Back to Journals » OncoTargets and Therapy » Volume 13
LncRNA GAS8-AS1 Inhibits Ovarian Cancer Progression Through Activating Beclin1-Mediated Autophagy
Authors Fang YJ, Jiang P, Zhai H, Dong JS
Received 5 June 2020
Accepted for publication 4 September 2020
Published 14 October 2020 Volume 2020:13 Pages 10431—10440
DOI https://doi.org/10.2147/OTT.S266389
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yao Dai
Ying-Ji Fang,1,* Ping Jiang,2,* Hui Zhai,1 Jing-Sen Dong1
1Department of Gynecology, Jinan Maternal and Child Care Hospital, Jinan, Shandong, People’s Republic of China; 2Department of Obstetrics, Yantai Mountain Hospital, Yantai, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ping Jiang Department of Obstetrics, Yantai Mountain Hospital, Yantai, Shandong, People’s Republic of China
Email [email protected]
Background: Early detection and diagnosis of ovarian cancer (OC) is complicated due to the concealment of the ovarian anatomical position and the lack of clinical manifestations and specific indicators of early OC. Therefore, it is urgent to study the pathogenesis of OC, especially the molecular mechanism.
Results: LncRNA GAS8-AS1 was decreased in OC tissues and cell lines, and high expression of GAS8-AS1 indicated a higher 5-year survival rate of OC patients. Overexpression of GAS8-AS1 suppressed growth of OC cells, while deletion of GAS8-AS1 promoted the progression of OC cells. Further data indicated GAS8-AS1 activated autophagy in OC cells. Functional experiments showed that 3-MA removed the inhibitory effect of GAS8-AS1 in OC cells. On the contrary, Rapamycin reversed the promoting effect of GAS8-AS1 in OC cells. Furthermore, GAS8-AS1 bound with Beclin1 and promoted its expression, and silencing of Beclin1 reversed the inhibitory role of GAS8-AS1 in OC progression. In vivo tumorigenesis assay showed GAS8-AS1 suppressed OC progression and activated Beclin1 mediated autophagy.
Conclusion: Our study suggested GAS8-AS1 inhibited OC progression by activating autophagy via binding with Beclin1, and GAS8-AS1 might be a potential therapeutic target for OC clinical treatment.
Keywords: ovarian cancer, GAS8-AS1, autophagy, Beclin1, tumor progression
Introduction
Ovarian cancer (OC) is the most lethal female reproductive system malignant tumor, with a 5-year survival rate of less than 35%.1 Early detection and diagnosis of OC is complicated due to the concealment of the ovarian anatomical position and the lack of clinical manifestations and specific indicators of early OC.2 Patients are usually in the advanced stage when they are firstly detected, some of them even have peritoneal metastasis or distant metastasis.3 The recurrence rate of OC and the incidence of poor prognosis are relatively high.4 Patients with advanced OC have high resistance to postoperative chemotherapy and poor therapeutic effect. Therefore, it is urgent to study the pathogenesis of OC, especially the molecular mechanism.
Long non coding RNA (lncRNA) participates in regulating multiple activities of cells, such as cell growth, proliferation, apoptosis, epithelial-mesenchymal transformation, angiogenesis, and drug resistance.5,6 Studies have shown that lncRNAs are associated with the occurrence of human tumors and are considered as a potential prognostic marker for some tumors. LncRNA AC114812. 8 can promote the progression of bladder cancer,7 lncRNA MAGI2-AS3 can be used as a prognostic marker for gastric cancer,8 and high expression of lncRNA ZFAS1 can improve the malignant degree of glioblastoma.9 And in OC, upregulation of lncRNA is more likely to play a pro-cancer role, while downregulation of lncRNA tends to act as tumor suppressor. LncRNA MLK7-AS1 was significantly increased in OC cells, which endogenously competed with miR-375 and reduced its expression. And then MLK7-AS1 upregulated the expression of Yes related protein 1, promoted the proliferation and development of OC, and was associated with the survival and prognosis of OC patients.10 It has been reported that GAS8-AS1 contributed to the development of colorectal cancer,11 papillary thyroid carcinoma12 and hepatocellular carcinoma.13 However, the function of GAS8-AS1 in ovarian cancer is poorly defined.
In normal cells, autophagy removes damaged organelles and proteins from the cell to prevent disease and maintain energy balance in the absence of oxygen or nutrients.14 In apoptotic cells, autophagy prevents cell necrosis, a process that may exacerbate local inflammation and promote tumor growth.15 At present, it is believed that autophagy is closely related to gynecological tumors. Autophagy level is down-regulated in cervical squamous epithelial carcinoma and up-regulated in endometrial carcinoma.16,17 And in OC, there is a complex relationship between autophagy and the occurrence, development and emergence of drug resistance in OC. Increased autophagy may promote autophagic death of OC cells and may protect OC cells from death. The role of autophagy in the development and progression of OC is bidirectional.18
Beclin1 is one of the earliest autophagy genes identified mammals, which regulates autophagosome formation mainly by forming complexes with PI3K.19,20 Studies have shown that Beclin1 can regulate growth factor signaling, including the AKT and ERK pathways. Therefore, Beclin1 deficiency may cause dysregulation of growth factor receptor signaling, leading to the development of OC.21 Beclin1 expression is decreased and autophagy is weakened in ovarian serous carcinoma tissues, which may be related to the occurrence and development of serous carcinoma and poor prognosis.22 In addition, Beclin1 expression is also associated with the invasiveness of OC, and the higher Beclin1 level, the weaker the invasiveness of OC.23 Zhao et al showed that Beclin1 could prevent the development of OC.24 Therefore, we aimed to explore the role of GAS8-AS1 in OC, and whether Beclin1 mediated autophagy is involved in this process.
Methods
Clinical Samples
Fresh cancer tissue samples and para-tumor tissue samples were taken from 40 OC patients undergoing surgical procedures at Jinan Maternal and Child Care Hospital. All of the patients or their guardians (patient is too ill or poorly educated to sign) provided written consent. And the experiment was permitted by the Ethics Committee of Jinan Maternal and Child Care Hospital.
Microarray Analysis
LncRNAs in OC tissues were profiled using microarray analysis (Bio-tech, Shanghai, China). Differentially expressed lncRNAs were identified by Heatmap. Up-regulated or down-regulated lncRNAs were selected based on changes ≥2 fold threshold and P < 0.05.
RNA Immunoprecipitation (RIP)
RIP experiments were used to identify the relationship between GAS8-AS1 and Beclin1, and we purchased RIP RNA-Binding Protein Immunoprecipitation Kit from Millipore (USA). According to instructions, Beclin1 was transiently transfected into A2780 and SKOV3 cells. Cells were harvested and lysed using Beclin1 antibody, then detected by qRT-PCR analysis.
Cell Culture and Treatment
The OC cell lines (COC1, A2780 and SKOV3) and normal ovarian epithelial cells IOSE80 were purchased from the Science Cell Laboratory. Cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum and 100 μL/mL penicillin and streptomycin. 2 μg plasmid or 500 nM si-RNA or its NC was transfected into cells with Lipo 2000, respectively. Cells were treated with 3-MA (5 μM) or Rapamycin (50 nM) for 1 h, and treated with Bafilomycin A1 (0.5 μM) for 4 h. The sequence of the si-GAS8-AS1: 5′-GGCACAACGACAAAUGUCUTT-3′ (sense), 5′-AGACAUUUGUCGUUGUGCCTT-3′ (antisense).
RNA Isolation and qRT-PCR
RNA isolation, reverse transcription and quantitative expression were carried according to manufacturer’s instructions. All the kits were purchased from Vazyme, and gene expression was calculated using 2-ΔΔCt method.25 Primer sequence lists: GAS8-AS1 (F: 5′-CAACGAGCAAA CAAGAAGGAG-3′, R: 5′-TGAGCCAAACAGACCAGTCA-3′), Beclin1: (F: 5′- GACGGTGCCATGGAATTTGC −3′, R: 5′-GGCATAACGCATCTGGTTTT-3′) GADPH (F: 5′- ATGGGGAAGGTGAAGGTCGG −3′, R: 5′- GACGGTGCCATGGAATTTGC −3′)
Protein Isolation and Western Blot
Total protein was collected from cells with RIPA lysis Mix. Western blotting assay was performed as previously described.26 Briefly, 60 μg protein extractions were loaded via SDS-PAGE and transferred onto nitrocellulose membranes (absin, China); then, incubated with primary antibodies for 2 hrs at temperature, then plated at 4°C overnight, the membranes were incubated in 5% non-fat milk blocking buffer for horizontal mode 3 h. After incubation with secondary antibodies (Lincoln, Nebraska, USA), the membranes were scanned using an Odyssey, and data were analyzed with Odyssey software (LI-COR, USA). Primary antibody lists: LC3 I/II (L7543, Sigma), Beclin1 (ab210498, Abcam), GADPH (ab8245, Abcam).
MTT Assay
A2780 and SKOV3 were seeded in 96-well cell plates, and added MTT solution (Vazyme, China) at 48 h. 2 hours later, the OD value at 450 nm was measured.
Wound Healing Assay
A2780 and SKOV3 cells were planted in a 6-well plate, and when the cells grew to fuse, two vertical parallel lines were drawn with 10 μL suction head against the ruler. The floating cells were washed with PBS and cultured in serum-free medium for 24 hours. Images were taken at 0 and 24 hours of cell culture, respectively.
Transwell Assay
A2780 and SKOV3 in logarithmic growth phase were adjusted to 2 × 105 cells/well of medium (without serum) and plated into the upper chamber insert pre-coated with 1μg/μL Matrigel. Lower chamber was added with 500 μL of medium (with 10% FBS), and then incubate the chamber at 37°C for 48 h. Then the invading cells were visualized by the crystal violet and inverted microscope.
Clone Formation Assay
A2780 and SKOV3 were seeded into six well plates at a density of 100 cells/well and cultured for 14 days. Cells were fixed with 4% paraformaldehyde and stained with 0.1% Crystal Violet (Sigma-Aldrich, USA) at RT for 15min. Then, cells were rinsed with distilled water, and the colonies were visualized by inverted microscope.
Edu Assay
A2780 and SKOV3 cells were seeded in 24-well cell plates, and added Edu solution (Ribobio, China) according to the protocols. The cells were incubated for 2 h, and then treated and photographed.
In vivo Tumor Growth Assay
Animal experiments were permitted by the Animal Protection and Ethics Committee. BALB/c nude mice (6–8 weeks) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. For the experiment of Xenograft, A2780 cells (5 × 106) were suspended in 200 μL normal saline and subcutaneously injected into right flank. 1 week later, we injected lentivirus packaged GAS8-AS1 or pcDNA3.1 (Biowit Technology, Shenzhen, China) into tumors, and we measured tumor volume every 7 days. Tumor volume (mm3): V (mm3) = S2 (mm2) × L (mm)/2.27 After 28 days of injection, mice were intraperitoneally injected with 3% pentobarbital sodium and were killed by excessive anesthesia with a dose of 90 mL/kg, and the tumors were removed for follow-up study.
Institutional Animal Care and Use Committee Statement
All experimental protocols were approved by the Animal Research Ethical Committee of the Jinan Maternal and Child Care Hospital, and were performed in accordance with National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). All efforts were made to utilize only the minimum number of animals necessary to produce reliable scientific data. All animal experimentation was taken place in the Jinan Maternal and Child Care Hospital.
Statistical Analysis
All data are presented as a mean ± S.E.M. Statistical analysis was performed using Student’s t-test or Wilcoxon test or a one-way ANOVA through Graphpad 7.0 and SPSS 22.0.
Results
LncRNA GAS8-AS1 Was Downregulated in OC Tissues and Cells
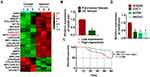
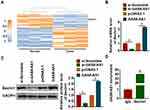
As shown in Figure 1A, GAS8-AS1 was significantly lower in OC comparing with para-tumor tissues. Then, we collected 40 OC tissues (Table 1), qRT-PCR also showed that GAS8-AS1 was decreased in OC tissues (Figure 1B). According to the median level of GAS8-AS1 in Figure 1B, OC patients were divided into low (n = 20) and high expression group (n = 20). Kaplan-Meier curves indicated 5-year survival rate of OC patients was significantly higher in high expression group than low expression group (Figure 1C). Then, we found a decrease of GAS8-AS1 in OC cell lines (COC1, A2780 and SKOV3) than that in human normal ovarian epithelial cells IOSE80 (Figure 1D).
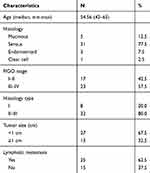 |
Table 1 Clinical Characteristics of OC Patients |
GAS8-AS1 Suppresssed the Growth of OC Cells
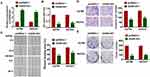
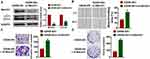
Then, we constructed GAS8-AS1 plasmid. As shown in Figure 2A, transfection of GAS8-AS1 significantly induced GAS8-AS1 expression in A2780 and SKOV3 cells. Then, MTT assay showed that GAS8-AS1 inhibited the cell viability of OC cells (Figure 2B). And forced expression of GAS8-AS1 inhibited the wound healing area, which indicated a low migrative ability of OC cells (Figure 2C). GAS8-AS1 also suppressed the invasive ability of OC cells comparing with pcDNA3.1 (Figure 2D). Furthermore, GAS8-AS1 decreased the clone numbers of OC cells (Figure 2E). These data indicated that upregulation of GAS8-AS1 inhibited the growth of OC cells.
Next, we constructed small interfering RNA of GAS8-AS1 (si-GAS8-AS1) to knockdown GAS8-AS1 level in OC cells (Figure 3A). Followed functional analysis showed that loss of GAS8-AS1 increased cell viability, promoted cell migration and invasion, and increased colony-forming capacity of OC cells (Figure 3B–E). These data indicated that upregulation of GAS8-AS1 inhibited the growth of OC cells, while downregulation of GAS8-AS1 showed the opposite effects.
GAS8-AS1 Inhibits Cancer Progression by Activating Autophagy of OC Cells
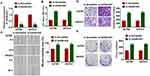
It has been proved that autophagy was involved in the process of OC, and we examined the effect of GAS8-AS1 on autophagy. Interestingly, GAS8-AS1 increased LC3 puncta in A2780 cells (Figure 4A). In addition, GAS8-AS1 induced ratio of LC3-II/LC3-I, while Bafilomycin A1 (inhibitor of autophagy) reversed the effect of GAS8-AS1 on autophagy (Figure 4B). Functional experiments showed that 3-MA (autophagy inhibitor) removed the inhibitory effect of GAS8-AS1 on migration and invasion in A2780 cells (Figure 4C and E and G). On the contrary, Rapamycin (autophagy inducer) reversed the promoting effect of GAS8-AS1 on migration and invasion in A2780 cells (Figure 4D and F and H). These data indicated that GAS8-AS1 inhibited OC development via activating autophagy.
GAS8-AS1 Interacted with Beclin1
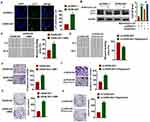
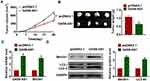
Beclin1 is a key molecule of autophagy process and OC progression, and we examined the effect of GAS8-AS1 on Beclin1. Microarray assay showed that Beclin1 was decreased in OC tissues (Figure 5A). qRT-PCR showed that si-GAS8-AS1 reduced Beclin1 level, while GAS8-AS1 induced Beclin1 expression in A2780 cells (Figure 5B). As well, Western blot analysis showed loss of GAS8-AS1 decreased Beclin1 expression, and overexpression of GAS8-AS1 increased Beclin1 expression in A2780 cells (Figure 5C). To further explore the relationship between GAS8-AS1 and Beclin1, we performed RIP assay. And the qRT-PCR analysis revealed a binding of GAS8-AS1 and Beclin1 (Figure 5D).
Then, we transfected si-Beclin1 or its NC in OC cells with the presence of GAS8-AS1. Western blot showed that silencing of Beclin1 inhibited the protein expression of Beclin1 and decreased the ratio of LC3-II/LC3-I in A2780 cells (Figure 6A). And functional analysis showed deficiency of Beclin1 removed the inhibitory effect of GAS8-AS1 on cell migration, invasion and colony formation in A2780 cells (Figure 6B–D).
GAS8-AS1 Inhibited OC Tumorigenesis in vivo
For further explore the function of GAS8-AS1 in OC, we set up xenograft nude mice model. Thirty mice were divided into two groups randomly, A2780 cells were subcutaneously injected into nude mice. One week later, we injected lentivirus packaged GAS8-AS1 or pcDNA3.1 into tumors, and we measured tumor volume. Injection of GAS8-AS1 showed a bigger tumor volume, and tumors grew faster (Figure 7A). The tumors were isolated at 28 days after injection, injection of GAS8-AS1 significantly increased tumor weight (Figure 7B). In addition, isolated tumor tissues had a higher GAS8-AS1 level after injection of lentivirus packaged GAS8-AS1, and injection of GAS8-AS1 increased Beclin1 expression (Figure 7C). As well, GAS8-AS1 promoted Beclin1 protein expression and the ratio of LC3-II/LC3-I (Figure 7D). Taken together, GAS8-AS1 inhibited the progression of OC via activating Beclin1-mediated autophagy.
Discussion
Ovarian cancer is the leading cause of death in female genital tumors.28 The first chemotherapy is effective for most patients, but about 70% of patients will relapse within a few months after the first chemotherapy, mainly due to the development of drug resistance.29 Resistance of ovarian cancer can be caused by a variety of mechanisms, including inadequate accumulation of chemotherapy drugs, inactivation of chemotherapy drugs, repair of DNA damage, and activation of survival signaling pathways. And studies have showed an essential role of lncRNAs in cancer progression. Present study firstly found an elevation of lncRNA GAS8-AS1 in OC tissues and cells. And overexpression of GAS8-AS1 suppressed growth of OC cells, while deletion of GAS8-AS1 promoted the progression of OC cells. Mechanismly, GAS8-AS1 interacted with beclin1 and activating autophagy process in OC cells.
Numerous studies have shown the outstanding function of lncRNAs in the development of OC.30 In particular, inhibiting the proliferation of tumor cells is the focus of anti-tumor research. The expression of lncRNA GAS5 was decreased in ovarian cancer, and the down-regulation of GAS5 led to the increase of tumor cell proliferation and colony formation, and the reduction of cell apoptosis.31 As an anticancer factor, lncRNA GAS5 is expected to become a new target for early detection, diagnosis and treatment of various tumors. And in present study, we performed microarray and found GAS8-AS1 was significantly decreased in OC tissues, which was then confirmed by qRT-PCR. Furthermore, high expression of GAS8-AS1 indicated a higher 5-year survival rate of OC patients. Our data showed a correlation between GAS8-AS1 and OC, which prompted us to further explore GAS8-AS1 function.
Tumor metastasis is one of the main causes of death in patients with malignant tumors, accounting for more than 90% of tumor mortality.32 For patients with malignant tumors, the poor prognosis of distant tissue or organ metastasis. LncRNA is a new class of regulatory factors of gene expression, which contributes to the invasion, metastasis and prognosis of tumor cells.33 GAS8-AS1 was shown to inhibit thyroid cancer cell proliferation.34 So, we tested the effects of GAS8-AS1 on OC cell migration and invasion. Followed functional analysis showed that overexpression of GAS8-AS1 suppressed proliferation, migration and invasion of OC cells, while deletion of GAS8-AS1 showed the opposite effects. Our data indicated that lncRNA GAS8-AS1 might inhibit the progression of OC cells.
As another apoptosis process of cells, autophagy has attracted extensive attention. Many scholars have found that autophagy plays an important role in the occurrence and development of ovarian cancer.35 Autophagy-related factors are closely related to the development of ovarian cancer, even the generation of drug resistance. Among them, Beclin1 and LC3 are most closely related to the development of ovarian cancer.22 Beclin1 is closely related to the occurrence of ovarian cancer, which can be used to determine the prognosis. Monitoring LC3 level during chemotherapy can determine the changes in autophagy level. Interestingly, our data showed GAS8-AS1 activated autophagy in OC cells, exhibiting an increase of LC3II expression. 3-MA and Rapamycin are two key drugs in studying autophagy,36,37 3-MA can block autophagy and Rapamycin can activate autophagy. Thus, we used 3-MA and Rapamycin to examine the effects of GAS8-AS1. Functional experiments showed that 3-MA removed the inhibitory effect of GAS8-AS1 on migration and invasion in A2780 and SKOV3 cells. On the contrary, Rapamycin reversed the promoting effect of GAS8-AS1 on migration and invasion in A2780 and SKOV3 cells. Furthermore, we found that GAS8-AS1 bound with Beclin1 and promoted its expression, silencing of Beclin1 reversed the inhibitory role of GAS8-AS1 on OC progression. Xenograft model is common used in exploring tumor occurrence and growth.38 Our tumorigenesis assay showed GAS8-AS1 suppressed OC progression and activated Beclin1 mediated autophagy.
In conclusion, our study suggested GAS8-AS1 inhibited OC progression by activating autophagy via binding with Beclin1. And GAS8-AS1 might be a potential therapeutic target for OC clinical treatment.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Li N, Zhan X, Zhan X. The lncRNA SNHG3 regulates energy metabolism of ovarian cancer by an analysis of mitochondrial proteomes. Gynecol Oncol. 2018;150(2):343–354. doi:10.1016/j.ygyno.2018.06.013
2. Singh A, Gupta S, Sachan M. Epigenetic biomarkers in the management of ovarian cancer: current prospectives. Front Cell Dev Biol. 2019;7:182.
3. Masoodi T, Siraj S, Siraj AK, et al. Genetic heterogeneity and evolutionary history of high-grade ovarian carcinoma and matched distant metastases. Br J Cancer. 2020;122(8):1219–1230. doi:10.1038/s41416-020-0763-4
4. Wu W, Gao H, Li X, et al. LncRNA TPT1-AS1 promotes tumorigenesis and metastasis in epithelial ovarian cancer by inducing TPT1 expression. Cancer Sci. 2019;110(5):1587–1598. doi:10.1111/cas.14009
5. Hosono Y, Niknafs YS, Prensner JR, et al. Oncogenic role of THOR, a conserved cancer/testis long non-coding RNA. Cell. 2017;171(7):1559–1572.e1520. doi:10.1016/j.cell.2017.11.040
6. Lin YH. Crosstalk of lncRNA and cellular metabolism and their regulatory mechanism in cancer. Int J Mol Sci. 2020;21(8):2947.
7. Li W, Li Y, Ma W, Zhou J, Sun Z, Yan X. Long noncoding RNA AC114812.8 promotes the progression of bladder cancer through miR-371b-5p/FUT4 axis. Biomed Pharmacother. 2020;121:109605. doi:10.1016/j.biopha.2019.109605
8. Li D, Wang J, Zhang M, et al. LncRNA MAGI2-AS3 is regulated by BRD4 and promotes gastric cancer progression via maintaining ZEB1 overexpression by sponging miR-141/200a. Mol Ther Nucleic Acids. 2020;19:109–123. doi:10.1016/j.omtn.2019.11.003
9. Večeřa M, Šána J, Bútová R, et al. [Dysregulation of long non-coding RNAs in glioblastoma multiforme and their study through use of modern molecular-genetic approaches]. Klinicka Onkologie: Casopis Ceske a Slovenske Onkologicke Spolecnosti. 2018;31:168–170.Czech.
10. Yan H, Li H, Li P, et al. Long noncoding RNA MLK7-AS1 promotes ovarian cancer cells progression by modulating miR-375/YAP1 axis. J Exp Clin Cancer Res. 2018;37(1):237. doi:10.1186/s13046-018-0910-4
11. Zhao Y, Chu Y, Sun J, S R, Li Y, Xu F. LncRNA GAS8-AS inhibits colorectal cancer (CRC) cell proliferation by downregulating lncRNA AFAP1-AS1. Gene. 2019;710:140–144. doi:10.1016/j.gene.2019.05.040
12. Chen N, Yin D, Lun B, et al. LncRNA GAS8-AS1 suppresses papillary thyroid carcinoma cell growth through the miR-135b-5p/CCND2 axis. Biosci Rep. 2019;39(1).
13. Pan W, Zhang N, Liu W, et al. The long noncoding RNA GAS8-AS1 suppresses hepatocarcinogenesis by epigenetically activating the tumor suppressor GAS8. J Biol Chem. 2018;293(44):17154–17165. doi:10.1074/jbc.RA118.003055
14. Kaamiranta K, Sinha D, Blasiak J, et al. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9(7):973–984. doi:10.4161/auto.24546
15. Booth LA, Roberts JL, Dent P. The role of cell signaling in the crosstalk between autophagy and apoptosis in the regulation of tumor cell survival in response to sorafenib and neratinib. Semin Cancer Biol. 2019.
16. Cui X, Wang X, Zhou X, et al. miR-106a regulates cell proliferation and autophagy by targeting LKB1 in HPV-16-associated cervical cancer. Mol Cancer Res. 2020.
17. Lin Q, H H, Zhang M, Xiong H, Jiang Q. Knocking down FAM83B inhibits endometrial cancer cell proliferation and metastasis by silencing the PI3K/AKT/mTOR pathway. Biomed Pharmacother. 2019;115:108939. doi:10.1016/j.biopha.2019.108939
18. Zhu J, Zheng Y, Zhang H, et al. Low concentration of chloroquine enhanced efficacy of cisplatin in the treatment of human ovarian cancer dependent on autophagy. Am J Transl Res. 2017;9(9):4046–4058.
19. Sahni S, Merlot AM, Krishan S, Jansson PJ, Richardson DR. Gene of the month: BECN1. J Clin Pathol. 2014;67(8):656–660. doi:10.1136/jclinpath-2014-202356
20. Bao L, Jaramillo MC, Zhang Z, et al. Induction of autophagy contributes to cisplatin resistance in human ovarian cancer cells. Mol Med Rep. 2015;11(1):91–98. doi:10.3892/mmr.2014.2671
21. Tang Maggie KS, Kwong A, Tam K-F, et al. BRCA1 deficiency induces protective autophagy to mitigate stress and provides a mechanism for BRCA1 haploinsufficiency in tumorigenesis. Cancer Letters. 2014;346(1):139–147. doi:10.1016/j.canlet.2013.12.026
22. Satyavarapu EM, Das R, Mandal C, Mukhopadhyay A, Mandal C. Autophagy-independent induction of LC3B through oxidative stress reveals its non-canonical role in anoikis of ovarian cancer cells. Cell Death Dis. 2018;9(10):934. doi:10.1038/s41419-018-0989-8
23. Valente G, Morani F, Nicotra G, et al. Expression and clinical significance of the autophagy proteins BECLIN 1 and LC3 in ovarian cancer. BioMed Res Int. 2014;2014:462658.
24. Zhao Z, Xue J, Zhao X, et al. Prognostic role of autophagy-related proteins in epithelial ovarian cancer: a meta-analysis of observational studies. Minerva medica. 2016;108(3):277–286.
25. Wang C, Zhu C, Xu J, et al. The lncRNA UCA1 promotes proliferation, migration, immune escape and inhibits apoptosis in gastric cancer by sponging anti-tumor miRNAs. Mol Cancer. 2019;18(1):115. doi:10.1186/s12943-019-1032-0
26. Chen Z, Chen X, Lu B, et al. Up-regulated LINC01234 promotes non-small-cell lung cancer cell metastasis by activating VAV3 and repressing BTG2 expression. J Hematol Oncol. 2020;13(1):7. doi:10.1186/s13045-019-0842-2
27. Nishino M, Dahlberg S, Cardarella S, et al. Volumetric tumor growth in advanced non-small cell lung cancer patients with EGFR mutations during EGFR-tyrosine kinase inhibitor therapy: developing criteria to continue therapy beyond RECIST progression. Cancer. 2013;119(21):3761–3768. doi:10.1002/cncr.28290
28. Coan M, Rampioni Vinciguerra GL, Cesaratto L, et al. Exploring the role of fallopian ciliated cells in the pathogenesis of high-grade serous ovarian cancer. Int J Mol Sci. 2018;19(9):2512.
29. Stover EH, Baco B, Cohen O, et al. Pooled genomic screens identify anti-apoptotic genes as targetable mediators of chemotherapy resistance in ovarian cancer. Mol Cancer Res. 2019;17(11):2281–2293. doi:10.1158/1541-7786.MCR-18-1243
30. Salamini-Montemurri M, Lamas-Maceiras M, Barreiro-Alonso A, et al. The challenges and opportunities of LncRNAs in ovarian cancer research and clinical use. Cancers. 2020;12(4):1020.
31. Long X, Song K, Hu H, et al. Long non-coding RNA GAS5 inhibits DDP-resistance and tumor progression of epithelial ovarian cancer via GAS5-E2F4-PARP1-MAPK axis. J Exp Clin Cancer Res. 2019;38(1):345. doi:10.1186/s13046-019-1329-2
32. Contartese D, Salamanna F, Veronesi F, Fini M. Relevance of humanized three-dimensional tumor tissue models: a descriptive systematic literature review. Cell Mol Life Sci. 2020;1–32. doi:10.1007/s00018-020-03513-y
33. Liang Y, Song X, Li Y, et al. LncRNA BCRT1 promotes breast cancer progression by targeting miR-1303/PTBP3 axis. Mol Cancer. 2020;19(1):85. doi:10.1186/s12943-020-01206-5
34. Qin Y, Sun W, Zhang H, et al. LncRNA GAS8-AS1 inhibits cell proliferation through ATG5-mediated autophagy in papillary thyroid cancer. Endocrine. 2018;59(3):555–564. doi:10.1007/s12020-017-1520-1
35. Shen FF, Dai SY, Wong NK, et al. Mediating K/H transport on organelle membranes to selectively eradicate cancer stem cells by a small molecule. J Am Chem Soc. 2020.
36. Lu HY, Zhu JS, Xie J. et al. Hydroxytyrosol and oleuropein inhibit migration and invasion via induction of autophagy in ER-positive breast cancer cell lines (MCF7 and T47D). Nutr Cancer. 2020:1–11. 10.1080/01635581.2020.1750661
37. Bao Y, Ding Z, Zhao P, et al. Autophagy inhibition potentiates the anti-EMT effects of alteronol through TGF-β/Smad3 signaling in melanoma cells. Cell Death Dis. 2020;11(4):223. doi:10.1038/s41419-020-2419-y
38. Ren X, Chen C, Luo Y, et al. lncRNA-PLACT1 sustains activation of NF-κB pathway through a positive feedback loop with IκBα/E2F1 axis in pancreatic cancer. Mol Cancer. 2020;19(1):35. doi:10.1186/s12943-020-01153-1
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.