Back to Journals » Cancer Management and Research » Volume 12
LncRNA MAFG-AS1 Accelerates Cell Migration, Invasion and Aerobic Glycolysis of Esophageal Squamous Cell Carcinoma Cells via miR-765/PDX1 Axis
Authors Qian C, Xu Z, Chen L, Wang Y, Yao J
Received 12 May 2020
Accepted for publication 22 July 2020
Published 5 August 2020 Volume 2020:12 Pages 6895—6908
DOI https://doi.org/10.2147/CMAR.S262075
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Cui-juan Qian1 ,* Zhu-rong Xu1 ,* Lu-yan Chen,1 Yi-chao Wang,2 Jun Yao1
1Institute of Tumor, School of Medicine, Taizhou University, Taizhou, Zhejiang 318000, People’s Republic of China; 2Department of Medical Laboratory, Taizhou Central Hospital, Taizhou University Hospital, Taizhou, Zhejiang 318000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Yao Email [email protected]
Background: LncRNA dysregulation is implicated in esophageal squamous cell carcinoma (ESCC) progression; However, the precise role and function of lncRNA MAFG-AS1 in ESCC remains unknown.
Materials and Methods: Expressions of MAFG-AS1, miR-765, PDX1, GLUT1 and LDH-A were detected via qRT-PCR or/and Western blot in ESCC tissues and cell lines. CCK-8, transwell and glycolysis assays were used to investigate the effects of MAFG-AS1 on ESCC cell proliferation, migration, invasion and aerobic glycolysis after knockdown or overexpression of MAFG-AS1, and bioinformatics analyses, RNA pull-down and dual luciferase reporter systems were applied to investigate the interaction between MAFG-AS1, miR-765 and PDX1.
Results: MAFG-AS1 was significantly up-modulated in ESCC tissues and cell lines. MAFG-AS1 significantly accelerated ESCC cell proliferation, migration, invasion and aerobic glycolysis. MAFG-AS1 competitively adsorbed miR-765, while miR-765 negatively modulated the expression of PDX1. miR-765 and PDX1 participated in the promotive effects of MAFG-AS1 on cell migration, invasion and aerobic glycolysis in ESCC cells.
Conclusion: Our research indicates that the MAFG-AS1/miR-765/PDX1 axis accelerates ESCC cell proliferation, migration, invasion and aerobic glycolysis.
Keywords: ESCC, MAFG-AS1, aerobic glycolysis, miR-765, PDX1
Introduction
Due to the development and improvement of surgery, radiotherapy, chemotherapy and comprehensive treatments, the curative effect on treating early-stage esophageal squamous cell carcinoma (ESCC) has made great progress, but the curative effect of ESCC in the middle and advanced stages is still unsatisfactory.1,2 The poor prognosis of patients with mid-advanced ESCC has been directly attributed to tumor invasion and metastasis.1,2 Therefore, it is of great clinical significance to investigate the mechanism of ESCC invasion and metastasis, and to seek new biomarkers for diagnosis and prognosis and new therapeutic targets for ESCC.
In recent years, with the in-depth study of gene expression modulation, especially in the field of epigenetics, long non-coding RNAs (lncRNAs) have been found to be important and even decisive factors in ESCC occurrence and development.3 For example, lncRNA taurine up-modulated gene 1 (TUG1) accelerates ESCC cell proliferation and invasion via miR-498.4 Similarly, lncRNA DLX6 antisense RNA 1 (DLX6-AS1) accelerates ESCC cell growth and tumor metastasis.5 MAFG antisense 1 (MAFG-AS1, Aliases: MAFG divergent transcript, MAFG-DT) is a newly discovered lncRNA, and a series of recent studies have demonstrated that MAFG-AS1 can accelerate cancer cell proliferation and invasion in hepatocellular carcinoma, breast cancer and colorectal cancer.6,7 However, the function and related mechanism of lncRNA MAFG-AS1 has not been systematically studied in ESCC. Importantly, we here provided strong evidences to support the tumor-promotive role of MAFG-AS1 in ESCC progression.
Normal cells mainly obtain energy via mitochondrial oxidation pathway, but most tumor cells still preferentially obtain energy via glycolysis pathway even under the condition of sufficient oxygen, which is called aerobic glycolysis or Warburg Effect.8 Moreover, increasing data have revealed that lncRNAs affect tumor invasion and metastasis via modulating aerobic glycolysis process.9 However, there have been no reports on the correlation between MAFG-AS1 and aerobic glycolysis. Interestingly, we here provided strong evidences to support the correlation between MAFG-AS1 and aerobic glycolysis in ESCC.
At present, the study focus of lncRNAs is on the unique function of “molecular sponge”.10,11 LncRNAs can specifically bind to the corresponding miRNAs, thus functioning as “molecular sponges” to influence the modulation of miRNAs on their target genes in ESCC.3,12 For instance, lncRNA prostate cancer associated transcript 1 (PCAT-1) accelerated ESCC progression via adsorbing miR-508-3p.12 lncRNA TTN antisense RNA 1 (TTN-AS1) induces fascin homolog 1 (FSCN1) expression via sponging miR-133b, which further accelerates ESCC invasion and metastasis.13 However, it is not clear which miRNA can be anchored via MAFG-AS1.
MiR-765 is believed to function as a tumor accelerator or suppressor gene in a variety of malignant tumors. For example, miR-765 was highly expressed in osteosarcoma (OS) tissues, and up-modulation of miR-765 accelerated OS cell proliferation, migration and invasion,14 whereas miR-765 restrained cell proliferation, invasion and metastasis in clear cell renal cell carcinoma via down-modulating proteolipid protein 2 (PLP2).15 Moreover, a previous study demonstrated that ectopic expression of lncRNA Linc00511 accelerated OS cell proliferation, colony formation and migration via down-modulation of miR-765,16 but so far the particular role and mechanism of miR-765 in ESCC has not been reported, and how MAFG-AS1 modulates expression and function of miR-765 still needs further systematic study. Here, we demonstrated that miR-765 played a tumor-repressive role, and was negatively modulated by MAFG-AS1 in ESCC.
In short, the researches on the role of lncRNAs in ESCC and how lncRNAs modulate the occurrence and development of ESCC are still in their preliminary stages. The current study examined the expression of a novel lncRNA MAFG-AS1 in ESCC tissues and cell lines, and then further investigated the effects of MAFG-AS1 on the proliferation, migration, invasion and aerobic glycolysis of ESCC cells via modulating miR-765/PDX1 axis. We confirmed here that miR-765 plays a tumor-repressive role in ESCC cells. More importantly, we supposed that MAFG-AS1 can specifically bind to miR-765, thus functioning as “molecular sponges” to influence the modulation of miR-765 on its downstream target gene PDX1. The current study provides experimental basis and theoretical support for seeking new biomarkers for early diagnosis and prognosis, and new therapeutic targets for ESCC.
Materials and Methods
Clinical Tissue Specimens
The current study was approved by the Medical Ethics Committee of Taizhou University Hospital. Forty paired ESCC and their paired paracancerous nontumor tissues were collected from surgical tumor resections performed at Taizhou University Hospital (Taizhou, Zhejiang, China). All ESCC patients signed the written informed consent. Tissues were snap-frozen in liquid nitrogen and stored at −80°C for subsequent analysis.
Cell Culture and Transfection
Human ESCC cell lines (EC9706, EC109, KYSE30 and KYSE150) and a human esophageal epithelial cell line (Het-1A) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). These cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum (FBS), 10,000 units/mL of penicillin and 10,000 g/mL of streptomycin. The culture conditions were 37°C and 5% CO2. Cells were harvested during logarithmic growth phase, and seeded in 6-well plates at 1×105 cells/well. The transfection products, including si-MAFG-AS1 (5′-GGGCAAUUCCAACCAAGAAAC-3′), si-NC (negative control siRNA; 5′-AAUUCUCCGAACGUGUCUCGUGU-3′),6 miR-765 mimics and control mimics were all synthesized by GenePharma (Shanghai, China). EC109 and EC9706 cells were transfected with 100 ng of the vector or 50 nM of the indicated oligonucleotide by Lipofectamine™ 2000 (Invitrogen, CA, USA). For overexpression, MAFG-AS1 was cloned into mammalian expression vector pcDNA3.1 (Invitrogen, CA, USA) to construct overexpressing plasmid p-MAFG-AS1. Vector pcDNA3.1 was used as a vector control. After 48 h of transfection, cells were used for the next detection.
Expressions of MAFG-AS1, miR-765 and PDX1 Detected via qRT-PCR
The transient transfection efficiencies of ESCC cells after 48 h were detected. Total RNA of ESCC cells and tissues was extracted with TRIzol reagent (Invitrogen, CA, USA), and then the first-strand cDNA was synthesized using the PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Dalian, China). qRT-PCR was performed using the SYBR Green qPCR Kit (Thermo Scientific, Waltham, MA, USA) with the 7500 Fast Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc). The relative quantification of genes was carried out via the 2−ΔΔCt method and normalized to the internal control GAPDH or U6. The sequences of all primers used in this experiment are listed in Table 1, and the RNA expression levels were analyzed as described previously.6,17,18
 |
Table 1 qRT-PCR Primer Sequences |
Expressions of PDX1, LDH-A and GLUT1 Detected via Western Blotting Analysis
Cells were collected and lysed using RIPA Lysis Buffer (Beyotime, Wuxi, China) supplemented with a protease inhibitor cocktail and phenylmethylsulfonyl fluoride (Roche, Pleasanton, CA, USA). The protein concentration was determined using a Bradford Protein Assay Kit (Beyotime, Wuxi, China). Approximately 50 µg of protein extract was separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred to polyvinylidene fluoride (PVDF) membranes (Merck KGaA, Darmstadt, Germany) and incubated with specific antibodies. The primary antibodies against PDX1 (1:1000, catalog no.ab ab47267; Abcam, Cambridge, MA, USA), LDH-A (1:1000, catalog no.ab125683; Abcam, Cambridge, MA, USA) and GLUT1 (1:1000, catalog no.ab15309; Abcam, Cambridge, MA, USA) were used. Following extensive washing, membranes were incubated with a horseradish peroxidase-conjugated goat polyclonal anti-rabbit IgG secondary antibody (1:2000, catalog no.7074; Cell Signaling Technology, Danvers, MA, USA) for 1 h at room temperature. Immunoreactivity was detected by enhanced a chemiluminescence system kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and visualized using a LAS-4000 imaging system (Fujifilm Holdings Corporation, Tokyo, Japan). GAPDH (1:1000, catalog no.ab181602; Abcam, Cambridge, MA, USA) served as a loading control.
Cell Proliferation Detected via CCK-8 Assay
After transfection, ESCC cell viability was assessed with the Cell Counting Kit-8 (CCK-8) kit (Abcam, Cambridge, MA, USA). In brief, ESCC cells were harvested at logarithmic growth phase, and seeded in a 96-well plate at 1×104 cells/well; 100 μL RPMI 1640 medium was added to the cells, and three parallel controls were set up in each group. Cells were transfected with si-MAFG-AS1, si-NC, p-MAFG-AS1 or vector control, respectively. 10 μL of CCK-8 solution was added to the cells, and then incubated in the incubator for 2 h before detection of the optical density OD at 450 nm. All operations were performed in accordance with the instructions of the CCK-8 detection kit.
Cell Migration and Invasion Detected via Transwell Assay
For transwell migration assay, ESCC cells were collected at logarithmic growth stage, and the cell density was adjusted to 2×105 cells/mL with RPMI 1640 medium supplemented with 1% FBS; 250 μL of cell suspension was added into the upper transwell insert chamber (Corning, USA), and 500 μL of RPMI 1640 medium containing 15% FBS was added into the lower chamber. After 24 h of incubation, cells in the upper chamber which did not migrate were removed with cotton swabs. Cells on the bottom side were stained with DAPI (0.5 μg/mL) for 5 min after methanol fixation. Then, stained cells were observed and photographed with a fluorescence microscope (Eclipse 80i; Nikon Corporation, Tokyo, Japan). The cells were counted in 5 random fields, and the average number was taken.
For transwell invasion assay, Matrigel was placed in the upper insert chamber and hydrated at room temperature for 30 min. The cell density was adjusted to 4×105 cells/mL with RPMI 1640 containing 1% FBS. After 24 h of incubation, the invasion cells were fixed and stained with 400 μL cell dye (0.1% crystal violet) for 30 min. Then the membrane at the bottom of the upper chamber which contained the stained invasion cells was completely cut off, and the membrane was lysed with 200 μL of lysis reagent (33% acetic acid). Finally, 100 μL of lysate was taken to a 96-well plate, and the absorbance value was measured at 560 nm with a microplate reader (550; Bio-Rad, USA).
Glucose Uptake and Lactate Production Detected via Glycolysis Assay
ESCC cells were inoculated into 6-well plates with 2×105 cells/well, and the culture supernatants were collected after 24 h of conventional culture. The concentrations of glucose and lactate in the culture supernatant of ESCC cells were detected with glucose uptake and lactate production assay kits (Nanjing Jiancheng Biology Company, China) strictly according to the instruction of the manufacturer.
Pull-Down Assay with Biotinylated miR-765
48 hours after transfection with biotin-labeled wild type miR-765 (Bio-miR-765-WT), mutated miR-765 (Bio-miR-765-MUT) or antagonistic miR-765 probe (GenePharma, Shanghai, China), EC109 cells were collected and lysed in specific lysis buffer (Ambion, Austin, TX, USA) for 10 min, and then mixed with Dynabeads M-280 Streptavidin (Sigma-Aldrich, St. Louis, MO, USA) for 3 hours at 4°C. TRIzol reagent (Invitrogen, CA, USA) was used to elute and purify the interacted RNA complex, and qRT-PCR was used to detect the expression level of MAFG-AS1.
Interaction Between miR-765 and MAFG-AS1/PDX1 Verified via Double Luciferase Reporter Gene Assay
The interactions between MAFG-AS1 and miR-765 or PDX1 were predicted by TargetScan (http://www.targetscan.org/) and miRDB (http://mirdb.org/), respectively. The partial sequences of MAFG-AS1 or PDX1 3′-untranslated region (UTR), which contains the putative miR-765-binding site, were amplified via PCR and constructed into the pmirGLO Luciferase vector (Promega, Madison, WI, USA) to generate wild-type MAFG-AS1 reporter (MAFG-AS1-WT) or PDX1 reporter (PDX1-WT). The GeneArt™ Site-Directed Mutagenesis System (Thermo Scientific, Waltham, MA, USA) was used to produce mutant-type MAFG-AS1 (miR-765 target site-mutation MAFG-AS1, MAFG-AS1-MUT) reporter or mutant-type PDX1 (miR-765 target site-mutation PDX1 3′UTR, PDX1-MUT) reporter. All constructs were verified via DNA sequencing. Subsequently, the luciferase reporter and miR-765 mimic or control mimic were co-transfected into ESCC cells. After 48 h of transfection, luciferase activity was detected using a dual-luciferase reporter assay system (Promega, Madison, WI, USA) following the manufacturer’s instructions, and the fluorescence value of the vector itself was used as the internal control.
Statistical Analysis
SPSS 18.0 software (IBM, New York, USA) was used for statistical analysis. All data statistics were expressed by mean ± standard deviation (SD). Pairwise comparison was performed using two sample means t test. A Kaplan–Meier curve was plotted for survival analysis, and the difference between the two groups was compared using a Log rank test. Spearman correlation analysis was used to determine the correlations between the expression levels of MAFG-AS1, miR-765 and PDX1 in ESCC tissues. The difference was considered statistically significant at P<0.05.
Results
MAFG-AS1 Expression is Elevated in ESCC Tissues and Cell Lines
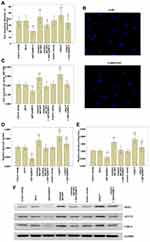
To investigate the role of MAFG-AS1 in ESCC progression, we first examined the expression of MAFG-AS1 in ESCC and matched adjacent nontumor tissues, and found that the expression of MAFG-AS1 in ESCC was significantly higher than that in matched adjacent nontumor tissues (Figure 1A; P<0.01). At the same time, qRT-PCR assay demonstrated that the expression of MAFG-AS1 in ESCC cell lines EC9706, EC109, KYSE30 and KYSE150 was significantly higher than that in the human normal esophageal epithelial cell line Het-1A (Figure 1B; P<0.01). Furthermore, high expression of MAFG-AS1 predicted an unfavourable prognosis (Figure 1C; P<0.05). These results suggested that the abnormal elevated expression of MAFG-AS1 might be closely related to the malignant biological behavior of ESCC.
MAFG-AS1 Modulates ESCC Cell Proliferation
qRT-PCR assay demonstrated that, compared with the control groups, transfection of si-MAFG-AS1 significantly reduced MAFG-AS1 expression in EC109 cells (Figure 2A; P<0.01), which expressed relatively high endogenous MAFG-AS1 (Figure 1B). CCK-8 assay demonstrated that MAFG-AS1 knockdown significantly restrained the cell proliferation of EC109 (Figure 2C; P<0.05) cells. The above results indicated that knockdown of MAFG-AS1 could restrain the ESCC cell proliferation.
Moreover, qRT-PCR assay demonstrated that, compared with the control groups, transfection of p-MAFG-AS1 significantly elevated MAFG-AS1 expression in EC9706 cells (Figure 2B; P<0.01), which expressed relatively low endogenous MAFG-AS1 (Figure 1B). CCK-8 assay demonstrated that MAFG-AS1 overexpression significantly accelerated the cell proliferation of EC9706 cells (Figure 2D; P<0.05). The above results indicated that overexpression of MAFG-AS1 could accelerate the ESCC cell proliferation.
MAFG-AS1 Knockdown Restrains EC109 Cell Migration, Invasion and Glycolysis
In order to find out the role of MAFG-AS1 on cell migration, invasion and glycolysis, we measured the cell migration and invasion via transwell assay, and detected the glucose uptake and lactate production levels via glycolysis assay in EC109 cells. The results of transwell assay without or with Matrigel demonstrated that MAFG-AS1 knockdown via si-MAFG-AS1 significantly restrained the cell migration (Figure 3A and B; P<0.01) and invasion (Figure 3C; P<0.01) of EC109 cells. The results of glycolysis assay demonstrated that after down-modulating MAFG-AS1 expression, the relative levels of glucose uptake (Figure 3D; P<0.01) and lactate production (Figure 3E; P<0.01) were both significantly decreased in EC109 cells. Consistently, Western blotting analysis showed that MAFG-AS1 knockdown reduced aerobic two glycolysis-associated proteins, GLUT1 and LDH-A, protein expression in EC109 cells (Figure 3F). The above results indicated that MAFG-AS1 knockdown could restrain the cell migration, invasion and glycolysis in ESCC cells.
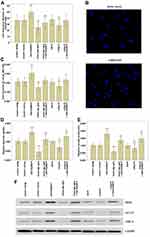
MAFG-AS1 Overexpression Accelerates EC9706 Cell Migration, Invasion and Glycolysis
In order to find out the role of MAFG-AS1 on cell migration, invasion and glycolysis, we measured the cell migration and invasion by transwell assay, and detected the glucose uptake and lactate production levels by glycolysis assay in EC9706 cells. The results of transwell assay without or with Matrigel showed that MAFG-AS1 overexpression by transfecting with p-MAFG-AS1 significantly accelerated the cell migration (Figure 4A and B; P<0.01) and invasion (Figure 4C; P<0.01) of EC9706 cells. The results of glycolysis assay showed that after up-regulating MAFG-AS1 expression, the relative levels of glucose uptake (Figure 4D; P<0.01) and lactate production (Figure 4E; P<0.01) were both significantly increased in EC9706 cells. We further detected the protein expression levels of LDHA and GLUT-1 via Western blot. The results showed that LDHA and GLUT1 expression levels were significantly increased in EC9706 cells with MAFG-AS1 overexpression (Figure 4F). The above results indicated that MAFG-AS1 overexpression could accelerate the cell migration, invasion and glycolysis in ESCC cells.
MAFG-AS1 Targets miR-765 in ESCC Cells
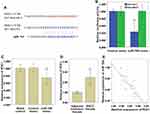
The bioinformatics database miRDB (http://mirdb.org/) was used to predict that miR-765 may be a potential target gene of MAFG-AS1 (Table 2), and suggested that miR-765 might share the binding sites with MAFG-AS1 (Figure 5A). RNA pull-down assay showed that MAFG-AS1 bound biotin-labeled wild type (wt) miR-765 (Bio-miR-765-WT) but not mutated (mt) miR-338-3p (Bio-miR-3924-MT) (Figure 5B). The results of the double luciferase reporter assay demonstrated that co-transfection of miR-765 mimics and pmirGLO-MAFG-AS1-WT vector (without mutation at the target site) could significantly reduce luciferase activity in EC109 cells (Figure 5C; P<0.01), while co-transfection of miR-765 mimics and pmirGLO-MAFG-AS1-mut vector (with mutations in the targeting site) did not affect luciferase activity. qRT-PCR was used to detect the expression level of miR-765 in EC109 after MAFG-AS1 knockdown via si-MAFG-AS1, and the results demonstrated that MAFG-AS1 knockdown significantly facilitated miR-765 expression (Figure 5D; P<0.01). Further, we tested the expression level of miR-765 in ESCC and matched adjacent nontumor tissues, and found that miR-765 was significantly lower expressed in ESCC tissue than that in matched adjacent nontumor tissues (Figure 5E; P<0.01), and there was a significant negative correlation between MAFG-AS1 and miR-765 expressions in tumor tissue samples (Figure 5F; r = −0.809, P<0.01).
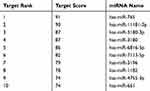 |
Table 2 The Predicted Targets of MAFG-AS1 |
MiR-765 Targets PDX1 in ESCC Cells
Target genes of MiR-765 were predicted via the bioinformatics database miRDB (http://mirdb.org/), PDX1 was found to be a potential target gene of miR-765 (Table 3), and PDX1 3′UTR might share the binding sites with miR-765 (Figure 6A). The luciferase reporter gene was subsequently used, and verified that miR-765 could bind to the 3′UTR target sequence of PDX1 (Figure 6B). The effect of ectopic expression of miR-765 via miR-765 mimic on PDX1 expression was detected via qRT-PCR (Figure 6C; P<0.01) and Western blot (Figure 4F), and the results demonstrated that ectopic expression of miR-765 significantly restrained the mRNA and protein expression of PDX1 in EC109 cells, suggesting miR-765 could restrain the expression of PDX1. We also tested the mRNA expression level of PDX1 in ESCC and matched adjacent nontumor tissues, and found that the mRNA expression level of PDX1 in ESCC tissue was significantly higher than that of matched nontumor tissue (Figure 6D; P<0.01), and there was a significant negative correlation between miR-765 and PDX1 expressions in tumor tissue samples (Figure 6E; r= −0.784, P<0.01).
 |
Table 3 The Predicted Targets for miR-765 |
MAFG-AS1 Modulates Cell Migration, Invasion and Glycolysis via miR-765 and PDX1 in ESCC Cells
To explore the roles of miR-765 and PDX1 in MAFG-AS1 knockdown modulating cell migration, invasion and glycolysis, rescue assays were carried out by down-modulating miR-765 or up-modulating PDX1 expression in EC109 cells with MAFG-AS1 knockdown. Transwell assays with or without Matrigel showed that the cell invasive and migrative abilities were significantly hampered by MAFG-AS1 knockdown, and then it was partially reversed after transfection with miR-765 inhibitor or p-PDX1 (Figure 3A-C). Meanwhile, the glucose uptake, lactate production and LDHA and GLUT1 expression were significantly hampered by MAFG-AS1 knockdown, and then it was partially reversed after transfection with miR-765 inhibitor or p-PDX1 (Figure 3D-F). Thus, the above results showed that miR-765 inhibition or PDX1 overexpression could partially reverse the inhibitory effects of MAFG-AS1 knockdown on cell migration, invasion and glycolysis in EC109 cells.
Moreover, to explore the role of miR-765 and PDX1 in MAFG-AS1 overexpression modulating cell migration, invasion and glycolysis, rescue assays were further carried out by up-modulating miR-765 or down-modulating PDX1 expression in EC9706 cells with MAFG-AS1 overexpression. Transwell assays with or without Matrigel showed that the cell invasive and migrative abilities were significantly facilitated by MAFG-AS1 overexpression, and then it was partially reversed after transfection with miR-765 mimic or si-PDX1 (Figure 4A-C). Meanwhile, the glucose uptake, lactate production and LDHA and GLUT1 expression were significantly facilitated by MAFG-AS1 overexpression, and then it was partially reversed after transfection with miR-765 mimic or si-PDX1 (Figure 4D-F). Thus, the above results showed that miR-765 overexpression or PDX1 knockdown could partially reverse the promotive effects of MAFG-AS1 overexpression on cell migration, invasion and glycolysis in EC9706 cells. Overall, these above findings suggested MAFG-AS1 might modulate the malignant behaviors of ESCC cells via miR-765/PDX1 axis.
Discussion
Currently, the regulatory roles and mechanisms of lncRNAs in ESCC are becoming more deeply understood.3 As reported, some lncRNAs function as molecular sponges to sequester target miRNAs and modulate their function during ESCC progression.3,19 LncRNA MAFG-AS1 has been reported to play an oncogenic role in some tumors. For instance, MAFG-AS1 was highly expressed in gastric cancer (GC) tissues and cell lines, and knockdown of MAFG-AS1 restrained GC cell proliferation, migration, and invasion.20 Similarly, MAFG-AS1 expression was up-modulated in lung adenocarcinoma (LUAD) tissues, and high MAFG-AS1 expression was associated with poor prognosis of patients.21 However, the specific role and mechanism behind MAFG-AS1 contributing to ESCC progression has not been elucidated. Thus, we performed clinical sample tests and loss-of-function experiments to investigate the biological function of MAFG-AS1 in ESCC tissues and cells. Here, we found that MAFG-AS1 was highly expressed in ESCC tissues and cell lines (Figure 1A and B), high expression of MAFG-AS1 was predictive of a poor overall survival in ESCC patients (Figure 1C). Knockdown of MAFG-AS1 restrained ESCC cell proliferation (Figure 2A,C), migration and invasion (Figure 3A-C), and meanwhile overexpression of MAFG-AS1 accelerated ESCC cell proliferation (Figure 2B and D), migration and invasion (Figure 4A-C), suggesting MAFG-AS1 may play a tumor-promotive role in ESCC by modulating the malignant biological behavior.
Furthermore, not only tumor cell growth, but also tumor metastasis were accelerated via aerobic glycolysis.22 Lactate produced via glycolysis facilitates cell motility, and the enhanced expression of lactate in tumor tissues strongly suggests the occurrence of tumor metastasis.23 In this study, we found that MAFG-AS1 not only modulated ESCC cell proliferation, migration and invasion, but also affected ESCC aerobic glycolysis by negatively regulating GLUT1 and LDH-A (Figures 3 and 4), implying that the acquisition of metastatic potential of ESCC induced by MAFG-AS1 might benefit from glucose metabolism. Thus, we supposed that MAFG-AS1 could facilitate the malignant biological behavior of ESCC via modulating aerobic glycolysis. Several previous studies have demonstrated that lncRNAs-miRNAs network could possibly be involved in Warburg effect in some tumors.24,25 Interestingly, our current bioinformatics analysis demonstrated that miR-765 may be a potential target of MAFG-AS1 (Table 2, Figure 5A). Here, the current clinical sample tests demonstrated that miR-765 was determined to be significantly down-modulated in ESCC tissues (Figure 5E), and miR-765 expression was found to be inversely correlated with MAFG-AS1 in ESCC tissues (Figure 5F). Further, the current loss-of-function and rescue experiments demonstrated that MAFG-AS1 knockdown elevated miR-765 expression (Figure 5D), and miR-765 contribute to the partial effects of MAFG-AS1 on cell migration, invasion and glycolysis (Figures 3 and 4). Therefore, we deduced that MAFG-AS1 may accelerate cell migration, invasion and glycolysis of ESCC cells via negatively modulating miR-765. However, other mechanisms may also exist, and more further studies are still needed.
The function of miRNAs is realized via modulating the expression of their target genes.26 So in this study, we conducted the bioinformatics analysis, and suggested that PDX1 may be one of the potential downstream targets of miR-765 (Table 3, Figure 6A). As a transcription factor, PDX1 changes its role from tumor suppressor to tumor promoter during the process of pancreatic tumorigenicity,27 and PDX1 was found to be frequently expressed in colorectal serrated adenocarcinoma.28 Herein, clinical sample tests demonstrated that PDX1 was identified to be significantly up-modulated in ESCC tissues (Figure 6D), and there was a significant negative correlation between miR-765 and PDX1 expressions in tumor tissue samples (Figure 6E). Further, gain-of-function experiments demonstrated and rescue experiments that ectopic expression of miR-765 restrained PDX1 expression in ESCC cells (Figures 3,4,6C). The above results suggested miR-765 may function as a tumor suppressor of ESCC cells via negatively modulating PDX1.
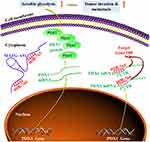
A previous study has indicated that FAM83H-AS1 could serve as a competing endogenous RNA (ceRNA) for miR-136-5p to mediate triple-negative breast cancer progression.29 Here, our current bioinformatics analyses predicated potential binding sites in MAFG-AS1 and miR-765 (Figure 5A), as well as miR-765 and PDX1 3′UTR (Figure 6A), suggesting the possibility that MAFG-AS1 functions as a molecular sponge for miR-765 to modulate the expression level of PDX1. Thus, we supposed that MAFG-AS1 may function as a ceRNA for miR-765 to modulate PDX1 expression during ESCC progression. To address this point, we conducted experiments to demonstrate our hypothesis. Herein, RNA pull-down and luciferase reporter assay indicated that MAFG-AS1 covalently targeted miR-765 (Figure 5B and C), and miR-765 covalently targeted PDX1 3′UTR (Figure 6B). Next, MAFG-AS1 expression was found to be inversely correlated with miR-765 in ESCC tissues (Figure 5F), while miR-765 expression was found to be inversely correlated with PDX1 in ESCC tissues (Figure 6E). And miR-765 and PDX1 contributed to the partial effects of MAFG-AS1 on cell migration, invasion and glycolysis (Figures 3 and 4), suggesting MAFG-AS1 may regulate the malignant behaviors of ESCC cells via miR-765/PDX1 axis. Taken together, our results indicated that MAFG-AS1 functions via a ceRNA mechanism via competing with endogenous miR-765, thus triggering PDX1 protein expression in ESCC (Figure 7).
Conclusions
Our current study demonstrated that lncRNA MAFG-AS1 could facilitate the cell proliferation, invasion and aerobic glycolysis activities of ESCC cells via modulating the expression of PDX1 via miR-765, thus enhancing the malignant phenotype of ESCC. Our results suggest that MAFG-AS1/miR-765/PDX1 axis may be an effective target for ESCC. However, more specific molecular mechanisms and the effects of targeted diagnosis and treatment of ESCC need to be confirmed by more detailed molecular mechanism and animal experiments in the future.
Ethics Approval
The study protocol was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Taizhou University Hospital (reference number 2019-014).
Disclosure
The authors declare they have no conflicts of interest for this work.
References
1. Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers. 2017;3(1):17048. doi:10.1038/nrdp.2017.48
2. Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360–373. doi:10.1053/j.gastro.2017.08.023
3. Feng Q, Zhang H, Yao D, et al. Emerging role of non-coding RNAs in esophageal squamous cell carcinoma. Int J Mol Sci. 2019;21(1):258. doi:10.3390/ijms21010258
4. Wang Z, Liu J, Wang R, et al. Long non-coding RNA Taurine Upregulated Gene 1 (TUG1) downregulation constrains cell proliferation and invasion through regulating cell division cycle 42 (CDC42) expression via mir-498 in esophageal squamous cell carcinoma cells. Med Sci Monit. 2020;26:e919714. doi:10.12659/MSM.919714
5. Wu SB, Wang HQ. Upregulation of long noncoding RNA DLX6-AS1 promotes cell growth and metastasis in esophageal squamous cell carcinoma via targeting miR-577. Eur Rev Med Pharmacol Sci. 2020;24(3):1195–1201. doi:10.26355/eurrev_202002_20171
6. Ouyang H, Zhang L, Xie Z, et al. Long noncoding RNA MAFG-AS1 promotes proliferation, migration and invasion of hepatocellular carcinoma cells through downregulation of miR-6852. Exp Ther Med. 2019;18(4):2547–2553. doi:10.3892/etm.2019.7850
7. Li H, Zhang GY, Pan CH, et al. LncRNA MAFG-AS1 promotes the aggressiveness of breast carcinoma through regulating miR-339-5p/MMP15. Eur Rev Med Pharmacol Sci. 2019;23(7):2838–2846. doi:10.26355/eurrev_201904_17561
8. Kobliakov VA. The mechanisms of regulation of aerobic glycolysis (Warburg Effect) by oncoproteins in carcinogenesis. Biochemistry (Mosc). 2019;84(10):1117–1128. doi:10.1134/S0006297919100018
9. Liu H, Luo J, Luan S, et al. Long non-coding RNAs involved in cancer metabolic reprogramming. Cell Mol Life Sci. 2019;76(3):495–504. doi:10.1007/s00018-018-2946-1
10. Tornesello ML, Faraonio R, Buonaguro L, et al. The role of microRNAs, long non-coding RNAs, and circular RNAs in cervical cancer. Front Oncol. 2020;10:150. doi:10.3389/fonc.2020.00150
11. Ghafouri-Fard S, Esmaeili M, Taheri M. Expression of non-coding RNAs in hematological malignancies. Eur J Pharmacol. 2020;875:172976. doi:10.1016/j.ejphar.2020.172976
12. Zang B, Zhao J, Chen C. LncRNA PCAT-1 promoted ESCC progression via regulating ANXA10 expression by sponging miR-508-3p. Cancer Manag Res. 2019;11:10841–10849. doi:10.2147/CMAR.S233983
13. Lin C, Zhang S, Wang Y, et al. Functional role of a novel long noncoding RNA TTN-AS1 in esophageal squamous cell carcinoma progression and metastasis. Clin Cancer Res. 2018;24(2):486–498. doi:10.1158/1078-0432.CCR-17-1851
14. Lv DB, Zhang JY, Gao K, et al. MicroRNA-765 targets MTUS1 to promote the progression of osteosarcoma via mediating ERK/EMT pathway. Eur Rev Med Pharmacol Sci. 2019;23(11):4618–4628. doi:10.26355/eurrev_201906_18040
15. Xiao W, Wang C, Chen K, et al. MiR-765 functions as a tumour suppressor and eliminates lipids in clear cell renal cell carcinoma by downregulating PLP2. EBioMedicine. 2020;51:102622. doi:10.1016/j.ebiom.2019.102622
16. Yan L, Wu X, Liu Y, et al. LncRNA Linc00511 promotes osteosarcoma cell proliferation and migration through sponging miR-765 [published online ahead of print, 2018 Dec 28]. J Cell Biochem. 2018. doi:10.1002/jcb.
17. Long S, Long S, He H, et al. MicroRNA-765 is upregulated in multiple myeloma and serves an oncogenic role by directly targeting SOX6. Exp Ther Med. 2019;17(6):4741–4747. doi:10.3892/etm.2019.7473
18. Ma J, Wang BB, Ma XY, et al. Potential involvement of heat shock proteins in pancreatic-duodenal homeobox-1-mediated effects on the genesis of gastric cancer: a 2D gel-based proteomic study. World J Gastroenterol. 2018;24(37):4263–4271. doi:10.3748/wjg.v24.i37.4263
19. Talebi A, Masoodi M, Mirzaei A, et al. Biological and clinical relevance of metastasis-associated long noncoding RNAs in esophageal squamous cell carcinoma: a systematic review. J Cell Physiol. 2020;235(2):848–868. doi:10.1002/jcp.29083
20. Li C, Wu R, Xing Y. MAFG-AS1 is a novel clinical biomarker for clinical progression and unfavorable prognosis in gastric cancer. Cell Cycle. 2020;19(5):601–609. doi:10.1080/15384101.2020.1728017
21. Sui Y, Lin G, Zheng Y, et al. LncRNA MAFG-AS1 boosts the proliferation of lung adenocarcinoma cells via regulating miR-744-5p/MAFG axis. Eur J Pharmacol. 2019;859:172465. doi:10.1016/j.ejphar.2019.172465
22. Yang J, Ren B, Yang G, et al. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol Life Sci. 2020;77(2):305–321. doi:10.1007/s00018-019-03278-z
23. de la Cruz-lópez KG, Castro-Muñoz LJ, Reyes-Hernández DO, et al. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol. 2019;9:1143. doi:10.3389/fonc.2019.01143
24. Wang Y, Zhang X, Wang Z, et al. LncRNA-p23154 promotes the invasion-metastasis potential of oral squamous cell carcinoma by regulating Glut1-mediated glycolysis. Cancer Lett. 2018;434:172–183. doi:10.1016/j.canlet.2018.07.016
25. Zhang L, Fu Y, Guo H. c-Myc-induced long non-coding RNA small nucleolar RNA host gene 7 regulates glycolysis in breast cancer. J Breast Cancer. 2019;22(4):533–547. doi:10.4048/jbc.2019.22.e54
26. Ali Syeda Z, Langden SSS, Munkhzul C, et al. Regulatory mechanism of MicroRNA expression in cancer. Int J Mol Sci. 2020;21(5):1723. doi:10.3390/ijms21051723
27. Tang ZC, Chu Y, Tan YY, et al. Pancreatic and duodenal homeobox-1 in pancreatic ductal adenocarcinoma and diabetes mellitus. Chin Med J (Engl). 2020;133(3):344–350. doi:10.1097/CM9.0000000000000628
28. Sakamoto N, Feng Y, Stolfi C, et al. BRAFV600E cooperates with CDX2 inactivation to promote serrated colorectal tumorigenesis. Elife. 2017;6:e20331. doi:10.7554/eLife.20331
29. Han C, Fu Y, Zeng N, et al. LncRNA FAM83H-AS1 promotes triple-negative breast cancer progression by regulating the miR-136-5p/metadherin axis. Aging (Albany NY). 2020;12(4):3594–3616. doi:10.18632/aging.102832
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.