Back to Journals » Clinical Interventions in Aging » Volume 15
lncRNA GAS5 Is Upregulated in Osteoporosis and Downregulates miR-21 to Promote Apoptosis of Osteoclasts
Authors Cong C, Tian J, Gao T, Zhou C, Wang Y, Cui X, Zhu L
Received 18 October 2019
Accepted for publication 10 June 2020
Published 16 July 2020 Volume 2020:15 Pages 1163—1169
DOI https://doi.org/10.2147/CIA.S235197
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Chunlei Cong, Jun Tian, Tianqi Gao, Changlin Zhou, Yuxiang Wang, Xintao Cui, Liyu Zhu
Department of Orthopedics, The Second Affiliated Hospital of Harbin Medical University, Harbin City, Heilongjiang Province 150001, People’s Republic of China
Correspondence: Jun Tian
Department of Orthopedics, The Second Affiliated Hospital of Harbin Medical University, Harbin City, Heilongjiang Province 150001, People’s Republic of China
Email [email protected]
Background: It has been reported that lncRNA growth arrest-specific transcript 5 (GAS5) interacts with miR-21, which plays critical roles in osteoporosis. The involvement of GAS5 in osteoporosis was investigated in this study.
Methods: Expression levels of GAS5 and miR-21 in plasma of both osteoporosis patients and healthy controls were determined by RT-qPCR. Diagnostic values of GAS5 and miR-21 for osteoporosis were analyzed by ROC curve analysis. Overexpression experiments were used to assess the interactions between GAS5 and miR-21. The roles of GAS5 and miR-21 in the apoptosis of osteoclasts were investigated by cell apoptosis assay.
Results: The present study aimed to investigate the roles of GAS5 in osteoporosis. The results showed that GAS5 was upregulated, while miR-21 was downregulated in plasma of osteoporosis patients. Expression levels of GAS5 and miR-21 were inversely correlated across plasma samples from osteoporosis patients but not the plasma samples from the controls. Altered expression of GAS5 and miR-21 distinguished osteoporosis patients from the controls. In osteoclasts, overexpression of GAS5 led to downregulation of miR-21, while overexpression of miR-21 did not affect the expression of GAS5. Overexpression of GAS5 led to promoted apoptosis of osteoclasts, while overexpression of miR-21 led to suppressed apoptosis of osteoclasts. In addition, overexpression of miR-21 attenuated the enhancing effects of overexpressing GAS5 on cell apoptosis.
Conclusion: GAS5 is upregulated in osteoporosis and may downregulate miR-21 to promote the apoptosis of osteoclasts.
Keywords: osteoporosis, GAS5, miR-21, apoptosis
Background
Osteoporosis, or porous bone, is caused by reduced density and quality of bones.1 With the development of osteoporosis, bones become more fragile and porous, leading to high risk of fracture.2 Currently, more than 200 million people are suffering from osteoporosis. It is estimated that more than 40% females and about 15–30% of males will develop osteoporosis during their lifetime.3,4 The direct medical costs of osteoporosis and indirect cost, such as reduced productivity and survival of the patients, have become a heavy burden on public health.5 Despite the development of therapeutic approaches, fragility fracture in osteoporosis remains to be under-treated in many countries of the world.1,6 Therefore, novel therapeutic and preventative approaches are of great importance.
Aging is the main risk factor for osteoporosis.7 Besides, genetic factors are also critical players in the molecular pathogenesis of osteoporosis.8 In effect, functional characterization of genetic factors involved in the pathogenesis of osteoporosis may provide novel insights into the development of targeted therapies.9 MiR-21 has been proven to play critical roles in osteoporosis.10 For instance, it has been reported that miR-21 can target reversion-inducing cysteine-rich protein with Kazal motifs (RECK) to suppress the progression of osteoporosis.10 It is known that miR-21 is regulated by certain long (>200 nt) non-coding RNAs (lncRNAs), such as growth arrest-specific transcript 5 (GAS5).11 Therefore, we hypothesized that miR-21 may also interact with GAS5 in osteoporosis. This study aimed to investigate the interactions between miR-21 and GAS5 in osteoporosis.
Methods
Osteoporosis Patients and Healthy Controls
This study enrolled 66 osteoporosis patients (42 females and 24 males, 40 to 69 years old, mean age 54.1 ± 5.3 years old) and 66 healthy controls (42 females and 24 males, 40 to 69 years old, mean age 54.3 ± 5.2 years old) who were admitted by the Second Affiliated Hospital of Harbin Medical University were between March 2017 and May 2019. Disease duration ranged from 3.6 to 11.9 years, and the mean duration was 8.4± 2.8 years. Healthy controls matched the distribution of age, gender, smoking and drinking habits, BMI and other clinical data distributions of the patient group. All patients received no therapy within 3 months before admission. No other bone diseases and other severe clinical disorders were observed among patients. Healthy controls had a T score range of −0.81 to 3.22 and the mean value was 1.27 ± 0.35. T score range of patient group was −2.76 to −4.93 and the mean value was −3.33 ± 0.41. So T score was lower in patients than that in the controls. This study passed the review of the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University. All patients and controls were informed of the principle of experimental design and informed consent was signed by all of them.
Treatment and Plasma
Therapeutic approaches were determined according to patients’ conditions. Treatment approaches, such as hormones (estrogen) and bisphosphonates (risedronate, alendronate, and zoledronic acid) or their combinations were used. Before the initiation of therapies, all participants were fasted overnight and blood (5 mL) was extracted. Blood samples were added into BD Vacutainer® PPT™ Plasma Preparation Tubes, followed by centrifugation at 1200 g for 15 min to separate plasma. All plasma samples were frozen in liquid nitrogen, followed by storage at −80°C before use.
Osteoclasts and Transient Transfections
Primary osteoblasts were purchased from Sigma-Aldrich (USA). Following the instructions from Sigma-Aldrich, osteoblasts were cultivated and collated at passage 3 to 6 to perform the following transfections. Vector expressing GAS5 was constructed using pcDNA3.1 vector (Sigma-Aldrich) as the backbone. MiR-21 mimic and negative control (NC) miRNA were synthesized by Takara (Dalian, China). Lipofectamine 2000 (Thermo Fisher Scientific) was used to transfect 10 nM vector (empty vector as NC group) or 50 nM miRNA (NC miRNA as NC group) into 106 osteoblasts. Untransfected cells were used as the Control (C) group. Subsequent experiments were carried out using cells harvested at 48 h post-transfection.
Qrt-Pcr
Trizol reagent (Invitrogen, USA) was used to extract total RNAs. RNA precipitation and washing were performed using 85% ethanol to retain miRNAs. Genomic DNA in RNA samples was removed using LookOut® DNA Erase (Sigma-Aldrich, USA). The SSRT IV system (Invitrogen, USA) was used to perform reverse transcriptions (RTs) with poly (T) as primer. The qPCR mixtures were prepared using Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent, USA). Expression levels of GAS5 were normalized to GAPDH endogenous control. For the quantification of miR-21, All-in-OneTM miRNA qRT-PCR Detection Kit (GeneCopoeia, Shanghai, China) was used to perform polyadenylation, RTs and qPCR assays. Expression levels of miR-21 were normalized to U6 endogenous control. Primer sequences were: 5ʹ-ACACTCCAGCTGGGTAGCTTATCAG-3ʹ (forward) and 5ʹ-TGGTGTCGTGGAGTCG-3ʹ (reverse) for miR-21; 5ʹ-CTTCTGGGCTCAAGTGATCCT-3ʹ (forward) and 5ʹ-TTGTGCCATGAGACTCCATCAG-3ʹ (reverse) for miR-21; 5ʹ-AAGGTGAAGGTCGGAGTCAA-3ʹ (forward) and 5ʹ-ATGAAGGGGTCATTGATGG-3ʹ (reverse) for GAPDH; 5ʹ-GCTTCGGCAGCACATATACTAAAATTGGA-3ʹ (forward) and 5ʹ-CTTCACGAATTTGCGTGTCATCCTTG-3ʹ (reverse) for U6. Three replications were set for each experiment. PCR reaction conditions were: 95°C for 1 min, followed by 40 cycles of 9 5°C for 10 s and 55°C for 45 s. 2−ΔΔCt method was used to calculate the fold changes of gene expression across samples.
Cell Apoptosis Assay
FITC/PI apoptosis kit (Life Technologies, USA) was used to assess the effects of transfections on cell apoptosis. Cells were harvested at 48 h post-transfection and were washed in PBS twice, followed by staining with PI and Annexin-V in dark at room temperature for 20 min. Flow cytometry was performed to separate apoptotic cells. Living, early stage, and late stages apoptotic cells were Annexin-V−/PI−, Annexin-V+/PI−, and Annexin-V+/PI+ cells, respectively.
Statistical Analysis
All data were expressed as mean values using data from 3 independent biological replicates. Unpaired t test was used to compare differences between two groups. ANOVA (one-way) combined with Tukey’s test were used to explore differences among multiple groups. Correlations were analyzed by Pearson’s correlation coefficient. Diagnostic analysis was performed by ROC curve. In ROC curve analysis, osteoporosis patients were true positive cases and healthy controls were true negative cases. p < 0.05 was statistically significant.
Results
GAS5 and miR-21 Showed Opposite Expression Pattern in Osteoporosis Patients
The differential expression of GAS5 and miR-21 in osteoporosis patients was assessed by measuring the expression levels of GAS5 and miR-21 in plasma derived from both osteoporosis patients (n = 66) and healthy controls (n = 66). Unpaired t-test analysis showed that GAS5 was significantly upregulated (Figure 1A) in plasma of osteoporosis patients, while miR-21 was downregulated (Figure 1B) in plasma of osteoporosis patients compared to that in healthy controls (p < 0.05).
GAS5 and miR-21 Were Inversely Correlated in Osteoporosis Patients
Correlations between the expression levels of GAS5 and miR-21 were analyzed by Pearson’s correlation coefficient. It showed that the expression levels of GAS5 were significantly and inversely correlated with the expression levels of miR-21 across plasma samples in osteoporosis patients (Figure 2A). In contrast, the correlation between GAS5 and miR-21 was not significant across plasma samples in healthy controls (Figure 2B).
Altered Plasma Levels of GAS5 and miR-21 Distinguished Osteoporosis Patients from Healthy Controls
The diagnostic values of plasma levels of GAS5 and miR-21 for osteoporosis were analyzed by performing ROC curve analysis. For plasma GAS5, the area under the curve was 0.91, with 95% confidence interval of 0.85–0.95 and standard error of 0.025 (Figure 3A). For plasma miR-21, the area under the curve was 0.95, with 95% confidence interval of 0.91–0.98, and standard error of 0.018 (Figure 3B).
GAS5 Could Bind with miR-21 at 3ʹ-UTR
From RNAs interaction detected by IntaRNA (http://rna.informatik.uni-freiburg.de/IntaRNA/), we obtained that lncRNA GAS5 might bind with miR-21 (Figure 4A) at 3ʹ-UTR. To confirm our finding from IntaRNA database, RNA pull-down assay was conducted to detect this interaction. lncRNA GAS5 could only be precipitated by miR-21 probe but it was undetectable in the product precipitated by control probe (Figure 4B, p < 0.001), indicating that miR-21 directly interacts with lncRNA GAS5 in osteoblasts.
Overexpression of GAS5 Downregulated miR-21 in Osteoblasts
Osteoblasts were transfected with GAS5 overexpression vector or miR-21 mimic. Overexpression of GAS5 and miR-21 was confirmed by qPCR at 24 h post-transfection (Figure 5A and B, p < 0.05). Compared to the NC and C groups, overexpression of GAS5 led to downregulation of miR-21 (Figure 5B, p < 0.05). In contrast, overexpression of miR-21 did not affect the expression of GAS5 (Figure 5C).
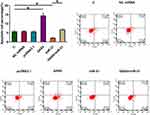
Overexpression of GAS5 Promoted Osteoclast Apoptosis Through miR-21
Compared to untransfected cells (C) and cells transfected with miRNA mimic or empty expression vector, overexpression of GAS5 led to promoted, while overexpression of miR-21 led to suppressed apoptosis of osteoclasts. In addition, overexpression of miR-21 attenuated the effects of overexpressing GAS5 (Figure 6, p < 0.05).
Discussion
This study mainly investigated the interactions between GAS5 and miR-21 in osteoporosis. We found that GAS5 was upregulated in osteoporosis patients and it could downregulate miR-21 to promote the apoptosis of osteoclasts.
Previous studies have characterized a considerable number of differentially expressed lncRNAs involved in the development and pathogenesis of osteoporosis.12 In fact, lncRNAs have been proven to be critical players in osteoporosis.13,14 For instance, lncRNA maternally expressed 3 (MEG3) is overexpressed in osteoporosis and can directly target miR-133a-3 to suppress osteogenic differentiation of bone marrow mesenchymal stem cells.13 In another study, lncRNA long intergenic non-protein coding RNA 311 (LINC00311) is reported to target delta-like ligand 3 (DLL3), thereby promoting the differentiation and proliferation of osteoclasts.14 GAS5 is a well-characterized tumor-suppressive lncRNA in different types of cancer with downregulated expression pattern.15,16 GAS5 plays tumor-suppressive roles mainly by regulating cell behaviors like enhancing cancer cell apoptosis.15,16 To the best of our knowledge, this study is the first to report the upregulation of GAS5 in osteoporosis. In addition, we observed increased apoptotic rate of osteoclasts after the overexpression of GAS5. Therefore, GAS5 might have different expression patterns in different types of disease but it plays similar roles in regulating cell apoptosis.
Osteoclasts mediate the continuous destruction of bone to promote the development and progression of osteoporosis.17 Therefore, osteoclast apoptosis may serve as a potential therapeutic target for the treatment of osteoporosis.17 MiR-21 was reported to play protective roles in osteoporosis by targeting RECK (reversion-inducing cysteine-rich protein with Kazal motifs).10 In this study, we observed decreased apoptotic rate of osteoclasts after the overexpression of miR-21. Therefore, miR-21 may also play enhancing roles in the development of osteoporosis. A recent study reported that GAS5 may suppress cell invasion and migration through the downregulation of miR-21.11 Our results also showed downregulated miR-21 after the overexpression of GAS5. In addition, overexpression of GAS5 reduced the inhibitory effects of overexpressing miR-21 on cell apoptosis. Therefore, GAS5 may downregulate miR-21 to increase the apoptotic rate of osteoclasts, thereby playing protective roles in osteoporosis.
In conclusion, GAS5 is upregulated in osteoporosis and may downregulate miR-21 to promote osteoclast apoptosis.
Data Sharing Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The present study was approved by the Ethics Committee of The Second Affiliated Hospital of Harbin Medical University. The research has been carried out in accordance with the World Medical Association Declaration of Helsinki. All patients and healthy volunteers provided written informed consent prior to their inclusion within the study.
Author Contributions
Chunlei Cong, Jun Tian designed the experiments and paper, collected the data and analyzed the data, prepared and revised the manuscript. Tianqi Gao, Changlin Zhou, Yuxiang Wang, Xintao Cui, Liyu Zhu collected the data and analyzed the data, edited the manuscript. All the authors gave final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors declare that they have no competing interests.
References
1. Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5(11):898–907. doi:10.1016/S2213-8587(17)30188-2
2. Cauley JA. Osteoporosis: fracture epidemiology update 2016. Curr Opin Rheumatol. 2017;29(2):150–156. doi:10.1097/BOR.0000000000000365
3. K N T, J D L, Wan CKV, et al. Osteoporosis: a review of treatment options. P T. 2018;43(2):92–104.
4. Chen P, Li Z, Hu Y. Prevalence of osteoporosis in China: a meta-analysis and systematic review. BMC Public Health. 2016;16(1):1039. doi:10.1186/s12889-016-3712-7
5. Vijayakumar R, Büsselberg D. Osteoporosis: an under-recognized public health problem. J Local Global Health Sci. 2016;2016(1):2. doi:10.5339/jlghs.2016.2
6. Qaseem A, Forciea MA, McLean RM, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818–839. doi:10.7326/M15-1361
7. Yu B, Wang CY. Osteoporosis: the result of an ‘aged’ bone microenvironment. Trends Mol Med. 2016;22(8):641–644. doi:10.1016/j.molmed.2016.06.002
8. Rosen CJ. The Epidemiology and Pathogenesis of Osteoporosis[M]//Endotext [Internet]. MDText. com, Inc; 2017.
9. Deal C. Future therapeutic targets in osteoporosis. Curr Opin Rheumatol. 2009;21(4):380–385. doi:10.1097/BOR.0b013e32832cbc2a
10. Zhao W, Dong Y, Wu C, et al. MiR-21 overexpression improves osteoporosis by targeting RECK. Mol Cell Biochem. 2015;405(1–2):125–133. doi:10.1007/s11010-015-2404-4
11. Hu L, Ye H, Huang G, et al. Long noncoding RNA GAS5 suppresses the migration and invasion of hepatocellular carcinoma cells via miR-21. Tumour Biol. 2016;37(2):2691–2702. doi:10.1007/s13277-015-4111-x
12. Hao L, Fu J, Tian Y, et al. Systematic analysis of lncRNAs, miRNAs and mRNAs for the identification of biomarkers for osteoporosis in the mandible of ovariectomized mice. Int J Mol Med. 2017;40(3):689–702. doi:10.3892/ijmm.2017.3062
13. Wang Q, Li Y, Zhang Y, et al. LncRNA MEG3 inhibited osteogenic differentiation of bone marrow mesenchymal stem cells from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed Pharmacother. 2017;89:1178–1186. doi:10.1016/j.biopha.2017.02.090
14. Wang Y, Luo TB, Liu L, et al. LncRNA LINC00311 promotes the proliferation and differentiation of osteoclasts in osteoporotic rats through the notch signaling pathway by targeting DLL3. Cell Physiol Biochem. 2018;47(6):2291–2306. doi:10.1159/000491539
15. Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta. 2013;1832(10):1613–1623. doi:10.1016/j.bbadis.2013.05.005
16. Shi X, Sun M, Liu H, et al. A critical role for the long non‐coding RNA GAS5 in proliferation and apoptosis in non‐small‐cell lung cancer. Mol Carcinog. 2015;54(S1):E1–E12. doi:10.1002/mc.22120
17. Teitelbaum SL. Osteoclasts, integrins, and osteoporosis. J Bone Miner Metab. 2000;18(6):344–349. doi:10.1007/s007740070007
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.