Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
LncRNA GAS5 Silencing Attenuates Oxygen-Glucose Deprivation/Reperfusion-Induced Injury in Brain Microvascular Endothelial Cells via miR-34b-3p-Dependent Regulation of EPHA4
Authors Shen B, Wang L, Xu Y, Wang H, He S
Received 16 January 2021
Accepted for publication 9 April 2021
Published 26 May 2021 Volume 2021:17 Pages 1667—1678
DOI https://doi.org/10.2147/NDT.S302314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Bin Shen,1 Lan Wang,2 Yuejun Xu,3 Hongwei Wang,1 Shiyi He1
1Jiangsu Vocational College of Medicine, Yancheng, 224005, Jiangsu Province, People’s Republic of China; 2Hubei University of Chinese Medicine, Wuhan, 430065, Hubei Province, People’s Republic of China; 3Wuchang University of Technology, Wuhan, 430223, Hubei Province, People’s Republic of China
Correspondence: Bin Shen
Jiangsu Vocational College of Medicine, Jinhua Garden, Chaosheng Road, Tinghu District, Yancheng City, 224005, Jiangsu Province, People’s Republic of China
Tel +86-515-88588608
Email [email protected]
Background: The aim of our study was to explore the role of long non-coding RNA (lncRNA) growth arrest-specific 5 (GAS5) in ischemic stroke using oxygen-glucose deprivation/reperfusion (OGD/R)-induced bEnd.3 cells as in vitro cell model.
Methods: Real-time quantitative polymerase chain reaction (RT-qPCR) and Western blot assay were adopted to analyze RNA and protein expression. Cell viability and apoptosis were analyzed by Cell Counting Kit-8 (CCK8) assay and flow cytometry. The levels of nitric oxide (NO) and endothelin-1 (ET-1) in culture supernatant were examined by their matching commercial kits. The intermolecular target interaction was predicted by starBase software and tested by dual-luciferase reporter assay and RNA immunoprecipitation (RIP) assay.
Results: OGD/R-induced apoptosis and dysregulation in vascular endocrine system were largely alleviated by the knockdown of GAS5. GAS5 interacted with microRNA-34b-3p (miR-34b-3p), and GAS5 silencing protected bEnd.3 cells from OGD/R-induced injury partly through up-regulating miR-34b-3p. EPH receptor A4 (EPHA4) was a target of miR-34b-3p. GAS5 acted as the molecular sponge of miR-34b-3p to up-regulate EPHA4 in bEnd.3 cells. GAS5 interference protected against OGD/R-induced damage in bEnd.3 cells partly through down-regulating EPHA4.
Conclusion: LncRNA GAS5 knockdown protected brain microvascular endothelial cells bEnd.3 from OGD/R-induced injury depending on the regulation of miR-34b-3p/EPHA4 axis.
Keywords: ischemic stroke, oxygen-glucose deprivation/reperfusion, GAS5, miR-34b-3p, EPHA4
Introduction
Ischemic stroke, featured by artery blockage, is a major type of stroke that accounts for 87% of all stroke cases.1 Artery blockage results in the absence of oxygen and nutrients in brain, and the subsequent reperfusion (oxygen-glucose deprivation/reperfusion (OGD/R)) induces cell apoptosis, cell viability suppression and oxidative stress, which makes the condition even worse.2 Vascular endothelial cells are major targets of ischemic vascular damage, and the injury of vascular endothelial cells eventually leads to vascular dysfunction.3,4 We established in vitro ischemic stroke cell model through exposing brain microvascular endothelial cells bEnd.3 to OGD/R to explore novel effective therapeutic targets.
Endothelial nitric oxide synthase (eNOs) is the precursor of nitric oxide (NO). NO is an important protective molecular that protects endothelial cells against ischemia-induced injury through accelerating vasodilation to allow the blood flow to brain.5 The levels of eNOs and NO are reduced during OGD. Restoring the levels of eNOs and NO is a promising method for attenuating ischemia-mediated injury.6
Long noncoding RNA (lncRNA) growth arrest-specific 5 (GAS5) was implicated in progression of several malignancies.7,8 Wen et al found that GAS5 suppressed the progression of cervical cancer through sponging microRNA-21 (miR-21).9 The vital role of GAS5 in regulating the development of ischemic stroke has also been reported. Chen et al demonstrated that GAS5 promoted ischemic stroke progression through regulating miR-137/Notch1 signaling.10 Nevertheless, the precise working mechanism of GAS5 in ischemic stroke remains to be illustrated.
LncRNAs are generally known to bind to target microRNAs (miRNAs) to release downstream genes from the inhibition of miRNAs.11 For example, SNHG12 accelerated the angiogenesis after ischemic stroke through targeting miR-150/VEGF axis.12 Based on the prediction of bioinformatic starBase database, miR-34b-3p was a possible target of GAS5. MiR-34b was reported to protect against focal cerebral ischemia-reperfusion (I/R) injury through regulating Keap1/Nrf2 signal pathway.13 Here, we tested the intermolecular binding relation between miR-34b-3p and GAS5 and explored their functional relevance in ischemic stroke.
Through using starBase database, EPH receptor A4 (EPHA4) was predicted to be a possible target of miR-34b-3p. EPHA4 was reported to accelerate the disruption of blood-brain barrier after OGD/R through regulating Rho/ROCK signal pathway.14 Li et al found that EPHA4 suppression attenuated OGD/R-induced apoptosis of CA1 pyramidal neurons.15 In this study, the target relation between miR-34b-3p and EPHA4 and their functional association in ischemic stroke were investigated.
In the current study, the level of GAS5 was observed to be aberrantly up-regulated in bEnd.3 cells exposed to OGD/R. The biological role of GAS5 in OGD/R-induced injury in bEnd.3 cells and its associated mechanism were subsequently explored.
Materials and Methods
Cell Culture
Mouse brain microvascular endothelial cell line bEnd.3 was obtained from Peking Union Medical College Cell Bank (Beijing, China). bEnd.3 cells were maintained in Dulbecco’s modified Eagle medium (DMEM, Gibco, Carlsbad, CA, USA) plus 10% fetal bovine serum (FBS, Gibco) and 10% penicillin/streptomycin (Gibco) at a 37°C incubator containing 5% CO2 and 95% O2.
Oxygen-Glucose Deprivation/Reperfusion (OGD/R) Cell Model Establishment
To mimic OGD, bEnd.3 cells were cultured with Earle’s balanced salt solution (Leagene, Beijing, China) in the sealed Anaero container with an Anaero Pack (Mitsubishi, Tokyo, Japan) for 4 h followed by culturing with normal culture medium for 24 h under normoxic condition.
Cell Transfection
GAS5 small interfering RNAs (si-GAS5#1, si-GAS5#2 or si-GAS5#3), siRNA negative control (si-NC), miR-34b-3p mimics (miR-34b-3p), miR-NC, miR-34b-3p inhibitor (anti-miR-34b-3p), anti-NC, EPHA4 ectopic expression plasmid (EPHA4) and vector were purchased from Genepharma (Shanghai, China) and Sangon (Shanghai, China). When the confluence of bEnd.3 cells in the logarithmic growth phase reached about 80%, transfection was implemented with Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA).
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Reverse transcription was carried out with miRcute miRNA First-Strand complementary DNA (cDNA) Synthesis Kit (for miR-34b-3p; TIANGEN, Beijing, China) and TaqMan reverse transcription kit (for GAS5; Applied Biosystems, Rotkreuz, Switzerland). cDNA was amplified with TransStarts Green qPCR SuperMix (Transgen, Beijing, China). U6 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as the internal references for miR-34b-3p and GAS5, respectively. The primers for GAS5, miR-34b-3p and internal controls were listed as follows. Mouse GAS5, Forward: 5ʹ-GGATAACAGAGCGAGCGCAAT-3ʹ, Reverse: 5ʹ-CCAGCCAAATGAACAAGCATG-3ʹ. Mouse miR-34b-3p, Forward: 5ʹ-AATCACTAACTCCACTGCCATC-3ʹ, Reverse: 5ʹ-GATGGCAGTGGAGTTAGTGATT-3ʹ. Mouse U6, Forward: 5ʹ-CGCTTCGGCAGCACATATACTA-3ʹ, Reverse: 5ʹ-CGCTTCACGAATTTGCGTGTCA-3ʹ. Mouse GAPDH, Forward: 5ʹ-GGAGCGAGACCCCACTAACAT-3ʹ, Reverse: 5ʹ-GTGAGTTGTCATATTTCTCGTGG-3ʹ.
Cell Viability Detection via Cell Counting Kit-8 (CCK8) Assay
bEnd.3 cells were seeded into 96-well plates. 10 μL CCK8 solution (Beyotime, Shanghai, China) was pipetted into each well, and bEnd.3 cells were incubated for 4 h. Subsequently, the absorbance was immediately measured at 450 nm via TECAN infinite M200 Multimode Microplate Reader (Tecan, Mechelen, Belgium).
Flow Cytometry
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Dojindo, Shanghai, China) was used in this study. bEnd.3 cells were re-suspended in 100 μL binding buffer. Annexin V-FITC and PI were then added into the reaction mixture to incubate for 15 min. FITC+ and PI± cells were considered to be apoptotic cells, and these apoptotic cells were identified by flow cytometer within 1 h of cell collection.
Western Blot Assay
bEnd.3 cells were disrupted using Radio Immunoprecipitation Assay (RIPA) lysis buffer (Beyotime) plus 1% protease inhibitor (Invitrogen). Protein concentration was assessed using BCA protein assay kit (Invitrogen). 20 μg protein samples were loaded onto sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and then electro-transferred onto polyvinylidene fluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA), which was blocked with 5% skim milk for 1 h. After that, the membrane was incubated with primary antibodies, including anti-B cell leukemia/lymphoma 2 (anti-Bcl-2; ab59348; Abcam, Cambridge, MA, USA), anti-Bcl-2 associated X, apoptosis regulator (anti-Bax; ab182733; Abcam), anti-Cleaved caspase 3 (anti-Cleaved-cas3; ab231289; Abcam), anti-endothelial nitric oxide synthase (anti-eNOs, ab199956; Abcam), anti-EPHA4 (E6900; Sigma, St. Louis, MO, USA) and anti-GAPDH (ab8245; Abcam). After washing three times, horse radish peroxidase (HRP)-labeled secondary antibody (Abcam) was incubated with the membrane for 2 h. Protein signal was measured by the enhanced chemiluminescent visualization (ECL) system (Pierce, Rockford, IL, USA).
NO and Endothelin-1 (ET-1) Abundance Detection
NO level in culture supernatant was detected by NO assay kit (JianCheng Biotechnology, Nanjing, China). ET-1 level in culture supernatant was detected via Endothelin-1 Quantikine Enzyme-linked immunosorbent assay (ELISA) Kit (R&D Systems, Minneapolis, MN, USA).
Establishment of GAS5/miR-34b-3p/EPHA4 Axis
starBase software was used for exploring the candidate targets of GAS5 and miR-34b-3p.
Dual-Luciferase Reporter Assay
The partial sequence of GAS5 or the 3ʹ untranslated region (3ʹUTR) fragment of EPHA4, containing the predicted miR-34b-3p binding sites, was amplified by PCR and inserted to the psiCHECK-2 plasmid (Promega, Madison, WI, USA) to obtain reconstructed luciferase reporter plasmid, termed as GAS5 wild type (GAS5 WT) or EPHA4 WT. The matching counterparts, containing the mutant binding sites with miR-34b-3p, were also inserted to psiCHECK-2 plasmid (Promega) to generate GAS5 MUT and EPHA4 MUT. After transfection for 48 h, luciferase activities were assessed via the dual luciferase reporter assay system (Promega).
RNA Immunoprecipitation (RIP) Assay
bEnd.3 cells were harvested and disrupted using 25 mM Tris-HCl buffer (pH 7.5) plus RNase inhibitor (Sigma). Cell lysate was then incubated with Sepharose beads (Bio-Rad) that pre-coated with anti-Argonaute 2 (anti-Ago2; Bio-Rad) or anti-Immunoglobulin G (anti-IgG; Bio-Rad) for 3 h. RNA was isolated using TRIzol solution (Invitrogen), and RT-qPCR was applied to detect the expression of GAS5 and miR-34b-3p.
Statistical Analysis
Data from three independent experiments were shown as mean ± standard deviation (SD). Datasets with two groups were analyzed by Student’s t-test, and comparison in multiple groups was analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s test. P<0.05 was considered as a significant difference.
Results
OGD/R-Induced Injury in bEnd.3 Cells is Largely Attenuated by GAS5 Interference
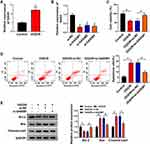
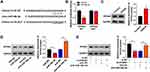
Vascular endothelial cell injury model was established through exposing immortal vascular endothelial cell line bEnd.3 to OGD/R. OGD/R treatment markedly increased the expression of GAS5 in bEnd.3 cells (Figure 1A). We designed three specific small interfering RNAs against GAS5 to perform loss-of-function experiments. As shown in Figure 1B, GAS5 level was notably reduced in bEnd.3 cells transfected with si-GAS5#1, si-GAS5#2 or si-GAS5#3, especially in si-GAS5#1 group. Thus, si-GAS5#1 was selected for further assays. OGD/R treatment suppressed the viability and promoted the apoptosis of bEnd.3 cells, and the injury was largely alleviated by GAS5 silencing (Figure 1C and D), demonstrating that the up-regulation of GAS5 was essential for OGD/R-induced injury. The role of GAS5 on the apoptosis of OGD/R-induced bEnd.3 cells was further confirmed by Western blot assay. The expression of anti-apoptotic protein Bcl-2 was down-regulated upon OGD/R exposure, and GAS5 silencing recovered Bcl-2 expression in bEnd.3 cells (Figure 1E). The expression trend of pro-apoptotic proteins (Bax and Cleaved-cas3) exhibited an opposite phenomenon to Bcl-2 (Figure 1E). These results demonstrated that OGD/R induced the apoptosis of bEnd.3 cells partly through up-regulating GAS5.
OGD/R-Induced Dysregulation in Vascular Endocrine System is Alleviated by GAS5 Silencing in bEnd.3 Cells
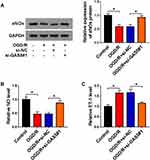
NO is a key factor derived from vascular endothelial cells to reduce blood pressure, and ET-1 exerts an opposite role to constrict the vessel. eNOs is the precursor of NO. We measured the levels of eNOs, NO and ET-1 in GAS5-silenced bEnd.3 cells during OGD/R insult to explore if GAS5 functioned in OGD/R-induced vascular dysfunction. OGD/R treatment reduced the levels of intracellular eNOs and extracellular NO, and GAS5 knockdown largely recovered the levels of eNOs and NO (Figure 2A and B). Furthermore, OGD/R-induced up-regulation of ET-1 in cell culture supernatant was largely attenuated by GAS5 silencing (Figure 2C). Overall, OGD/R induced the dysregulation of eNOs, NO and ET-1 in bEnd.3 cells partly through up-regulating GAS5.
GAS5 Interacts with miR-34b-3p in bEnd.3 Cells
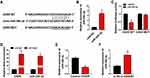
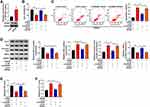
Through using starBase software, we predicted the candidate miRNA targets of GAS5. Among all these predicted targets, six miRNAs were screened out due to their vital roles in regulating cerebral ischemia-reperfusion injury, including miR-34b-3p,13 miR-485-3p,16 miR-211-5p,17 miR-217-5p,18 miR-874-3p19 and miR-338-5p.20 We tested the regulatory relationships between GAS5 and these candidate targets. As shown in Supplementary Figure 1A, with the silencing of GAS5, the levels of miR-34b-3p, miR-217-5p and miR-338-5p were markedly up-regulated. Additionally, the negative regulatory relation between GAS5 and miR-34b-3p was the most significant (Supplementary Figure 1A). Thus, miR-34b-3p was selected for further analysis. The putative binding sites between GAS5 and miR-34b-3p are shown in Figure 3A. RT-qPCR assay confirmed the high overexpression efficiency of miR-34b-3p in bEnd.3 cells (Figure 3B). MiR-34b-3p overexpression markedly reduced the luciferase activity of wild-type luciferase reporter plasmid (GAS5 WT) (Figure 3C). Besides, luciferase activity of mutant luciferase reporter plasmid (GAS5 MUT) remained almost unchanged when co-transfected with miR-NC or miR-34b-3p (Figure 3C), suggesting that GAS5 interacted with miR-34b-3p via the putative sites. The results of RIP assay revealed that GAS5 and miR-34b-3p were both enriched in anti-Ago2 group (Figure 3D), suggesting that there was spatial interaction between GAS5 and miR-34b-3p in RNA-induced silencing complex (RISC). OGD/R treatment down-regulated miR-34b-3p expression in bEnd.3 cells (Figure 3E). GAS5 silencing markedly up-regulated miR-34b-3p in bEnd.3 cells (Figure 3F), suggesting the negative regulatory relationship between GAS5 and miR-34b-3p. Taken together, GAS5 negatively regulated miR-34b-3p level via binding to it in bEnd.3 cells.
GAS5 Silencing-Mediated Influences in OGD/R-Treated bEnd.3 Cells are Overturned by miR-34b-3p Interference
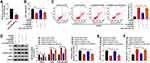
The silencing efficiency of anti-miR-34b-3p was high in bEnd.3 cells (Figure 4A). To investigate if GAS5 functioned through negatively regulating miR-34b-3p expression, we performed rescue experiments. GAS5 silencing recovered cell viability and suppressed the apoptosis of bEnd.3 cells upon OGD/R exposure, and the addition of anti-miR-34b-3p induced cell injury again (Figure 4B and C). Consistently, Western blot assay revealed that GAS5 knockdown attenuated OGD/R-induced apoptosis in bEnd.3 cells partly through up-regulating miR-34b-3p (Figure 4D). Si-GAS5#1 transfection rescued the levels of eNOs and NO in OGD/R-exposed bEnd.3 cells, and the introduction of anti-miR-34b-3p down-regulated the levels of eNOs and NO again (Figure 4D and E). The level of ET-1 in culture supernatant exhibited an opposite tendency to NO and eNOs (Figure 4F), suggesting that OGD/R disrupted homeostasis in vascular endocrine system through up-regulating GAS5 and down-regulating miR-34b-3p. Overall, OGD/R induced injury of vascular endothelial cells and vascular endocrine system dysfunction through targeting GAS5/miR-34b-3p axis.
EPHA4 is a Target of miR-34b-3p in bEnd.3 Cells
We predicted the possible messenger RNA (mRNA) targets of miR-34b-3p using starBase database. EPHA4,15 MCL1,21 PDE4D,22 BCL2L13,23 GATA324 and ROCK125 were chosen due to their important regulatory functions in cerebral ischemia-reperfusion injury. EPHA4 was chosen for further analysis due to its the most significant negative regulatory relationship with miR-34b-3p (Supplementary Figure 1B). The predicted binding sequence between miR-34b-3p and EPHA4 3ʹUTR is shown in Figure 5A. bEnd.3 cells were co-transfected with miR-NC or miR-34b-3p and EPHA4 WT or EPHA4 MUT. Luciferase activity was dramatically reduced in EPHA4 WT group with the co-transfection of miR-34b-3p compared with EPHA4 WT and miR-NC group, while miR-34b-3p transfection did not cause significant difference in luciferase activity in EPHA4 MUT group compared with miR-NC and EPHA4 MUT group (Figure 5B), suggesting that EPHA4 was a target of miR-34b-3p in bEnd.3 cells. OGD/R treatment markedly up-regulated EPHA4 protein expression in bEnd.3 cells (Figure 5C). We transfected miR-34b-3p mimics or anti-miR-34b-3p along with their negative controls into bEnd.3 cells to explore the modulatory relationship between miR-34b-3p and EPHA4 in bEnd.3 cells. As shown in Figure 5D, miR-34b-3p overexpression notably reduced EPHA4 protein expression, whereas miR-34b-3p interference markedly up-regulated EPHA4 protein level in bEnd.3 cells, suggesting the negative regulatory relation between miR-34b-3p and EPHA4 in bEnd.3 cells. Given the negative regulatory relation between miR-34b-3p and GAS5 or EPHA4, we further analyzed the regulatory relationship between GAS5 and EPHA4 in bEnd.3 cells. GAS5 silencing down-regulated EPHA4 protein expression, and this suppressive effect was partly alleviated by miR-34b-3p interference in bEnd.3 cells (Figure 5E). These findings suggested that miR-34b-3p interacted with EPHA4, and GAS5 up-regulated EPHA4 via sponging miR-34b-3p in bEnd.3 cells.
GAS5 Interference-Mediated Effects in OGD/R-Treated bEnd.3 Cells are Alleviated by EPHA4 Overexpression
Rescue experiments were carried out through transfecting si-GAS5#1 alone or together with EPHA4 ectopic expression plasmid into OGD/R-induced bEnd.3 cells. We firstly assessed the overexpression efficiency of EPHA4 overexpression plasmid in bEnd.3 cells. As shown in Figure 6A, the transfection of EPHA4 ectopic expression plasmid markedly up-regulated EPHA4 protein level. Si-GAS5#1-mediated protective role in bEnd.3 cells was attenuated by the addition of EPHA4 overexpression plasmid (Figure 6B and C). The introduction of EPHA4 plasmid down-regulated the level of anti-apoptotic protein Bcl-2 in GAS5-silenced bEnd.3 cells during OGD/R insult (Figure 6D). The levels of pro-apoptotic proteins (Bax and Cleaved-cas3) revealed an opposite phenomenon to Bcl-2 (Figure 6D). The overexpression of EPHA4 also reduced the levels of eNOs and NO and increased the level of ET-1 in GAS5-silenced bEnd.3 cells upon OGD/R exposure (Figure 6D–F). Overall, GAS5 silencing alleviated OGD/R-induced cell injury partly through down-regulating EPHA4 in bEnd.3 cells.
Discussion
Ischemic stroke is one of the deadly diseases globally, which occurs due to artery blockage, resulting in the immediate absence of oxygen and nutrients in brain.26 The injury of brain microvascular endothelial cells triggered by OGD/R is the initial stage of the disruption of blood-brain barrier, causing dismal outcome of ischemic stroke patients.27,28
LncRNAs were originally thought to be the products derived from transcriptional background noise.29,30 Accumulating articles have demonstrated the important roles of lncRNAs in cellular physiological and pathological responses, including cell viability, proliferation, apoptosis and inflammatory response. For instance, lncRNA GAS5 suppressed the proliferation of colorectal cancer cells through targeting miR-182-5p/FOXO3a signaling.31 Currently, lncRNAs have been reported be implicated in the cerebrovascular pathophysiology by previous works, including stroke.32 For instance, lncRNA TUG1 accelerated the apoptosis of neurons via miR-9/Bcl2l11 axis under ischemic condition.33 LncRNA MALAT1 facilitated the angiogenesis in brain microvascular endothelial cells (BMECs) to protect these cells from OGD-induced injury through regulating miR-145, VEGF-A and ANGPT2.34 Here, we explored the role of GAS5 in regulating cerebral ischemia-reperfusion injury using OGD/R-induced mouse brain microvascular endothelial cells bEnd.3 as in vitro cell model. The roles of GAS5 in regulating the phenotypes of neurons in ischemic stroke have been reported by former articles.10,35 Zhou et al found that GAS5 accelerated the apoptosis of neurons through elevating PUMA expression via its miRNA sponge role for miR-221 in ischemic stroke.35 Chen et al reported that GAS5 promoted OGD-induced injury in neurons through targeting miR-137/Notch1 pathway.10 In vascular endothelial cells, Chen et al found that macrophages-derived exosomal GAS5 promoted the apoptosis of vascular endothelial cells in atherosclerosis.36 However, the biological role of GAS5 in cerebral ischemia-reperfusion-induced injury in vascular endothelial cells remains largely unknown. We found that OGD/R insult markedly up-regulated the level of GAS5 in bEnd.3 cells. To explore its biological role, we performed loss-of-function experiments. OGD/R-induced apoptosis was largely attenuated by the silencing of GAS5, suggesting that OGD/R-induced injury in bEnd.3 cells was largely based on the up-regulation of GAS5. eNOs is the precursor of NO, and ischemia decreases the level of eNOs. NO is key factor that is released from vascular endothelial cells to promote vasodilation to allow blood flow to the brain.5 Meanwhile, NO also restrained the inflammation and thrombosis upon ischemic injury.5 Therefore, restoring the levels of eNOs and NO is considered as a potential therapeutic strategy.6 ET-1 played an opposite role with NO to constrict the vessel. OGD/R exposure reduced the levels of eNOs and NO whereas induced the expression of ET-1, and these effects were largely counteracted by the knockdown of GAS5, which further demonstrated that OGD/R-mediated injury in bEnd.3 cells was partly based on the up-regulation of GAS5.
MiRNAs have also emerged as crucial regulators in ischemic stroke. For instance, MiR-191 suppressed the process of angiogenesis after ischemic stroke through suppressing VEZF1.37 MiR-130a played a neuroprotective role against ischemic stroke-induced injury through targeting PTEN/PI3K/AKT signaling.38 MiR-34b-3p was reported as a tumor suppressor in several cancers. For instance, miR-34b-3p restrained the proliferation and cell cycle and triggered the apoptosis of non-small-cell lung cancer through regulating CDK4.39 Tan et al found that miR-34b-3p suppressed the multidrug-chemoresistance of bladder cancer cells through targeting CCND2 and P2RY1.40 MiR-34b-3p has also been demonstrated to protect against focal cerebral ischemia-reperfusion (I/R) Injury through regulating Keap1.13 In this study, miR-34b-3p was verified as a target of GAS5 in bEnd.3 cells. OGD/R treatment down-regulated the enrichment of miR-34b-3p, and miR-34b-3p was negatively regulated by GAS5 in bEnd.3 cells. Through performing rescue experiments, we found that GAS5 contributed to OGD/R-induced injury of bEnd.3 cells through down-regulating miR-34b-3p.
MiRNAs are implicated in the regulation of cellular biological behaviors through restraining the translational process of mRNAs or degrading mRNAs by binding to their 3ʹUTR.41 To explore the mechanism by which GAS5/miR-34b-3p functioned in OGD/R-induced injury in bEnd.3 cells, we intended to seek the downstream molecules of miR-34b-3p. EPHA4 was confirmed as a target of miR-34b-3p in bEnd.3 cells. Cai et al found that miR-145 protected human cerebral cortical neurons from OGD-induced damage through reducing EPHA4 expression,42 suggesting that EPHA4 accelerated the progression of ischemic stroke. Chen et al demonstrated that the activation of EPHA4 accelerated the disruption of blood-brain barrier after OGD/R through regulating Rho/ROCK axis.14 Here, EPHA4 expression was elevated by OGD/R treatment in bEnd.3 cells. GAS5 was found to up-regulate EPHA4 expression through acting as a molecular sponge for miR-34b-3p in bEnd.3 cells. Rescue experiments revealed that GAS5 promoted OGD/R-mediated damage in bEnd.3 cells through up-regulating EPHA4.
In summary, lncRNA GAS5 contributed to OGD/R-induced injury in mouse brain microvascular endothelial cells bEnd.3 through up-regulating EPHA4 via sponging miR-34b-3p. GAS5/miR-34b-3p/EPHA4 axis might provide new insight for preventing and treating ischemic stroke.
Abbreviations
OGD/R, oxygen-glucose deprivation/reperfusion; lncRNA, long non-coding RNA; GAS5, growth arrest-specific 5; Bcl-2, B cell leukemia/lymphoma 2; Cleaved-cas3, Cleaved caspase 3; Bax, Bcl-2 associated X, apoptosis regulator; eNOs, endothelial nitric oxide synthase; EPHA4, EPH receptor A4; NO, nitric oxide; ET-1, endothelin-1.
Data Sharing Statement
All data generated or analyzed during this study are included in this article.
Ethical Approval
The conducted research is not related to either human or animal use.
Funding
This work was supported by Yancheng Development Project of Medical Science and Technology in 2020 (YK2020061).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Tressera-Rimbau A, Arranz S, Eder M, Vallverdú-Queralt A. Dietary polyphenols in the prevention of stroke. Oxid Med Cell Longev. 2017;2017:7467962. doi:10.1155/2017/7467962
2. Liu H, Wei X, Kong L, et al. NOD2 is involved in the inflammatory response after cerebral ischemia-reperfusion injury and triggers NADPH oxidase 2-derived reactive oxygen species. Int J Biol Sci. 2015;11(5):525–535. doi:10.7150/ijbs.10927
3. Ross MD, Malone E, Florida-James G. Vascular ageing and exercise: focus on cellular reparative processes. Oxid Med Cell Longev. 2016;2016:3583956. doi:10.1155/2016/3583956
4. Liu S, Kong X, Ge D, et al. Identification of new small molecules as apoptosis inhibitors in vascular endothelial cells. J Cardiovasc Pharmacol. 2016;67(4):312–318. doi:10.1097/FJC.0000000000000355
5. Zhang B, Li J. Phoenixin-14 protects human brain vascular endothelial cells against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced inflammation and permeability. Arch Biochem Biophys. 2020;682:108275. doi:10.1016/j.abb.2020.108275
6. Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci. 2004;27(5):283–289. doi:10.1016/j.tins.2004.03.009
7. Ye K, Wang S, Zhang H, Han H, Ma B, Nan W. Long noncoding RNA GAS5 suppresses cell growth and epithelial-mesenchymal transition in osteosarcoma by regulating the miR-221/ARHI pathway. J Cell Biochem. 2017;118(12):4772–4781. doi:10.1002/jcb.26145
8. Zhang Z, Zhu Z, Watabe K, et al. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013;20(11):1558–1568. doi:10.1038/cdd.2013.110
9. Wen Q, Liu Y, Lyu H, et al. Long noncoding RNA GAS5, which acts as a tumor suppressor via microRNA 21, regulates cisplatin resistance expression in cervical cancer. Int J Gynecol Cancer. 2017;27(6):1096–1108. doi:10.1097/IGC.0000000000001028
10. Chen F, Zhang L, Wang E, Zhang C, Li X. LncRNA GAS5 regulates ischemic stroke as a competing endogenous RNA for miR-137 to regulate the Notch1 signaling pathway. Biochem Biophys Res Commun. 2018;496(1):184–190. doi:10.1016/j.bbrc.2018.01.022
11. Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505(7483):344–352. doi:10.1038/nature12986
12. Zhao M, Wang J, Xi X, Tan N, Zhang L. SNHG12 promotes angiogenesis following ischemic stroke via regulating miR-150/VEGF pathway. Neuroscience. 2018;390:231–240. doi:10.1016/j.neuroscience.2018.08.029
13. Huang R, Ma J, Niu B, et al. MiR-34b protects against focal cerebral ischemia-reperfusion (I/R) injury in rat by targeting keap1. J Stroke Cerebrovasc Dis. 2019;28(1):1–9. doi:10.1016/j.jstrokecerebrovasdis.2018.08.023
14. Chen F, Liu Z, Peng W, et al. Activation of EphA4 induced by EphrinA1 exacerbates disruption of the blood-brain barrier following cerebral ischemia-reperfusion via the Rho/ROCK signaling pathway. Exp Ther Med. 2018;16(3):2651–2658. doi:10.3892/etm.2018.6460
15. Li J, Liu N, Wang Y, Wang R, Guo D, Zhang C. Inhibition of EphA4 signaling after ischemia-reperfusion reduces apoptosis of CA1 pyramidal neurons. Neurosci Lett. 2012;518(2):92–95. doi:10.1016/j.neulet.2012.04.060
16. Hu C, Bai X, Liu C, Hu Z. Long noncoding RNA XIST participates hypoxia-induced angiogenesis in human brain microvascular endothelial cells through regulating miR-485/SOX7 axis. Am J Transl Res. 2019;11(10):6487–6497.
17. Peng Z, Li M, Tan X, et al. miR-211-5p alleviates focal cerebral ischemia-reperfusion injury in rats by down-regulating the expression of COX2. Biochem Pharmacol. 2020;177:113983. doi:10.1016/j.bcp.2020.113983
18. Duan X, Gan J, Peng DY, et al. Identification and functional analysis of microRNAs in rats following focal cerebral ischemia injury. Mol Med Rep. 2019;19(5):4175–4184. doi:10.3892/mmr.2019.10073
19. Jiang D, Sun X, Wang S, Man H. Upregulation of miR-874-3p decreases cerebral ischemia/reperfusion injury by directly targeting BMF and BCL2L13. Biomed Pharmacother. 2019;117:108941. doi:10.1016/j.biopha.2019.108941
20. Yi X, Fang Q, Li L. MicroRNA-338-5p alleviates cerebral ischemia/reperfusion injury by targeting connective tissue growth factor through the adenosine 5ʹ-monophosphate-activated protein kinase/mammalian target of rapamycin signaling pathway. Neuroreport. 2020;31(3):256–264. doi:10.1097/WNR.0000000000001404
21. Frieler RA, Chung Y, Ahlers CG, et al. Genetic neutrophil deficiency ameliorates cerebral ischemia-reperfusion injury. Exp Neurol. 2017;298(3):104–111. doi:10.1016/j.expneurol.2017.08.016
22. Zhang G, Wang Q, Su D, Xie Y. Long non-coding RNAMALAT1 knockdown alleviates cerebral ischemia/reperfusion injury of rats through regulating the miR-375/PDE4D axis. Front Neurol. 2020;11:578765. doi:10.3389/fneur.2020.578765
23. Lu Y, Han Y, He J, Zhou B, Fang P, Li X. LncRNA FOXD3-AS1 knockdown protects against cerebral ischemia/reperfusion injury via miR-765/BCL2L13 axis. Biomed Pharmacother. 2020;132:110778. doi:10.1016/j.biopha.2020.110778
24. Yao P, Li YL, Chen Y, Shen W, Wu KY, Xu WH. Overexpression of long non-coding RNA Rian attenuates cell apoptosis from cerebral ischemia-reperfusion injury via Rian/miR-144-3p/GATA3 signaling. Gene. 2020;737:144411. doi:10.1016/j.gene.2020.144411
25. Chen H, Li X. LncRNA ROR is involved in cerebral hypoxia/reoxygenation-induced injury in PC12 cells via regulating miR-135a-5p/ROCK1/2. Am J Transl Res. 2019;11(9):6145–6158.
26. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. doi:10.1161/STR.0000000000000158
27. Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185. doi:10.1124/pr.57.2.4
28. Lakhan SE, Kirchgessner A, Tepper D, Leonard A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol. 2013;4:32. doi:10.3389/fneur.2013.00032
29. Hung T, Wang Y, Lin MF, et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43(7):621–629. doi:10.1038/ng.848
30. Hudson WH, Ortlund EA. The structure, function and evolution of proteins that bind DNA and RNA. Nat Rev Mol Cell Biol. 2014;15(11):749–760. doi:10.1038/nrm3884
31. Cheng K, Zhao Z, Wang G, Wang J, Zhu W. lncRNA GAS5 inhibits colorectal cancer cell proliferation via the miR‑182‑5p/FOXO3a axis. Oncol Rep. 2018;40(4):2371–2380. doi:10.3892/or.2018.6584
32. Bao MH, Szeto V, Yang BB, Zhu SZ, Sun HS, Feng ZP. Long non-coding RNAs in ischemic stroke. Cell Death Dis. 2018;9(3):281. doi:10.1038/s41419-018-0282-x
33. Chen S, Wang M, Yang H, et al. LncRNA TUG1 sponges microRNA-9 to promote neurons apoptosis by up-regulated Bcl2l11 under ischemia. Biochem Biophys Res Commun. 2017;485(1):167–173. doi:10.1016/j.bbrc.2017.02.043
34. Ren L, Wei C, Li K, Lu Z. LncRNA MALAT1 up-regulates VEGF-A and ANGPT2 to promote angiogenesis in brain microvascular endothelial cells against oxygen-glucose deprivation via targetting miR-145. Biosci Rep. 2019;39(3). doi:10.1042/BSR20180226
35. Zhou XB, Lai LF, Xie GB, Ding C, Xu X, Wang Y. LncRNAGAS5 sponges miRNA-221 to promote neurons apoptosis by up-regulated PUMA under hypoxia condition. Neurol Res. 2020;42(1):8–16. doi:10.1080/01616412.2019.1672382
36. Chen L, Yang W, Guo Y, et al. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS One. 2017;12(9):e0185406. doi:10.1371/journal.pone.0185406
37. Du K, Zhao C, Wang L, et al. MiR-191 inhibit angiogenesis after acute ischemic stroke targeting VEZF1. Aging (Albany NY). 2019;11(9):2762–2786. doi:10.18632/aging.101948
38. Zheng T, Shi Y, Zhang J, et al. MiR-130a exerts neuroprotective effects against ischemic stroke through PTEN/PI3K/AKT pathway. Biomed Pharmacother. 2019;117:109117. doi:10.1016/j.biopha.2019.109117
39. Feng H, Ge F, Du L, Zhang Z, Liu D. MiR-34b-3p represses cell proliferation, cell cycle progression and cell apoptosis in non-small-cell lung cancer (NSCLC) by targeting CDK4. J Cell Mol Med. 2019;23(8):5282–5291. doi:10.1111/jcmm.14404
40. Tan Y, Zhang T, Zhou L, Liu S, Liang C. MiR-34b-3p represses the multidrug-chemoresistance of bladder cancer cells by regulating the CCND2 and P2RY1 genes. Med Sci Monit. 2019;25:1323–1335. doi:10.12659/MSM.913746
41. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi:10.1146/annurev-biochem-060308-103103
42. Cai D, Wei D, Chen S, et al. MiR-145 protected the cell viability of human cerebral cortical neurons after oxygen-glucose deprivation by downregulating EPHA4. Life Sci. 2019;231:116517. doi:10.1016/j.lfs.2019.05.073
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.