed in Tca8113 and SCC-9 cells compared with untransfected group or anti-miR-NC group. Then, the effects of MALAT1 on the proliferation, migration, and invasion of TSCC cells were detected by CCK-8, Transwell migration, and invasion assay. The results showed that downregulation of MALAT1 by si-MALAT1 significantly suppressed the proliferation (Figure 3C and D), migration (Figure 3E), and invasion (Figure 3F) in both Tca8113 and SCC-9 cells. To further investigate whether the effects of MALAT1 in TSCC progression were mediated by miR-140-5p, miR-140-5p inhibitor was introduced into si-MALATA–transfected TSCC cells. The results showed that si-MALATA–induced inhibition of proliferation (Figure 3C and D), migration (Figure 3E), and invasion (Figure 3F) was greatly attenuated by knockdown of miR-140-5p. Taken together, these data indicate that MALAT1 exerted its oncogenic effects partially through suppressing miR-140-5p expression.
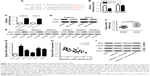
MALAT1 knockdown suppressed PAK1 expression by upregulating miR-140-5p
It is well known that miRNAs exert their functions by modulating the expression of target genes. By using miRcode website, the complementary sequences between miR-140-5p and PAK1 3′UTR were identified (Figure 4A). Subsequent dual-luciferase reporter assay showed that miR-140-5p overexpression decreased the luciferase activity of PAK1-WT reporter, while little change was observed in the luciferase activity of PAK1-MUT reporter between miR-NC and miR-140-5p groups (Figure 4B). To explore the effect of miR-140-5p on PAK1 expression, miR-140-5p mimic was transfected into TSCC cells. As expected, transfection of miR-140-5p mimic resulted in an obvious elevation of miR-140-5p level in Tca8113 and SCC-9 cells (Figure 4C). Western blot analysis revealed that the ectopic expression of miR-140-5p dramatically suppressed PAK1 protein expression in Tca8113 and SCC-9 cells (Figure 4D). PAK1 expression was found to be markedly upregulated in six randomly selected TSCC tissues compared with corresponding normal tissues (Figure 4E). Moreover, a notable upregulation of PAK1 mRNA level was observed in 40 cases of TSCC tissues compared with normal tissues (Figure 4F). Additionally, PAK1 mRNA level was markedly increased in several TSCC cells (Tca8113, SCC-2, SCC-4, SCC-9, and Cal-27) compared with NHOK control cells (Figure 4G). Subsequent correlation analysis revealed that PAK1 mRNA level was negatively associated with PAK1 level in TSCC tissues (Figure 4H). Furthermore, MALAT1 knockdown resulted in a prominent decline of PAK1 protein expression in both Tca8113 and SCC-9 cells, while this effect was markedly weakened by miR-140-5p inhibitor (Figure 4I). Overall, these data indicate that MALAT1 could act as a molecular sponge of miR-140-5p to sequester miR-140-5p from its target gene PAK1, resulting in the upregulation of PAK1 level in TSCC cells.
miR-140-5p repressed the proliferation, migration, and invasion through targeting PAK1 in TSCC cells
To further explore whether miR-140-5p affected TSCC development by regulating PAK1, the transfection efficiency of PAK1 overexpression plasmid (pcDNA3.1-PAK1) was first investigated in TSCC cells. As displayed in Figure 5A and B, PAK1 protein level was markedly enhanced in Tca8113 and SCC-9 cells following transfection with pcDNA3.1-PAK1 (Figure 5A and B), suggesting its applicable values in subsequent gain-of-function experiments. Then, TSCC cells were co-transfected with miR-140-5p mimics and pcDNA3.1-PAK1. Results showed that miR-140-5p overexpression led to a significant decrease in proliferation, migration, and invasion of Tca8113 and SCC-9 cells, while these effects were substantially abated following increase of PAK1 expression (Figure 5C–F). All these data suggest that miR-140-5p suppressed the proliferation, migration, and invasion via downregulating PAK1 in TSCC cells.
MALAT1 knockdown inhibited the growth of TSCC xenograft tumors through upregulating miR-140-5p and downregulating PAK1 in vivo
First, MALAT1 expression level was measured in Tca8113 cells infected with sh-NC or sh-MALAT1 lentiviruses. Results showed that MALAT expression was strikingly reduced in Tca8113 cells infected with sh-MALAT1 lentiviruses compared with sh-NC group (Figure 6A), indicating that sh-MALAT1–infected Tca8113 cells could be used for the following in vivo loss-of-function experiments. Then, the roles and molecular mechanisms of MALAT1 on the growth of TSCC xenograft tumors were further tested. Results displayed that MALAT1 knockdown inhibited tumor growth in xenograft models of TSCC, as evidenced by the reduction of tumor volume (Figure 6B) and tumor weight (Figure 6C) in MALAT-depleted mice. Moreover, downregulated MALAT1 level (Figure 6D), increased miR-140-5p expression (Figure 6E), and decreased PAK1 expression (Figure 6F) were observed in MALAT1-silenced xenograft tumors of TSCC. These data verified that MALAT1 contributed to tumor growth via miR-140-5p/PAK1 regulatory axis in vivo.
Discussion
Accumulating evidence indicates that abnormal expression of lncRNAs and miRNAs is closely associated with the development and progression of human carcinomas.25 Moreover, some reports pointed out that lncRNAs could act as potential tumor suppressors or oncogenic factors in TSCC. For instance, lncRNA AFAP1 antisense RNA 1 (AFAP1-AS1) facilitated proliferation, migration, and invasion via activating Wnt/β-catenin pathway in TSCC cells.26 NKILA impaired cell migratory and invasive capacities by inhibiting EMT via regulation of NF-κB activity in TSCC.27 Moreover, the carcinogenic effects of MALAT1 on TSCC have been highlighted in several reports.14,28 Nevertheless, the underlying molecular mechanisms of MALAT1 in TSCC progression are far from being elucidated.
A growing body of literature points out that lncRNAs can serve as miRNA decoys or sponges to affect their expression.29 miR-140-5p has been found to inhibit cell migration and invasion in TSCC.22 Moreover, previous studies showed that MALAT1 promoted proliferation and invasion of uveal melanoma cells through silencing miR-140,30 and MALAT1 knockdown enhanced the blood–tumor barrier permeability by increasing miR-140 expression.31
In the present study, it is found that MALAT1 expression was markedly upregulated in TSCC tissues and cells. Moreover, MALAT1 expression was associated with tumor size, LNM, and TNM stage in TSCC. Consistently, Fang et al pointed out that TSCC patients (n=59) with LNM have higher MALAT1 expression compared with patients without LNM (n=68).14 Zhou et al disclosed that OSCC patients (n=19) with higher MALAT1 expression have a shorter survival time compared with patients with lower MALAT1 expression (n=35).15 Also, miR-140-5p expression was notably downregulated in TSCC tissues and cells. Moreover, there existed a significant inverse correlation between MALAT1 and miR-140-5p expression in TSCC tissues. Subsequent bioinformatics analysis showed that MALAT1 contained binding sequences of miR-140-5p. Luciferase reporter experiments, RIP, and pull-down assay further validated the interplay between MALAT1 and miR-140-5p. Furthermore, MALAT1 knockdown strikingly facilitated miR-140-5p expression in TSCC cells. All these data indicate that MALAT1 reduced miR-140-5p expression by direct interaction. Then, functional analysis showed that downregulation of MALAT1 hampered the proliferation, migration, and invasion of TSCC cells, while these effects were obviously abrogated by inhibition of miR-140-5p. Overall, MALAT1 exerted its oncogenic effects through silencing miR-140-5p expression in TSCC cells.
Then, web-based tools demonstrated that PAK1-3′UTR possessed possible binding sites of miR-140-5p. Moreover, luciferase reporter and Western blot assays confirmed that PAK1 was a direct target of miR-140-5p. Furthermore, PAK1 expression was markedly increased in TSCC tissues and cells and negatively correlated with miR-140-5p expression in TSCC tissues. Additionally, MALAT1 knockdown inhibited PAK1 expression by upregulating miR-140-5p in TSCC cells.
Subsequent functional analysis clarified that miR-140-5p repressed the proliferation, migration, and invasion in TSCC cells, whereas these effects were dramatically reversed after overexpressing PAK1, suggesting that the tumor-suppressive roles of miR-140-5p were mediated by PAK1. PAK1, a prototype of group I PAK family, has been elucidated as the cause for several oncogenic processes.32,33 Several documents verified that PAK1 was a vital target of miRNA signal pathways. For example, miR-494 repressed the proliferation, migration, and invasion of breast carcinoma cells by targeting PAK1.34 miR-98 directly targeted the 3′UTR of PAK1 to downregulate its expression, thus inhibiting cell proliferation and invasion in non-small-cell lung carcinoma.35 In this study, it is further demonstrated that MALAT1 knockdown inhibited the growth of TSCC xenograft tumors via upregulating miR-140-5p and downregulating PAK1 in vivo.
Previous studies showed that MALAT1 facilitated OSCC development by regulating miR-125b/signal transducer and activator of transcription 3 (STAT3) axis.36 MALAT1 could interact with miR-320d by SRSF1, and miR-320d knockdown induced CCR7 expression in OSCC.37 Moreover, Zhang et al pointed out that MALAT1 knockdown inhibited the tumorigenesis and progression of tongue cancer in vitro and in vivo by miR-124/jagged1 (JAG1) pathway.28 MALAT1 could interact with miR-30a in human head and neck squamous cell carcinoma.38 Moreover, some studies have shown that miR-124 could exert its antitumor effect by targeting STAT3 in hepatocellular carcinoma,39 and that miR-140-5p suppressed glioma cell proliferation and invasion by targeting JAG1.40 ADAM10, LAMC1, HDAC7, and PAX6 have been reported as the targets of miR-140-5p in tongue cancer.22 Furthermore, Lee et al pointed out that PAK1 suppressed ADAM17 association and lipid raft transfer by phosphorylating paxillin, and HIV-1 and cancer cells could exploit a paxillin/integrin-controlled mechanism to release ADAM17/ADAM10-containing vesicles, hinting the link of PAK1 and ADAM10 (a target of miR-140-5p).41 These data indicate that some downstream signaling pathways of MALAT1 were mutually relevant.
Moreover, bioinformatics analysis by miRcode website suggests that MALAT1 has a chance to interact with multiple miRNAs such as miR-503, miR-96, and miR-17-5p. Additionally, prediction results from miRwalk show that ADAM2, WNT7B, PAQR7, and P2RY2 are the potential targets of miR-140-5p.
Taken together, this study demonstrated that MALAT1 promoted the development of TSCC partly via regulating miR-140-5p/PAK1 pathway in vitro and in vivo. This study provides a novel insight into the involvement of MALAT1 in TSCC development and elucidates a promising therapeutic candidate for TSCC treatment.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No 81402253, No 81572668).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Sgaramella N, Gu X, Boldrup L, et al. Searching for new targets and treatments in the battle against squamous cell carcinoma of the head and neck, with specific focus on tumours of the tongue. Curr Top Med Chem. 2018;18(3):214–218. | ||
Christopherson K, Morris CG, Kirwan JM, et al. Radiotherapy alone or combined with chemotherapy for base of tongue squamous cell carcinoma. Laryngoscope. 2017;127(7):1589–1594. | ||
Keshavarzi M, Darijani M, Momeni F, et al. Molecular imaging and oral cancer diagnosis and therapy. J Cell Biochem. 2017;118(10):3055–3060. | ||
Peng WX, Koirala P, Mo YY. lncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–5667. | ||
Gao W, Chan JY, Wong TS. Long non-coding RNA deregulation in tongue squamous cell carcinoma. Biomed Res Int. 2014;2014(2):1–10. | ||
Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031–8041. | ||
Gutschner T, Hämmerle M, Diederichs S. MALAT1 – a paradigm for long noncoding RNA function in cancer. J Mol Med. 2013;91(7):791–801. | ||
Liu J, Wx P, Yy M, Luo D. MALAT1-mediated tumorigenesis. Front Biosci (Landmark Ed). 2017;22:66–80. | ||
Wang Y, Xue D, Li Y, et al. The long noncoding RNA MALAT-1 is a novel biomarker in various cancers: a meta-analysis based on the Geo database and literature. J Cancer. 2016;7(8):991–1001. | ||
Wei Y, Niu B. Role of MALAT1 as a prognostic factor for survival in various cancers: a systematic review of the literature with meta-analysis. Dis Markers. 2015;2015:164635. | ||
Liang J, Liang L, Ouyang K, Li Z, Yi X. MALAT1 induces tongue cancer cells’ EMT and inhibits apoptosis through Wnt/β-catenin signaling pathway. J Oral Pathol Med. 2017;46(2):98–105. | ||
Fang Z, Zhang S, Wang Y, et al. Long non-coding RNA MALAT-1 modulates metastatic potential of tongue squamous cell carcinomas partially through the regulation of small proline rich proteins. BMC Cancer. 2016;16(1):706. | ||
Zhou X, Liu S, Cai G, et al. Long non coding RNA MALAT1 promotes tumor growth and metastasis by inducing epithelial–mesenchymal transition in oral squamous cell carcinoma. Sci Rep. 2015;5(1):15972. | ||
Duchaine TF, Fabian MR. Mechanistic insights into microRNA-mediated gene silencing. Cold Spring Harb Perspect Biol. 2018:a032771. | ||
Markopoulos GS, Roupakia E, Tokamani M, et al. A step-by-step microRNA guide to cancer development and metastasis. Cell Oncol (Dordr). 2017;40(4):303–339. | ||
Fang Z, Yin S, Sun R, et al. miR-140-5p suppresses the proliferation, migration and invasion of gastric cancer by regulating YES1. Mol Cancer. 2017;16(1):139. | ||
Yan X, Zhu Z, Xu S, et al. MicroRNA-140-5p inhibits hepatocellular carcinoma by directly targeting the unique isomerase Pin1 to block multiple cancer-driving pathways. Sci Rep. 2017;7(1):45915. | ||
Flamini V, Jiang WG, Cui Y. Therapeutic role of miR-140-5p for the treatment of non-small cell lung cancer. Anticancer Res. 2017;37(8):4319–4327. | ||
Lan H, Chen W, He G, Yang S. miR-140-5p inhibits ovarian cancer growth partially by repression of PDGFRA. Biomed Pharmacother. 2015;75:117–122. | ||
Kai Y, Peng W, Ling W, Jiebing H, Zhuan B. Reciprocal effects between microRNA-140-5p and ADAM10 suppress migration and invasion of human tongue cancer cells. Biochem Biophys Res Commun. 2014;448(3):308–314. | ||
Phatak P, Donahue J. Biotinylated micro-RNA pull down assay for identifying miRNA targets. Bio Protoc. 2017;7(9). | ||
Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol. 2014;34:9–14. | ||
Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta. 1859;2016:169–176. | ||
Wang ZY, Hu M, Dai MH, et al. Upregulation of the long non-coding RNA AFAP1-AS1 affects the proliferation, invasion and survival of tongue squamous cell carcinoma via the Wnt/β-catenin signaling pathway. Mol Cancer. 2018;17(1):3. | ||
Huang W, Cui X, Chen J, et al. Long non-coding RNA NKILA inhibits migration and invasion of tongue squamous cell carcinoma cells via suppressing epithelial–mesenchymal transition. Oncotarget. 2016;7(38):62520–62532. | ||
Zhang TH, Liang LZ, Liu XL, et al. Long non-coding RNA MALAT1 interacts with miR-124 and modulates tongue cancer growth by targeting JAG1. Oncol Rep. 2017;37(4):2087–2094. | ||
Chan J, Tay Y. Noncoding RNA:RNA regulatory networks in cancer. Int J Mol Sci. 2018;19(5):1310. | ||
Sun L, Sun P, Zhou QY, Gao X, Han Q. Long noncoding RNA MALAT1 promotes uveal melanoma cell growth and invasion by silencing of miR-140. Am J Transl Res. 2016;8(9):3939–3946. | ||
Ma J, Wang P, Yao Y, et al. Knockdown of long non-coding RNA MALAT1 increases the blood–tumor barrier permeability by up-regulating miR-140. Biochim Biophys Acta. 2016;1859(2):324–338. | ||
King H, Nicholas NS, Wells CM. Role of p-21-activated kinases in cancer progression. Int Rev Cell Mol Biol. 2014;309:347–387. | ||
Eswaran J, Li DQ, Shah A, Kumar R. Molecular pathways: targeting p21-activated kinase 1 signaling in cancer – opportunities, challenges, and limitations. Clin Cancer Res. 2012;18(14):3743–3749. | ||
Zhan MN, Yu XT, Tang J, et al. MicroRNA-494 inhibits breast cancer progression by directly targeting PAK1. Cell Death Dis. 2017;8(1):e2529. | ||
Yang G, Zhang X, Shi J. miR-98 inhibits cell proliferation and invasion of non-small cell carcinoma lung cancer by targeting PAK1. Int J Clin Exp Med. 2015;8(11):20135–20145. | ||
Chang SM, Hu WW. Long non-coding RNA MALAT1 promotes oral squamous cell carcinoma development via microRNA-125b/STAT3 axis. J Cell Physiol. 2018;233(4):3384–3396. | ||
Xu Z, Han X, Tang Z, et al. Interaction between MALAT-1, CCR7 and correlated genes in oral squamous cell carcinoma. Int J Clin Exp Pathol. 2017;10(11):10730–10739. | ||
Wang Y, Wu C, Zhang C, et al. TGF-β-induced STAT3 overexpression promotes human head and neck squamous cell carcinoma invasion and metastasis through malat1/miR-30a interactions. Cancer Lett. 2018;436:52–62. | ||
Lu Y, Yue X, Cui Y, Zhang J, Wang K. MicroRNA-124 suppresses growth of human hepatocellular carcinoma by targeting STAT3. Biochem Biophys Res Commun. 2013;441(4):873–879. | ||
Yang HL, Gao YM, Zhao JA. miR-140-5p inhibits human glioma cell growth and invasion by targeting JAG1. Mol Med Rep. 2017;16(3):3634–3640. | ||
Lee JH, Wittki S, Bräu T, et al. HIV Nef, paxillin, and Pak1/2 regulate activation and secretion of TACE/ADAM10 proteases. Mol Cell. 2013;49(4):668–679. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.