Back to Journals » OncoTargets and Therapy » Volume 13
LncRNA HCG11 Suppresses Cell Proliferation and Promotes Apoptosis via Sponging miR-224-3p in Non-Small-Cell Lung Cancer Cells
Authors Wang G, Liu L, Zhang J, Huang C, Chen Y, Bai W, Wang Y, Zhao K, Li S
Received 30 December 2019
Accepted for publication 19 May 2020
Published 3 July 2020 Volume 2020:13 Pages 6553—6563
DOI https://doi.org/10.2147/OTT.S244181
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Guige Wang,1,2 Lei Liu,1,2 Jiaqi Zhang,1,2 Cheng Huang,1,2 Yeye Chen,1,2 Wenliang Bai,1,2 Yanqing Wang,1,2 Ke Zhao,1,2 Shanqing Li1,2
1Department of Thoracic Surgery, Peking Union Medical College Hospital, Beijing 100730, People’s Republic of China; 2Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing 100730, People’s Republic of China
Correspondence: Shanqing Li
Department of Thoracic Surgery, Peking Union Medical College Hospital, Shuaifuyuan No. 1 Dongcheng District, Beijing 100730, People’s Republic of China
Email [email protected]
Introduction: Studies have found that Lnc-HCG11 is an important regulator of cancer. However, the function of Lnc-HCG11 in NSCLC is not known. Therefore, this experimental design was based on Lnc-HCG11 to explore the pathogenesis of NSCLC.
Methods: RT-qPCR was used to detect the expression of Lnc-HCG11 and miR-224-3p in NSCLC. The effects of Lnc-HCG11 and miR-224-3p on proliferation and apoptosis of NSCLC cells were detected by CCK-8 assay, Edu assay and Annexin V-FITC/PI assay. Target gene prediction and screening, luciferase reporter assays were used to verify downstream target genes for lnc-HCG11 and miR-224-3p. Western blotting was used to detect the protein expression of caspase-3. The tumor changes in mice were detected by in vivo.
Results: Lnc-HCG11 was significantly reduced in NSCLC. Lnc-HCG11 significantly inhibited cell proliferation of NSCLC cells and induced apoptosis. miR-224-3p was significantly elevated in the NSCLC cell line. Moreover, miR-224-3p significantly increased cell proliferation and inhibited apoptosis of NSCLC cells. Furthermore, Lnc-HCG11 was negatively correlated with miR-224-3p expression. Lnc-HCG11 over-expression was up-regulated the expression levels of c-caspase-3 and caspase-3. Finally, the results of in vivo animal models confirmed that Lnc-HCG11 inhibited tumor growth by modulating the miR-224-3p/c-caspase-3 axis.
Conclusion: Lnc-HCG11 could inhibit the progression of NSCLC by modulating the miR-224-3p/caspase-3 axis, and Lnc-HCG11 may be a potential therapeutic target for NSCLC.
Keywords: Lnc-HCG11, miR-224-3p, non-small-cell lung cancer, proliferation, apoptosis
Introduction
Lung cancer is one of the most extremely high mortality diseases, with the number of people on the scene and the number of deaths at the top of the list1,2. Non-small cell lung cancer (NSCLC) is the most common pathological type of lung cancer.3,4 In recent years, with the development of medical and scientific technology, such as surgical resection, radiotherapy, chemotherapy, molecular targeted therapy, the therapeutic effect of lung cancer has made certain progress.5,6 However, due to the lack of effective early diagnosis methods, most lung cancer patients have entered the middle and late stages of diagnosis and lost the best chance of surgery.7,8 The main treatments are radiotherapy, chemotherapy and molecular targeted therapy.9,10 These treatments have many drawbacks, such as large side effects, easy recurrence, and poor patient tolerance.11,12 Therefore, finding new therapeutic targets is very important for the treatment of lung cancer.
With the deepening of research, Long-chain non-coding RNA (lnc RNA) play a critical role in the regulation of transcriptional, transcriptional, such as genomic rearrangement, chromosome modification, and X-chromosome silencing.13 Lnc RNA plays different roles in various biological processes of cancer cells.14 Lnc RNA take part in NSCLC, and they regulate the development of NSCLC by stimulating or inhibiting biological processes.15 LncRNA HCG11 is a recently discovered lncRNA.16 LncRNA HCG11 has been reported as a tumor suppressor of prostate cancer, and has been shown to have gene regulation in breast cancer.17 Studies have found that lncRNA HCG11 inhibits apoptosis of liver cancer cells.18 However, the development and mechanism of lncRNA HCG11 in NSCLC is still unclear.
In recent years, the relationship of lncRNA-miRNAs has attracted the attention19. The interaction regulation of lncRNAs and miRNAs is currently a research hotspot.20 Micro RNAs (miRNAs) are an important member of the non-coding RNA family.21 Studies have shown that miRNAs are prevalent in malignant tumors.22 Moreover, the expression level of miRNAs is closely related to the tumor. At present, miRNAs have become important markers for tumor neoplasia, progression, metastasis and prognosis, and have become a research hotspot for tumor targeted therapy.23 miR-224-3p is the most common miRNA, which can participate in multiple malignant transformation processes in cells.24 Abnormally high expression of miR-224-3p in multiple human malignancies has been discovered and confirmed.25 And it has been found that miR-224-3p is increased in cervical cancer tissues.26 However, its function and mechanism in NSCLC is not clear. LncRNA may regulate downstream target genes through miRNA adsorption processes.27 The core component of apoptosis is cysteinyl aspartate specific proteinase (Caspase). Activated Caspase can degrade structural and functional proteins of cells, leading to apoptosis. Caspase-3 is downstream of the ordered cascade of apoptosis and is the most important effector Caspase.28 Therefore, it was hypothesized that LncRNA-HCG11 may regulate the progression of NSCLC through miR-224-3p/Caspase-3. The main purpose of this study was to investigate the mechanism of lncRNA-HCG11 in the regulation of NSCLC, and provide a scientific basis for the search for new drug targets.
Patients and Methods
Research Object
In this study, NSCLC of 20 patients were between 41 and 69 years old with an average age of 53 years. Patients were confirmed to NSCLC by pathology were not treated with chemotherapy or radiotherapy before enrollment. Pairs of NSCLC and paired precancerous tissue specimens were collected from 30 patients undergoing primary surgical resection. All patients provided written informed consent. The study was approved by the ethical committee of Peking Union Medical College Hospital. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of “ Ethics Committee of Peking Union Medical College Hospital” University and approved by the Animal Ethics Committee of “Animal Ethical and Welfare Committee (AEWC)”.
Cell Culture
Human lung cancer A549, SPC-A1, H1299, H1650, H1975 and PC-9 cells and human normal lung epithelial cells 16HBE were obtained from the American Type Culture Collection (ATCC, USA). All cells were subculture in RPMI 1640 medium containing 10% fetal bovine serum (FBS, FBS, Jitai, Shanghai, China).
Plasmid Construction and Transfection
The miR-224-3p mimetic, miR-224-3p inhibitor (anti-miR-224-3p) and the corresponding negative control miR (miR-NC, anti-miR-NC) were purchased from RiboBio (Guangzhou, China). The HCG11 cDNA sequence was amplified and introduced into a pcDNA vector (ABM, Canada) to construct plasmid complementary DNA HCG11. The shRNA sequences targeting LINC01194 and sh-NC were purchased from Genepharma Co., Ltd (Shanghai). Cells were transiently transfected with RNAiMax and Lipofectamine 3000 with Plus Reagent (Thermo Fisher Scientific). Transfection efficiency was determined by qRT-PCR.
RNA Isolation, Reverse Transcription and Quantitative Real-Time PCR (qRT-PCR)
Total RNA in NSCLC cells was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA). qRT-PCR was performed by a ViiATM 7 real-time PCR system (Jinuo, Shanghai, China). GAPDH and U6 were used as internal references. The expression level of lnc-HCG11 and miR-224-3p was detected by SYBR Premix Ex Taq II (Takara Biotechnology).29 The primers used in this study are listed in Table 1.
 |
Table 1 Sequences of Primers Used in qRT-PCR |
CCK-8 Assay
Cell suspensions (100 μL/well) were seeded in 96-well plates at 2000 cells per well. The medium was changed to a medium on the second day. The cells were starved for 48 h, and then replaced with PDGF-BB or 15% FBS. It was detected by CCK8 kit before and after stimulation. 10 μL of CCK8 solution was added. The absorbance at 450 nm was measured with a microplate reader. CCK-8 assay was repeated 3 times.
Edu Assay
In the logarithmic growth phase, the cells were seeded in 96-well plates at 1×105 cells per well. Edu labeling, red fluorescent staining and Hochest 33342 nuclear counterstaining were performed according to the Cell-LightTM Ed U Apollo® 567 In Vitro Imaging Kit instructions. Five fields were randomly selected under a fluorescence microscope. EdU-labeled cells (red fluorescently labeled cells) and non-labeled cells were counted, and the percentage of EdU-labeled cells was calculated. Edu assay was repeated 3 times.
Annexin V-FITC-PI Analysis
The transfected cells were harvested, and stained with Annexin V-FITC-PI Assay Kit (Solarbio, Beijing, China). Analysis was performed by a FACS flow cytometer (Jiyuan, Guangzhou, China). Annexin V-FITC-PI analysis was repeated 3 times.
Subcutaneous Xenograft Mouse Model
Fifteen male athymic BALB/c nude mice were randomly divided into three groups. 2×106 A549 cells transfected with empty vector pcDNA3.0 or plasmid pcDNA3.0-HCG11 or miR-224-3p mimics were resuspended and injected into the right side of nude mice (n=5). Then a mouse xenograft model was constructed. Tumor size was measured twice a week. All nude mice were sacrificed after 2 weeks and tumors were dissected. The pathological changes and apoptosis of tumor tissues were observed by TUNEL method. Animal experiments were carried out in strict accordance with the recommendations of the National Institutes of Health Laboratory Animal Care and Use Guidelines and approved by the Scientific Ethics Committee of Peking Union Medical College Hospital.
TUNEL Staining
Paraffin sections were incubated with TUNEL solution for 60 min and alkaline phosphatase antibody for 30 min. The sections were re-stained with hematoxylin. The blue-black nucleus was positive under light microscope. Six samples were taken from each group.
Ki67 Immunohistochemical Staining
Immunohistochemical staining SP method was used to stain Ki67 (Ki67 monoclonal antibody, 1:100, Myry Company). Conventional paraffin sections were dewaxed; 3% H2O2 was added to the sections for 10 min. After antigen repairing, normal goat serum sealing solution was added for 20 min. Then, one anti-50 mL was added for 1 h, and two anti-50 mL was added for 1 h at room temperature. After that, it was dyed with DAB for 5 min, re-stained with hematoxylin, and dehydrated with conventional paraffin.
Luciferase Reporter Assay
The lnc-HCG11 wild type or mutant binding miR-224-3p was inserted into the pMIR Basic vector (OBiO Biology, Shanghai) and designated as pMIR-REPOR-HCG11-wt or pMIR-REPOR-HCG11-mt. After 24 h of culture, cells were transfected with miR-224-3p mimic or mock control or miR-224-3p inhibitor or inhibitor NC (GenePharma, Shanghai, China) and co-transfected with empty pMIR-. After 48 h of transfection, luciferase activity was measured using a dual luciferase assay system (Promega).
Western Blot
The transfected cells were collected, total protein was extracted, and the protein concentration was quantified using the BCA Protein Assay Kit. Then it was incubated with anti-c-caspase-3 antibody (1:1000, Shidai, Shanghai, China), caspase-3 antibody (1:500, Shidai, Shanghai, China) and anti-GAPDH antibodies (1:1000, Shidai, Shanghai, China) overnight. After that, 1:5000 labeled anti-rabbit secondary antibody for 1 h.30
Statistical Method
The monitoring data were analyzed by SPSS19.0 statistical software. The results of data analysis were shown as mean ± standard deviation (mean ±SD). Multigroup data analysis was based on one-way ANOVA. LSD test was used for subsequent analysis. P < 0.05 indicated the difference was significant.
Results
The Role of lncRNA HCG11 in NSCLC Tumorigenesis
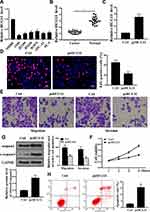
As shown in Figure 1A, compared with 16HBE cells, the expression levels of lncRNA HCG11 were significantly reduced in the NSCLC lines (A549, H1975, H1299, H1650, SPC-A1 and PC-9) (P < 0.05). There were no significant differences in HCG11 expression levels in NSCLC lines, A549 cells were selected for further experiments. As shown in Figure 1B, the expression level of lncRNA HCG11 in NSCLC tissues was significantly reduced than that in adjacent normal tissues (P< 0.05).
As shown in Figure 1C, compared with the control group, the expression level of HCG11 was significantly increased in the pc-HCG11 group (P<0.05), indicating successful transfection. As shown in Figure 1D–F, compared with the control group, HCG11 overexpression was significantly inhibited cell proliferation and DNA synthesis (P<0.05). In addition, HCG11 overexpression was significantly inhibited cell invasion and migration. Compared with the control group, overexpression of HCG11 was significantly up-regulated the protein levels of caspase-3 and c-caspase 3 (Figure 1G, P<0.05). And HCG11 overexpression was significantly induced apoptosis (Figure 1H, P<0.05). These data indicated that HCG11 was capable of consistent NSCLC proliferation and induced apoptosis in NSCLC.
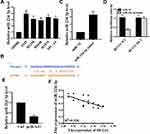
miR-224-3p Was the Target of Lnc-HCG11
The results are shown in Figure 2A. Compared with 16HBE cells, the expression levels of miR-224-3p in the NSCLC line (A549, H1975, H1299, H1650, SPC-A1 and PC-9) were significantly increased (P < 0.05). We predicted by two online prediction tools (miRanda and Starbase v2.0) and miR-224-3p was identified as a potential target for lnc-HCG11 (Figure 2B). In addition, compared with the control group, the expression level of miR-224-3p was significantly increased in the miR-224-3p overexpressing group (P < 0.05), indicating successful transfection (Figure 2C). To validate the predicted results, luciferase reporter assay was performed using WT-HCG11 or mutant (MUT)-HCG11 luciferase reporter plasmid. Luciferase activity of pGL3-REPOR-HCG11-WT was reduced by miR-224-3p mimic, but there was no significant change in the luciferase activity of pGL3-REPOR-HCG11-MUT (Figure 2D). In addition, as shown in Figure 2E, the expression level of miR-224-3p was significantly reduced in the pcHCG11 group compared with the control group (P < 0.05). In addition, a significant negative correlation (R2=0.536) between lnc-HCG11 and miR-224-3p was also observed (Figure 2F). These results indicated that lnc-HCG11 might exert its biological function through miR-224-3p.
Lnc-HCG11 Interacted with miR-224-3p to Regulate Cell Proliferation and Apoptosis in NSCLC Cells
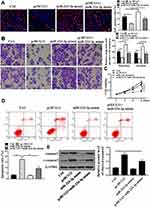
In order to further analyze whether Lnc-HCG11 acts on NSCLC cells via miR-224-3p, miR-224-3p mimic and pc HCG11 were transfected into the A549 cell line. The results showed that pc HCG11 was significantly inhibited proliferation and DNA synthesis of A549 cells compared with the control group, and miR-224-3p mimic significantly induced proliferation and DNA synthesis of A549 cells. Co-transfection with miR-224-3p mimic partially abolished the effect of pc HCG11 on cell proliferation and DNA synthesis (P < 0.01, Figure 3A and C). And pc HCG11 was significantly inhibited invasion and migration of A549 cells compared with the control group, and miR-224-3p mimic significantly induced invasion and migration of A549 cells. Co-transfection with miR-224-3p mimic partially abolished the effect of pc HCG11 on cell invasion and migration (P < 0.01, Figure 3B). As shown in Figure 3D and E, compared with the control group, pc HCG11 was significantly induced apoptosis of A549 cells and up-regulated the protein levels of caspase-3 and c-caspase 3. MiR-224-3p mimic was significantly inhibited apoptosis of A549 cells, and down-regulated the protein levels of caspase-3 and c-caspase 3. Co-transfection with miR-224-3p mimic partially abolished the effect of pc HCG11 on apoptosis and protein levels of caspase-3 and c-caspase 3 (P <0.01). These results indicated that lnc-HCG11 was promoted apoptosis by proliferating NSCLC cells by modulating miR-224-3p.
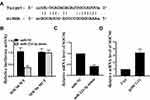
miR-224-3p Directly Interacted with SOCS6
Based on these results, it was aimed to identify the major target genes for miR-224-3p. We predicted by bioinformatics and SOCS6 was identified as a potential target for miR-224-3p (Figure 4A). The luciferase reporter gene assay is shown in Figure 4B, the expression level of SOCS6 was significantly inhibited the luciferase activity of WT- miR-224-3p, but it had no significant effect on the luciferase activity of MUT miR-224-3p. In addition, compared with the miR-NC group, miR-224-3p mimic significantly was reduced cleaved-caspase3 mRNA expression, while pc HCG11 was significantly increased the mRNA expression of SOCS6 in A549 cells (P<0.01, Figure 4C and D). These results indicated that SOCS6 was a direct target of miR-224-3p.
HCG11 Displayed the Strong Therapeutic Effect on NSCLC in Mice
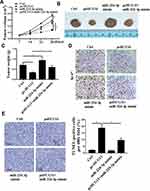
The effect of HCG11 on the progression of NSCLC was further determined in vivo. Compared with the control group, the tumor volume and weight of mice in the pc HCG11 group were significantly reduced, and the tumor volume and weight of the mice in the miR-224-3p mimic group were significantly increased, while co-transfection with miR-224-3p mimics was reversed the effect of pc HCG11 on tumor volume and weight of mice (P <0.01, Figure 5A–C). In addition, Ki-67 immunochemical staining results showed that there were fewer Ki67-positive cells in the tumor tissue of pc HCG11 group, more Ki67-positive cells in the tumor tissue of miR-224-3p mimic group, and Ki67-positive cells in tumor tissues were significantly increased after co-transfection of miR-224-3p mimics (Figure 5D). Moreover, TUNEL staining showed that pcHCG11 was significantly induced apoptosis, miR-224-3p mimic was significantly inhibited apoptosis, and reversed the apoptosis by co-transfection with miR-224-3p mimic (Figure 5E).
Discussion
Malignant tumors have become the killer of human health.31 NSCLC accounts for the vast majority of all types of lung cancer. Therefore, NSCLC has become the focus and difficulty of global cancer prevention and treatment.32 About 60% of lung cancers have undergone distant metastasis at the time of diagnosis, and their five-year survival rate is only 4%.33 The NSCLC transfer is complex, multi-factor dynamic process, and its molecular mechanism is still unclear. Therefore, the mechanism of tumor metastasis is great significance of therapeutic targets for the development of NSCLC.
In recent years, studies have shown that the occurrence of NSCLC is closely related to the abnormal expression of genes.34 The abnormal expression of genes is the intrinsic factor and molecular basis of the initiation, deterioration and metastasis of lung cancer. LncRNAs play an important role in biological behaviors.35 Some tumors are also inextricably linked to lncRNA.36 In recent years, it has been found that lncRNAs play a role in promoting or suppressing in NSCLC. For example, HOTAIR is higher in NSCLC tissues, and some patients with poor prognosis, such as brain metastasis, have higher expression levels of HOTAIR.37 Further studies have found that HOTAIR can promote cell proliferation of NSCLC.38 Previous studies have found that lncRNA HCG11 is involved in the development of lung cancer, liver cancer and other tumors.18 Studies have found that the down-regulation of HCG11 expression in prostate tissue is associated with a low survival rate in PCA.18 Our study found that the expression of lncRNA HCG11 in NSCLC was significantly decreased, and lncRNA HCG11 overexpression could inhibit the proliferation and induced cell apoptosis. And lncRNA HCG11 overexpression was inhibited tumor growth in mice, so lncRNA HCG11 as a tumor suppressor gene could up-regulate its expression to achieve the goal of controlling NSCLC development.
As a target of lncRNA, miRNA is the most widely studied non-coding RNA, which can regulate cell proliferation, differentiation and apoptosis by degrading target mRNA or inhibiting its translation. miRNAs play a part in the regulation of various biological behaviors of tumor cells, such as apoptosis, migration and play the role of oncogenes or tumor suppressor genes.39 Studies have confirmed that lncRNA HOXA11-AS can promote the development of NSCLC by miR-200b in NSCLC.40 miR-224-3p acts as a tumor activator in many types of cancer.41 In addition, overexpression of miR-224-5p can increase its resistance by down-regulating PRKCD in ovarian cancer cells.42 Further studies have shown that miR-224-5p plays a role in tumor-promoting genes in NSCLC, its high expression contributes to cell proliferation and metastasis, and participates in the regulation of NSCLC formation and progression.43 This study was consistent with the literature, and miR-224-5p was the target gene for lncRNA HCG11. The expression of miR-224-5p was significantly increased in NSCLC cells, and lncRNA HCG11 regulated its expression by targeting the 3ʹUTR of the miR-224-5p gene. LncRNA HCG11 was significantly negatively correlated with miR-224-5p. miR-224-5p mimic partially abolished the effect of lncRNA HCG11 on cell proliferation and apoptosis. These results indicated that lncRNA HCG11 might inhibit the growth of NSCLC cells by regulating miR-224-5p.
In recent years, there have been reports of interactions between miRNA and lncRNA, targeting and regulating related pathways.44 Caspase-3 is the most important executor and terminal cleavage enzyme in the process of apoptosis.45 Activated caspase-3 can cleave poly (ADP-ribose) polymerase PARP, which in turn increases endonuclease activity and cleaves DNA between nucleosomes to induce apoptosis.46 In this study, it was found that cleaved-caspase3 was a potential target for miR-224-5p, and miR-224-5p could regulate its expression by targeting the 3ʹUTR of the cleaved-caspase3 gene. In addition, miR-224-3p mimic was significantly reduced the expression level of cleaved-caspase3, while pcHCG11 significantly increased the expression level of cleaved-caspase3. Moreover, co-transfection of pcHCG11 with miR-224-3p mimic partially cleared the effect of pcHCG11 on the protein level of caspase3 and cleaved-caspase3. These indicated that lncRNA HCG11 could inhibit the proliferation of NSCLC by modulating the miR-224-3p/cleaved-caspase3 axis.
Conclusion
Lnc RNA HCG11 promote apoptosis of NSCLC by regulating miR-224-3p/caspase3 axis, suggesting that lncRNA HCG11 might be a potential tumor suppressor gene of NSCLC. It would provide theory bases for the clinical prognosis of this tumor and further targeted intervention therapy.
Disclosure
The authors declare that they have no competing interests.
References
1. Kwak EL, Bang YJ, Camidge DR, Shaw AT. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703.
2. Med N. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409.
3. Antonia SJ, Villegas A, Daniel D, Vicente D. Durvalumab after chemoradiotherapy in stage iii non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919.
4. Masters GA, Sarah T, Azzoli CG, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract. 2017;33(30):832–837.
5. Moya-Horno I, Viteri S, Karachaliou N, Rosell R. Combination of immunotherapy with targeted therapies in advanced non-small cell lung cancer (NSCLC). Ther Adv Med Oncol. 2018;10:175883401774501.
6. Sarcev T, Penakaran S, Ilic A, et al. Clinico-pathological prognostic factors for relapse in early stage non-small cell lung cancer after radical surgical resection. Eur Respir J. 2013.
7. Fan TWM, Zhang X, Wang C, et al. Exosomal lipids for classifying early and late stage non-small cell lung cancer. Anal Chim Acta. 2018;1037:256–264. doi:10.1016/j.aca.2018.02.051
8. Sobieszczyk MJ. Non-small cell lung cancer in the elderly: a practical approach to screening, diagnosis, and treatment. Curr Geriatr Rep. 2018;7(3):1–9.
9. Costanzo R, Piccirillo MC, Sandomenico C, Carillio G, Morabito AJJOB. Gefitinib in non small cell lung cancer. Biomed Res Int. 2014;2011(1110–7243):815269.
10. Mohammed N, Carruthers R, Brisbane I, Hicks J, O’Rourke N. Toxicity of hypofractionated accelerated radiotherapy concomitant with cisplatin/vinorelbine chemotherapy for non small cell lung cancer. Clin Oncol. 2010;67(10):S36–S36.
11. Couñago F, Rodríguez A, Calvo P, Luna J, Monroy JL, Taboada B. Targeted therapy combined with radiotherapy in non-small-cell lung cancer: a review of the Oncologic Group for the Study of Lung Cancer (Spanish Radiation Oncology Society). Clin Transl Oncol. 2017;19(1):31–43.
12. Yuan D, Song YJMJ. Treatment of non-small cell lung cancer comes to the age of immunotherapy. Med J Chin People’s Liberation Army. 2017.
13. Hao Y, Wu W, Shi F, et al. Prediction of long noncoding RNA functions with co-expression network in esophageal squamous cell carcinoma. BMC Cancer. 2015;15(1):168.
14. Iguchi T, Uchi R, Nambara S, Saito T, Research K. A long noncoding RNA, lncRNA-ATB, is involved in the progression and prognosis of colorectal cancer. Anticancer Res. 2015;35(3):1385.
15. Wang X, Yong C, Yu K, Yu R, Cai SJ. Long noncoding RNA (lncRNA) n379519 promotes cardiac fibrosis in post-infarct myocardium by targeting miR-30. Med Sci Monit. 2018;24:3958–3965.
16. Chen X, Liu P. Expression of Lnc RNA HCG11 and mi R-590-3p in squamous carcinoma of cervix and their relationship with prognosis. Cancer Res Prev Treat. 2018;45(03):148–153.
17. Xu Y, Zheng Y, Liu H, Li TJ. Modulation of IGF2BP1 by long non-coding RNA HCG11 suppresses apoptosis of hepatocellular carcinoma cells via MAPK signaling transduction. Int J Oncol. 2017;51(3):791–800.
18. Zhang Y, Zhang P, Wan X, Su X, Kong Z, Zhai Q. Downregulation of long non-coding RNA HCG11 predicts a poor prognosis in prostate cancer. Biomed Pharmacother. 2016;83:936–941.
19. Tang S, Tan G, Jiang X, et al. An artificial lncRNA targeting multiple miRNAs overcomes sorafenib resistance in hepatocellular carcinoma cells. Oncotarget. 2016;7(45):73257–73269.
20. Wang J-Y, Cui Y-H, Xiao L, et al. Regulation of intestinal epithelial barrier function by lncRNA\r uc.173\r through interaction with miR-29b. MCB.00010–00018. Mol Cell Biol. 2018;38(13):e00010–18
21. Gebert LFR. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21–37.
22. Tian F, Jia L, Chu Z, et al. MicroRNA-519a inhibits the proliferation and promotes the apoptosis of ovarian cancer cells through targeting signal transducer and activator of transcription 3. Mol Cell Biol. 2018;15(2):1819–1824.
23. Baulande S, Criqui A, Duthieuw M. Circulating miRNAs as a new class of biomedical markers. Med Sci. 2014;30(3):289.
24. Feng X, Zhao L, Gao S, et al. Increased fucosylation has a pivotal role in multidrug resistance of breast cancer cells through miR-224-3p targeting FUT4. Gene. 2016;578(2):232–241.
25. Sun B, Hu S, Li Y, et al. Retraction note: triptolide contributes to decrease in TLR4 expression by upregulating miR-224-3p to inhibit the inflammatory reaction in diabetic nephropathy. Oncotarget. 2016;7(51):85675. doi:10.18632/oncotarget.14017
26. Chang YH, Yin F, Fan GF, Zhao M. Down-regulation of miR-329-3p is associated with worse prognosis in patients with cervical cancer. Eur Rev Med Pharmacol Sci. 2017:4045–4049.
27. Zhu M, Chen Q, Liu X, et al. lncRNA H19/miR‐675 axis represses prostate cancer metastasis by targeting TGFBI. Febs J. 2015;281(16):3766–3775. doi:10.1111/febs.12902
28. Li H, Zhu H, Xu C-J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the fas pathway of apoptosis. Cell. 1998;94(4):491–501. doi:10.1016/S0092-8674(00)81590-1
29. Liang W, Xiaoxing Z, Rebeca C-L, Lingjiao W, Weihong S, Xueyuan Y. Selection and evaluation of reference genes for qRT-PCR analysis in Euscaphis konishii Hayata based on transcriptome data. Plant Methods. 2018;14(1):42.
30. Kazakova OA, Khapchaev AY, Ragimov AA, Salimov EL, Shirinsky VP. Western blotting-based quantitative measurement of myosin II regulatory light chain phosphorylation in small amounts of non-muscle cells. Biochemistry (Mosc). 2019;84(1):11–19.
31. Dougherty TJ. Photosensitizers: therapy and detection of malignant tumors. Photochem Photobiol. 2010;45(S1):879–889.
32. Herbst RS, Morgensztern D, Boshoff CJN. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446.
33. Hu Q, Li B, Garfield D, et al. Prognostic factors for survival in a Chinese population presenting with advanced non-small cell lung cancer with an emphasis on smoking status: a regional, single-institution, retrospective analysis of 4552 patients. Thorac Cancer. 2012;3(2):162–168.
34. Guo YP. Advancement and the value of the targeted gene related to lung cancer. J Int Oncol. 2013;269(1):68–73.
35. Liu S, Zhang M, Qu P. Expression level and clinical significance of HOX transcript antisense intergenic RNA in cervical cancer: a meta-analysis. Sci Rep. 2016;6(1):38047. doi:10.1038/srep38047
36. Zang W, Wang T, Huang J, et al. Long noncoding RNA PEG10 regulates proliferation and invasion of esophageal cancer cells. Cancer Gene Ther. 2015;22(3):138–144.
37. Jiang C, Yang Y, Yang Y, et al. LncRNA-HOTAIR affects tumorigenesis and metastasis of non-small cell lung cancer by up-regulating miR-613. Oncol Res. 2017.
38. Tan N, Lihong LI, Bai L, Zhao KJ. Expression of serum LncRNA HOTAIR in non-small cell lung cancer and its clinical significance. Chin J Lung Cancer. 2017;20(6):402–406.
39. Kurozumi A, Goto Y, Matsushita R, et al. Tumor-suppressive microRNA-223 inhibits cancer cell migration and invasion by targeting ITGA3/ITGB1 signaling in prostate cancer. Cancer Sci. 2016;107(1):84–94.
40. Li B, Wang W, Li Z, et al. MicroRNA-148a-3p enhances cisplatin cytotoxicity in gastric cancer through mitochondrial fission induction and cyto-protective autophagy suppression. Cancer Lett. 2017;410:212–227. doi:10.1016/j.canlet.2017.09.035
41. Fang W, Shu S, Yongmei L, Endong Z, Lirong Y, Bei S. miR-224-3p inhibits autophagy in cervical cancer cells by targeting FIP200. Sci Rep. 2016;6(1):33229.
42. Zhao H, Bi T, Qu Z, Jiang J, Cui S, Wang Y. Expression of miR-224-5p is associated with the original cisplatin resistance of ovarian papillary serous carcinoma. Oncol Rep. 2014;32(3):1003.
43. Tang H, Jianjie Z, Wenwen D, et al. CPNE1 is a target of miR-335-5p and plays an important role in the pathogenesis of non-small cell lung cancer. J Exp Clin Cancer Res. 2018;37(1):131.
44. Jili S, Eryong L, Lijuan L, Chao Z. RUNX3 inhibits laryngeal squamous cell carcinoma malignancy under the regulation of miR‐148a‐3p/DNMT1 axis. Cell Biochem Funct. 2016;34(8):597–605. doi:10.1002/cbf.3233
45. Zhou M, Liu X, Li Z, Huang Q, Li F, Li C-Y. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int J Cancer. 2018;143(4):921–930. doi:10.1002/ijc.31374
46. Sergeeva TF, Shirmanova MV, Zlobovskaya OA, et al. Relationship between intracellular pH, metabolic co-factors and caspase-3 activation in cancer cells during apoptosis. Biochim Biophys Acta Mol Cell Res. 2017;1864(3):604.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.